High-resolution and high-accuracy topographic and transcriptional maps of the nucleosome barrier
Figures
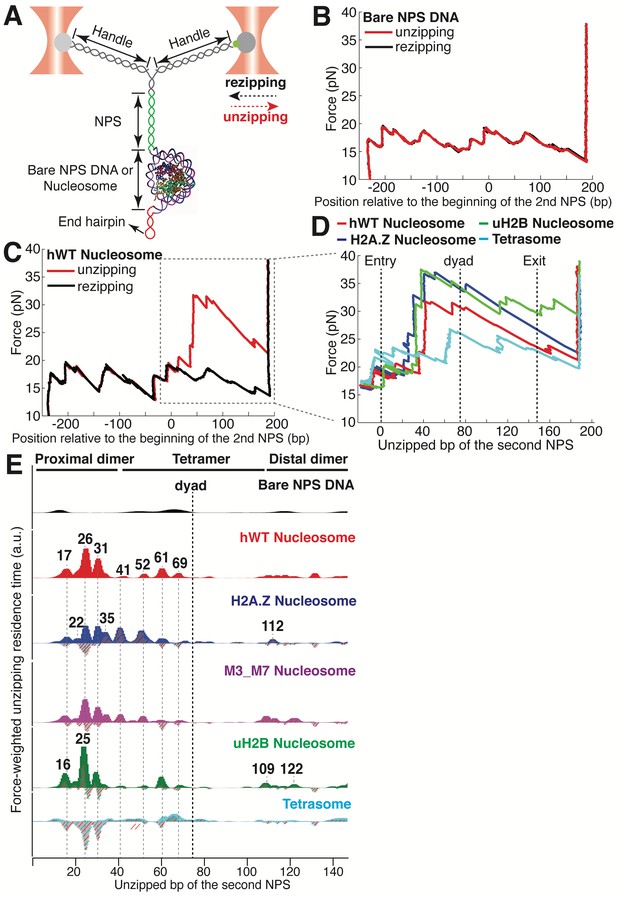
Dual-trap Optical Tweezers Single-molecule Unzipping Assay Unwinds Nucleosomal DNA and Maps Histone-DNA Interactions.
(A) Geometry of the single-molecule unzipping assay. Dashed arrows denote directions of trap movement (20 nm/s) during unzipping (red arrow) or rezipping (black arrow). Two DNA handles connect to the template DNA, which consists of two tandem NPS repeats and an end hairpin. Diagram illustrates nucleosome unzipping, with the second NPS replaced with a pre-assembled mononucleosome. For simplicity, linkers and restriction sites flanking the NPS are not shown. (B, C) Unzipping (red) and rezipping (black) traces of bare NPS DNA (B) and a single WT human nucleosome (C). The presence of the nucleosome on the second NPS causes characteristic high force (20–40 pN) transitions that correspond to disruption of histone-DNA contacts. The unzipped basepairs (bp) are normalized to the beginning of the second NPS. The nucleosome rezipping trace matches that of bare NPS DNA, indicating complete histone removal during unzipping. (D) Representative unzipping traces of tetrasome (cyan), WT (red), H2A.Z (blue), and uH2B (green) nucleosomes. For clarity, only the region after entering the second NPS (corresponding to the boxed region in (C)) is shown, with the unzipped bp normalized to the beginning of the second NPS. The three dashed lines are entry, dyad, and exit of the second NPS, respectively. Rezipping traces, identical to those of B and C, are not shown. (E) Topography maps are plotted as force-weighted residence time (RT) histograms of the unzipping fork along bare NPS DNA, tetrasome and different types of nucleosomes during unzipping at constant trap separation speed of 20 nm/s. The gray histograms with colored stripes (excluding Bare NPS DNA and WT Nucleosome) are residual plots found by subtracting the WT nucleosome RTs. Unzipped bp are normalized to the beginning of the second NPS core. Major peaks are highlighted with gray dashed lines, with the peak positions (in bp) labeled above the peaks. (Left to right: 17, 22, 26, 31, 35, 41, 52, 61, 69, 109, 112, 122 bp). n = 34, 41, 34, 39, 35, 10, respectively for NPS DNA, hWT, H2A.Z, M3_M7, uH2B nucleosome and tetrasome. See also Figure 1—figure supplement 1 for representative unzipping traces and analysis.
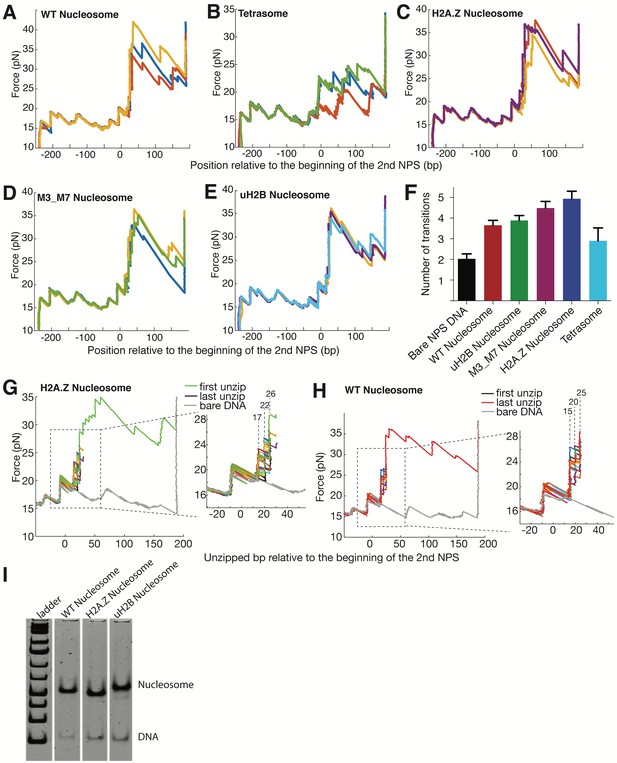
Unzipping Traces of Single Human WT, H2A.Z, M3_M7, uH2B Nucleosomes and Tetrasomes.
(A–E) Representative unzipping traces of WT nucleosomes (A), tetrasomes (B), H2A.Z nucleosomes (C), M3_M7 nucleosomes (D) and uH2B nucleosomes (E). Rezipping traces are not shown and they match bare NPS DNA rezipping traces. The unzipped bp (basepairs) are normalized to the beginning of the second NPS core. (F) Number of transitions per trace at the second NPS region. H2A.Z nucleosomes have on average one more transition per trace than WT or uH2B nucleosomes. A transition event is counted when the residence time peak is above an arbitrary threshold. (G–H) Partial unzipping of H2A.Z (G) and WT (H) nucleosomes reveals no lateral mobility induced by multiple rounds of unzipping-rezipping. The unzipping fork repeatedly propagates to the proximal dimer region followed by rezipping (not shown for clarity). The inset shows zoomed-in view of the boxed region, where the position of initial force rise remains unchanged. The dwelling of the unzipping fork in alternative positions (labeled above the dashed lines in bp) is consistent with hopping observed in this region. (I) Native PAGE gels showing homogenous WT, H2A.Z and uH2B nucleosome samples used for single-molecule unzipping experiments.
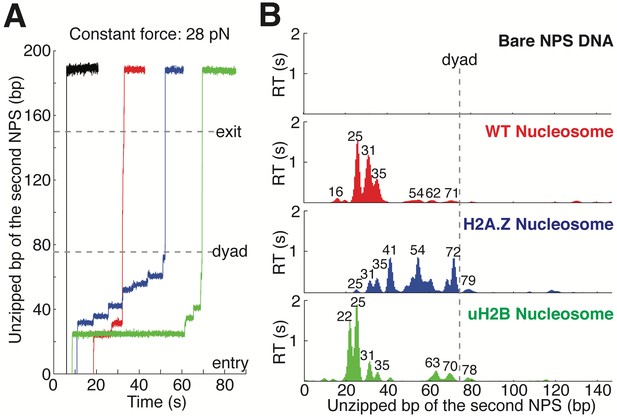
Topography Maps of the Nucleosome Revealed by Nucleosome Unzipping at Constant Force.
(A) Representative unzipping traces of bare NPS DNA (black), WT (red), H2A.Z (blue) and uH2B (green) nucleosomes at 28 pN constant force. Unzipped bp are normalized to the beginning of the second NPS. Dashed lines mark entry, dyad and exit regions of the second NPS. Traces are shifted horizontally for clarity. (B) Mean residence time (RT) histogram of the unzipping fork along bare NPS DNA (black), WT (red), H2A.Z (blue) and uH2B (green) nucleosomes during unzipping at a constant force of 28 pN. Bare NPS RTs are too short to see on the axes shown. Unzipped bp are normalized to the beginning of the second NPS core. Major peak positions are indicated above each peak (in bp). n = 33, 17, 20, 20, respectively for NPS DNA, WT, H2A.Z and uH2B nucleosomes. See also Figure 2—figure supplement 1 on assembly cooperativity of H2A.Z nucleosomes.
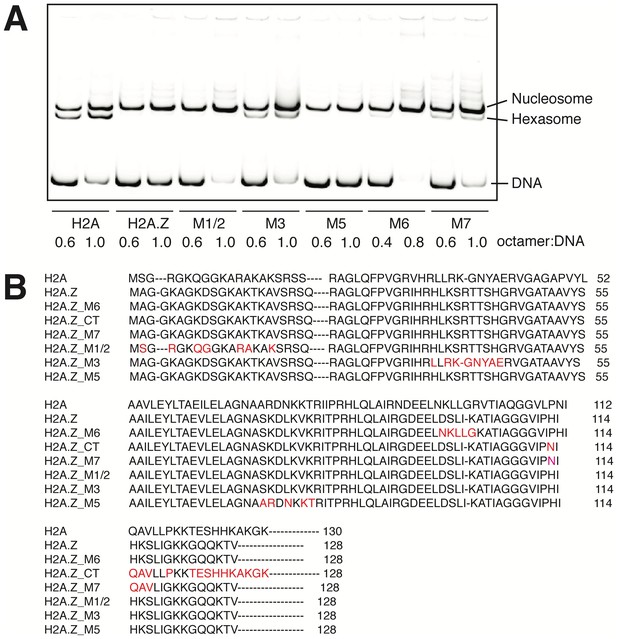
H2A.Z Nucleosomes Assemble More Cooperatively than WT nucleosomes.
(A) Sequence swaps between H2A and H2A.Z reveal important regions for hexasome formation. The native PAGE gel shows the propensity to form hexasomes during assembly of H2A, H2A.Z and swapped mutant nucleosomes. DNA is Cy5-labeled 70N0 where ‘N’ denotes the 601 NPS. We found that this DNA configuration is more prone to hexasome formation due to the asymmetric nature of the 601 sequence. Two octamer-to-DNA ratios are tested for each sample and are shown below its corresponding lanes. The nucleosome, hexasome or DNA bands are indicated on the right. (B) Sequence alignment of H2A and H2A.Z swap mutants. Nomenclature of the swap mutants follows Clarkson et al. (1999).
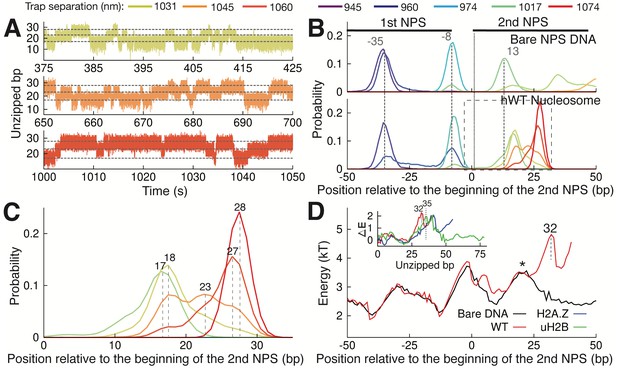
Observation of Multiple Nucleosomal States at the Proximal Dimer Region.
(A) Time traces of number of base pairs unzipped (relative to beginning of the second NPS) with hWT nucleosome for fixed trap separations of 1031 nm, 1045 nm, and 1060 nm (top to bottom). Color indicates increasing trap separation (purple to red), corresponding to clusters in Figure 3—figure supplement 1F. Gray dashed lines indicate 17, 23, and 28 base pairs unzipped. (B) Probability distributions for the number of DNA bps unzipped, computed from force-extension data in Figure 3—figure supplement 1F. Each curve is from a different trap separation, matching colors in A and Figure 3—figure supplement 1F. Distributions are shown for both bare DNA (top) and WT nucleosome (bottom). Vertical black dotted line indicates the start of the second NPS. Vertical gray dashed lines indicate peak positions for bare DNA (with position in bp labeled), showing that WT nucleosome shifts the first peak within the NPS, and gives rise to an additional peak at 28 bp. See Figure 3—figure supplement 1F for force-extension data. (C) Zoomed-in view of the black dashed box in (B). Peak positions are labeled in bp. (D) DNA unzipping energy computed by assuming the unzipped bp distributions from data in Figure 3—figure supplement 1F (including distributions in B) correspond to equilibrium Boltzmann statistics. Inset ∆E shows the DNA-octamer interaction energy, computed as the difference between unzipping energies in the presence of WT (red), H2A.Z (blue), and uH2B (green) nucleosomes and unzipping energies for bare DNA only (black). Vertical black dashed lines and * indicate peak positions (labeled in bp). See also Figure 3—figure supplement 1 on hopping traces and analysis of energy landscape from equilibrium data.
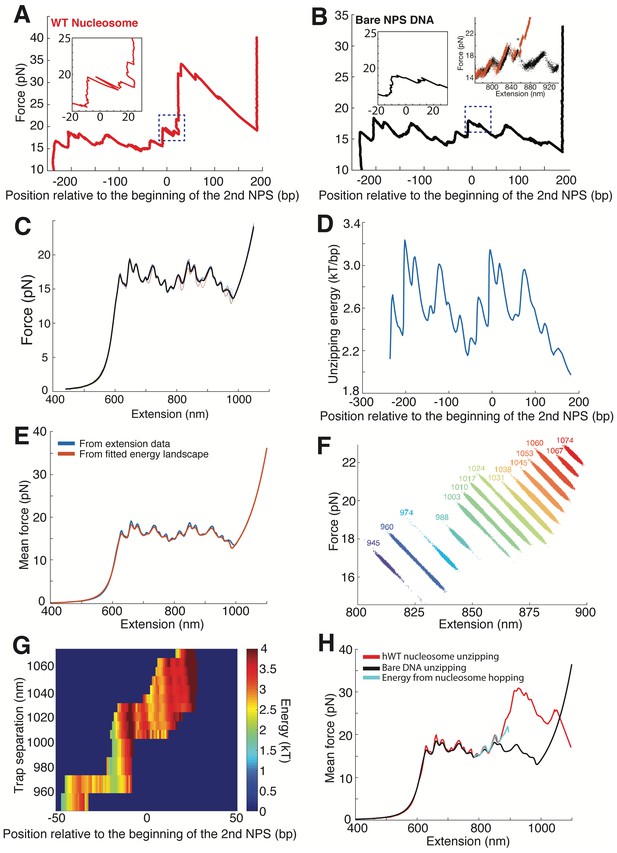
Hopping of the Unzipping Fork Near the Proximal Dimer Region of the Nucleosome.
(A, B) Unzipping traces of human WT nucleosome (A) and bare NPS DNA (B). The slow hopping event near the proximal dimer region of WT nucleosome is indicated with a dashed blue square box; no similar slow hopping was observed in the corresponding region during unzipping of bare NPS DNA. Insets are the zoomed-in view of the dashed square boxes. Raw force-extension unzipping curves of hWT nucleosome (orange dots) and bare DNA (black dots) near the hopping region are plotted at maximum bandwidth (800 Hz), with the asterisk highlighting the nucleosome-specific slow hopping event (top right inset in B). Unzipped bp is normalized to the beginning of the second NPS. Rezipping traces are not shown for clarity. (C) Aligned individual force-extension curves (thin colored curves) and mean force-extension curve (thick black curve), for bare DNA. (D) Energy of DNA unzipping for each base pair, calculated from mean force-extension curve. (E) Comparison of experimental mean force-extension curve (blue) to the force-extension calculated from the extracted DNA unzipping energy (red). (F) Force-extension traces obtained at fixed trap separations with WT nucleosome. Color indicates increasing trap separation (purple to red), with number indicating the trap separation in nm. (G) DNA unzipping energy for each base pair, calculated from equilibrium hopping data at multiple fixed trap separations as in (F). (H) Comparison of experimental mean force-extension curve for bare DNA (black) and DNA with a WT nucleosome (red) to the force-extension curve predicted by the apparent DNA unzipping energy from equilibrium hopping data for the WT nucleosome (cyan).
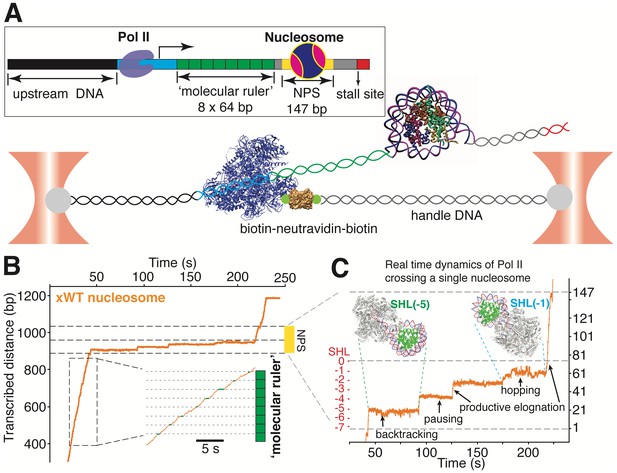
A ‘Molecular Ruler’ Gauges the Positions of an Elongating Pol II with Near-Basepair Accuracy.
(A) Experimental design of an improved single-molecule nucleosomal transcription assay. A single biotinylated Pol II (purple molecular structure) is tethered between two optical traps. Pol II transcription is measured as increases in distance between the two beads at 10 pN constant force. The inset box shows the composition of the template, which is constructed by ligating Pol II stalled complex (cyan), the molecular ruler (green), NPS DNA (or nucleosome, yellow-gray), and a short inter-strand crosslinked DNA (for stalling Pol II, red). The ‘molecular ruler’ consists of eight tandem repeats of a 64 bp DNA (green), each harboring a single sequence-encoded pause site. (B) A representative trace of a single Pol II transcribing through a Xenopus WT nucleosome. The three black dashed lines indicate NPS entry, dyad and NPS exit, respectively. The inset shows a zoomed-in view of the boxed region, highlighting the repeating pause patterns within the ‘molecular ruler’. The gray dashed lines are the predicted pause sites, whereas the short green lines mark the actual pauses of Pol II. (C) Zoomed-in view of Pol II dynamics within the NPS region of (B). The three black dashed lines indicate NPS entry, dyad and NPS exit, respectively. The right y-axis (in bp) is normalized to the beginning of the NPS. The left y-axis shows regions preceding the dyad as SHL in red. Black arrows indicates representative events of backtracking, pausing, productive elongation, and hopping. Regions corresponding to Pol II located at SHL(−5) and SHL(−1) are indicated with green and cyan dashed lines, with the corresponding Pol II-nucleosome complex structures plotted on top (PDB 6A5P for PolII-SHL(−5), 6A5T for PolII-SHL(−1)). Pol II, histones, template DNA, non-template DNA are colored in gray, green, red and blue, respectively. See also Figure 4—figure supplement 1 on detailed characterization of the ‘molecular ruler’.
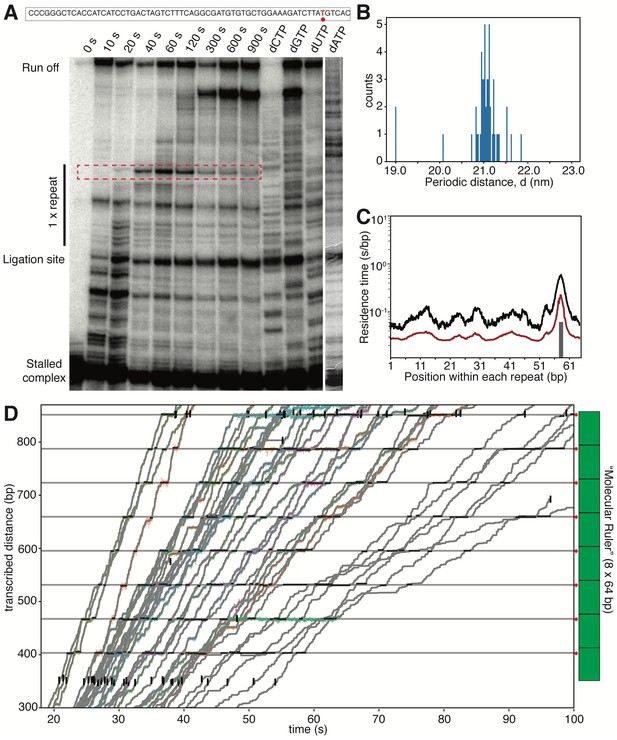
Biochemical and Single-molecule Characterization of the ‘Molecular Ruler’.
(A) In vitro transcription assay identifies a major pause site within a single repeat sequence (64 bp). The band corresponding to the pause site is highlighted with a dotted red box. The sequence of the single repeat template DNA is shown above the gel, with the identified pause site highlighted in red. (B) Histogram of the length of one repeat unit (periodicity, (d). From aligned traces of Pol II transcription through xWT nucleosomes, d is calculated to be 21.1 ± 0.3 nm. (C) Mean (black) and median (red) residence time (in log scale) of Pol II transcribing through the repeat sequence confirms a single major pause site at 59 bp in the repeat sequence, matching the site identified in (A). (D) Zoomed in view of the alignment of traces using the ‘molecular ruler’ (cartoon on the right). The major pause site within each repeat sequence is marked with a gray horizontal line and a red dot next to the ‘molecular ruler’. Short horizontal black lines indicate identified pauses and vertical black lines (with the exception of few cases where the tether breaks in the middle) indicate the entry and exit of the ‘molecular ruler’.
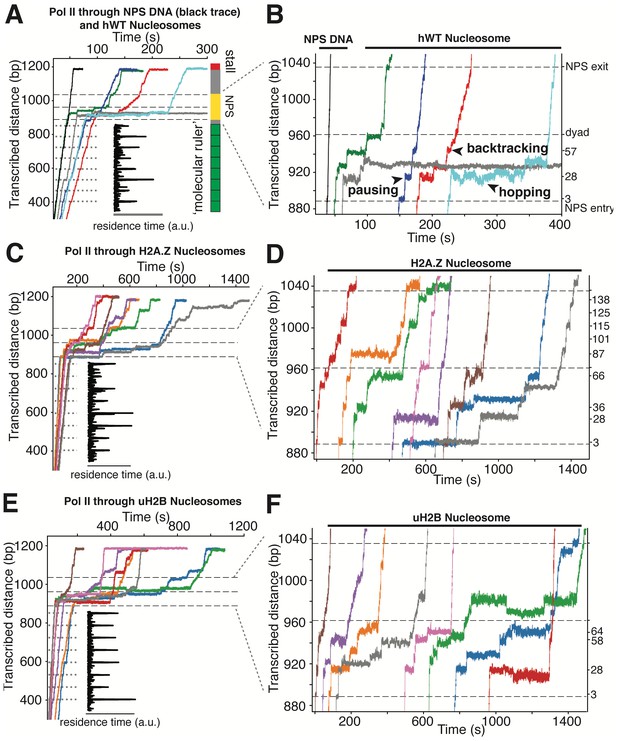
High-resolution Trajectories of Individual Pol II Enzymes Transcribing through WT, H2A.Z and uH2B Nucleosomes.
(A, B) Representative traces of single Pol II enzymes transcribing through single human WT nucleosomes. The gray dotted lines are the pause sites within the ‘molecular ruler’. The inset (black) is the residence time of Pol II within the ‘molecular ruler’, highlighting repeating pausing signatures of Pol II. The three black dashed lines indicate NPS entry, dyad and NPS exit. Relative positions of Pol II on the template DNA are shown as a cartoon on the right. The traces in blue, green, red and cyan are examples of successful nucleosome crossing, while the trace in gray is an example of Pol II arrest in the nucleosome. For comparison, a trace of Pol II transcribing through bare NPS DNA (black) is shown on the left. Zoomed in traces of high-resolution Pol II dynamics within the NPS are shown in (B), highlighting (black arrowheads) long-lived pausing, backtracking and hopping events. The traces are shifted horizontally for clarity. The right y-axis is normalized to the beginning of the NPS, with the major pause positions marked (in bp) on the right. (C, D) Representative traces of single Pol II enzymes transcribing through single human H2A.Z nucleosomes. (C) shows the full traces and (D) is a zoomed-in view of the high-resolution dynamics within the NPS region. (E, F) Representative traces of single Pol II enzymes transcribing through single human uH2B nucleosomes. (E) shows the full traces and (F) is a zoomed-in view of the high-resolution dynamics within the NPS region. See also Figure 5—figure supplement 1 on backtracking and hopping dynamics.
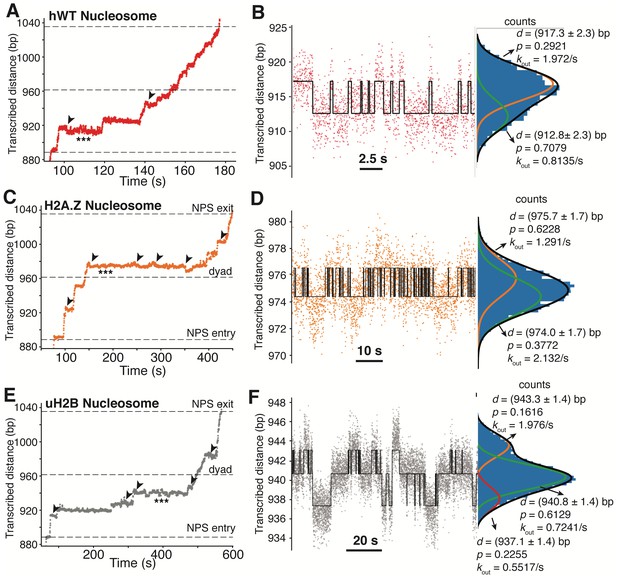
Long-lived Pauses of Pol II in the Nucleosome are Associated with Backtracking and Hopping Dynamics.
(A, B) Representative traces of backtracking (A) and hopping (B) dynamics of Pol II during transcription through an hWT nucleosome. The trace is the same as the red trace in Figure 5A and B. The black arrowheads in (A) highlight backtracking events right before long-lived pauses. The triple-stars highlight the region where Pol II has hopping dynamics, the zoomed in view of which is shown in (B). The hopping trace is fitted as two states with a classic hidden Markov model (red is raw data, black is fitted trace). The fitted parameters and histogram of Pol II position counts are shown to the right side. d is the distance transcribed, p is the probability in that state, and kout is the rate of transitioning to the other state. (C, D) Representative traces of backtracking (C) and hopping (D) dynamics of Pol II during transcription through an H2A.Z nucleosome. The trace is the same as the orange trace in Figure 5C and D. The data is analyzed and shown as in (A, B). (E, F) Representative traces of backtracking (E) and hopping (F) dynamics of Pol II during transcription through an uH2B nucleosome. The trace is the same as the gray trace in Figure 5E and F. The data is analyzed and shown as in (A, B) except that the trace in (F) is fitted as three-states.
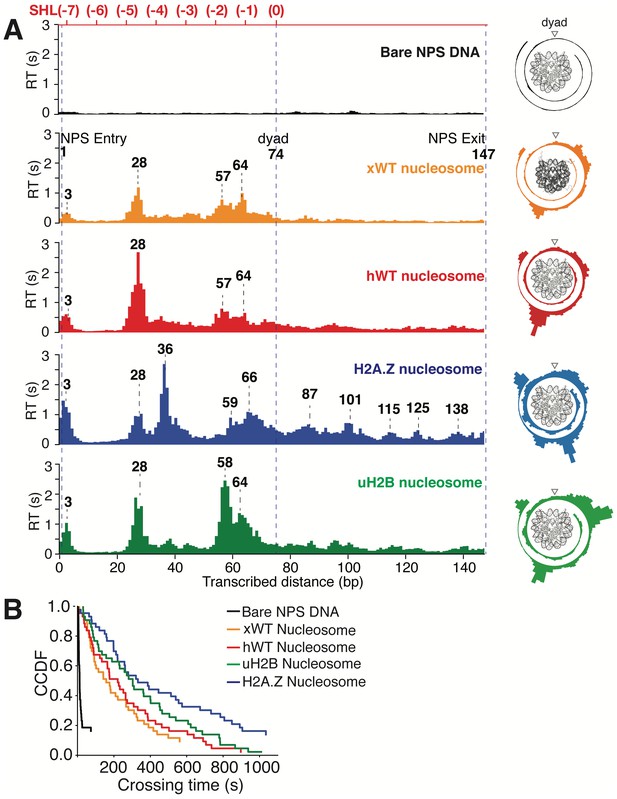
Transcriptional Maps of the Nucleosome Reveal that H2A.Z Enhances the Width and uH2B the Height of the Barrier.
(A) Median residence time histograms of Pol II transcription through bare NPS DNA (black), xWT (orange), hWT (red), H2A.Z (blue) and uH2B (green) nucleosomes. Bar width is 1 bp and major peak positions are labeled (in bp) above the corresponding peaks. NPS entry, dyad, NPS exit are marked with blue dashed lines. The polar plots on the right are the corresponding transcriptional maps of the nucleosome, formed by projecting the residence time histogram onto the surface of nucleosomal DNA. The top axis (red) indicates corresponding positions of the first half of nucleosome expressed as superhelical locations (SHL). n = 35, 23, 26, 21, 31, respectively for NPS DNA, xWT, hWT, H2A.Z and uH2B nucleosomes. (B) Crossing time (total time Pol II takes to cross the entire nucleosome region) distributions plotted using the complementary cumulative distribution function (CCDF, fraction of events longer than a given crossing time). Crossing times of Bare NPS DNA, Xenopus WT (xWT), human WT (hWT), uH2B and H2A.Z nucleosomes are plotted in black, orange, red, green and blue, respectively. See also Figure 6—figure supplement 1 on statistics of the crossing time, crossing probability, pause-free velocity and arrest position.
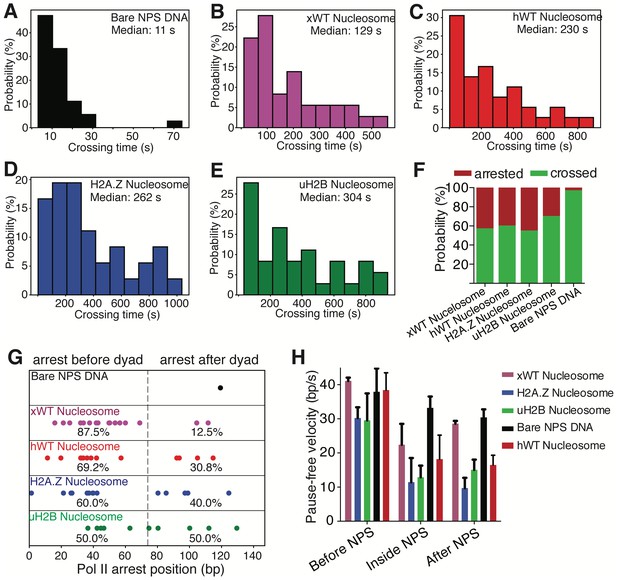
Crossing Time, Crossing Probability and Pause-free Velocity of Pol II during Transcription through NPS DNA or Nucleosomes.
(A–E) Histograms of crossing time of Pol II transcription through bare NPS DNA (A), xWT (B), hWT (C), H2A.Z (D) and uH2B (E) nucleosomes. (F) Relative percentage of Pol II molecules that are arrested or crossed during transcription through bare NPS DNA or nucleosomes. (G) Pol II arrest positions within the NPS. The positions are normalized to the beginning of the NPS. Each dot is a single arresting event. The percentages of arresting before or after dyad are shown below the dots. (H) Pause-free velocity of Pol II molecules before, inside and after NPS during transcription through bare NPS DNA, and xWT, hWT, H2A.Z and uH2B nucleosomes. Only traces that reached the stall site at the end of the template are considered. Pause-free velocities are calculated in three fastest regions (to partially correct for velocity differences due to sequence variations) up to100 bp before, inside, and up to 100 bp after NPS.
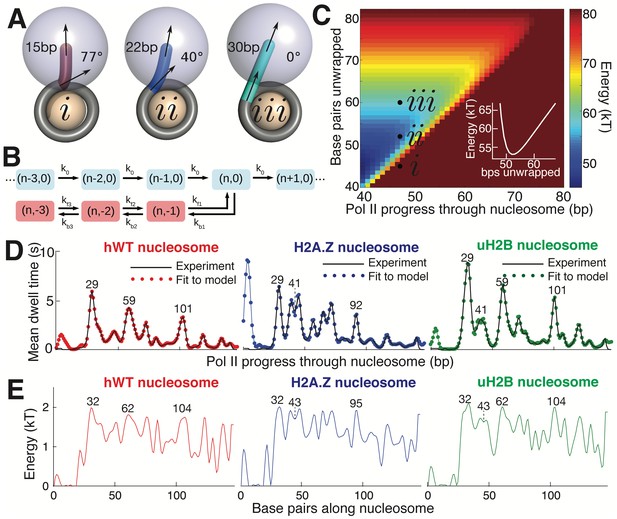
Mechanical Model for Pol II Transcription Through the Nucleosome.
(A) Schematic of the mechanical model, showing three different lengths of unwrapped DNA for a given polymerase position along the DNA sequence. The steric spheres are shown in purple (polymerase) and beige (nucleosome), while the DNA is shown as a tube. (i) shows a configuration with a short, sharply bent DNA linker connecting Pol II and the nucleosome, which are in contact and sterically pushing on each other. (ii) shows a medium-length straighter linker, with Pol II still pushing on the nucleosome. (iii) shows a long straight linker without contact between Pol II and the nucleosome. Linker DNA color corresponds to overall energy for each configuration (given in C). Black arrows represent tangent orientations of the DNA backbone at the point of polymerase binding (top) and for the last contact with the nucleosome (bottom). Linker length and bending angle (between indicated tangents) are labeled on each polymerase-nucleosome pair. (B) Model of Pol II dynamics. Pairs (p,q) indicate the Pol II state: p indicates the length of the RNA transcript, and q the number of base pairs backtracked from the most recent main pathway state. Pol II steps forward one base pair with rate k0 or can enter a backtracked pathway by stepping backward one base pair at rate kb1. From backtracked positions, Pol II can move forward a base pair with rate kfn or can backtrack another base pair at rate kbn. Moving forward from the first backtracked state returns Pol II to the main pathway. (C) Energy landscape of nucleosome-Pol II interaction, for constant DNA-nucleosome interaction energies of 1kBT per base pair. DNA unwrapping decreases the DNA linker conformational energy, while removing favorable DNA-nucleosome interactions, overall providing a minimum energy a few base pairs ahead of the front edge of Pol II. Forward Pol II steps are unfavorable as they shorten the DNA linker. Points i, ii, and iii correspond to configurations illustrated in A. Inset shows cross-section of energy landscape at Pol II position of 47 bp, highlighting the minimum in the energy landscape a few bps ahead of Pol II, at ~52 bps unwrapped. Pol II progress through the nucleosome is defined as the position of the Pol II center plus an additional 17 bp for consistency with the transcribed distance in Figure 6. (D) Dwell time profiles for human WT, H2A.Z, and uH2B nucleosomes. Solid black lines are experimental mean dwell times and colored dotted lines are the best fitted mean dwell times according to the mechanical model. (E) Estimated DNA-octamer interaction energy profiles for human WT, H2A.Z, and uH2B nucleosomes. The energy values are found such that they give the best fitted dwell times shown in (D). Peak positions referenced in the text are labeled in bp, relative to the start of the NPS. See also Figure 7—figure supplement 1 for fitting of nucleosome energy profiles based on Pol II dwell times.
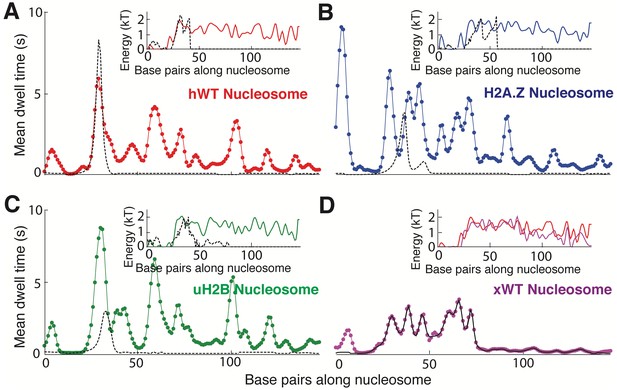
Fitting Nucleosome Energy Profiles Based on Pol II Dwell Times.
(A–C) Mean dwell times (colored dotted lines) calculated from best-fit mechanical model (see Figure 7D–E) whose corresponding nucleosome binding energies (insets, colored lines) are shown for (A) hWT, (B) H2A.Z, and (C) uH2B nucleosomes. The black dashed lines of the inset are binding energy data obtained from unzipping under equilibrium conditions (hopping, see Figure 3D inset), and black dashed lines of the main plots are dwell times calculated from these energy landscapes. The hopping data covers a narrow region of sequence, and therefore allows prediction of Pol II pausing only at the start of the NPS. (D) The experimental (black lines) and calculated mean dwell time (dotted line in magenta) for Xenopus WT (xWT) nucleosome, with the energy landscape extracted from the experimental mean dwell time using the same procedure as for hWT, H2A.Z, and uH2B (Figure 7D). Inset compares hWT (red) and xWT (magenta) energy landscapes.
Videos
Unzipping-rezipping of bare NPS DNA.
https://doi.org/10.7554/eLife.48281.004Unzipping-rezipping of hWT nucleosome.
https://doi.org/10.7554/eLife.48281.007Pol II transcription through bare NPS DNA.
The horizontal gray dashed lines indicate predicted pause sites in the molecular ruler, the three horizontal black dashed lines represent NPS entry, dyad, and NPS exit, respectively. This applies to all other videos.
Pol II transcription through bare NPS DNA, NPS zoom.
https://doi.org/10.7554/eLife.48281.013Pol II transcription through xWT nucleosome.
https://doi.org/10.7554/eLife.48281.018Pol II transcription through xWT nucleosome, NPS zoom.
https://doi.org/10.7554/eLife.48281.019Pol II transcription through hWT nucleosome.
https://doi.org/10.7554/eLife.48281.020Pol II transcription through hWT nucleosome, NPS zoom.
https://doi.org/10.7554/eLife.48281.021Pol II transcription through H2A.Z nucleosome.
https://doi.org/10.7554/eLife.48281.022Pol II transcription through H2A.Z nucleosome, NPS zoom.
https://doi.org/10.7554/eLife.48281.023Pol II transcription through uH2B nucleosome.
https://doi.org/10.7554/eLife.48281.024Pol II transcription through uH2B nucleosome, NPS zoom.
https://doi.org/10.7554/eLife.48281.025Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | NeutrAvidin (deglycosylated native avidin from egg whites) | Thermo Fisher | Cat# PI31000 | powder dissolved in PBS, 0.5 µM |
| Antibody | Anti-Digoxigenin from sheep | Sigma-Aldrich | Cat# 11333089001, RRID:AB_514496 | powder dissolved in PBS, 0.2 mg/ml |
| Strain | DH5α competent cells | Fisher Scientific | Cat# 18-265-017 | |
| Strain | BL21(DE3) competent cells | NEB | Cat# c2527H | |
| Strain | Agilent SURE 2 Supercompetent Cells | Fisher Scientific | Cat# 200152 | |
| Chemical compound | dNTP set 100 mM Solutions | Fisher Scientific | Cat# R0181 | |
| Chemical compound | NTP set 100 mM Solutions | Fisher Scientific | Cat# R0481 | |
| Chemical compound | 3'-Deoxyadenosine-5'-Triphosphate | TriLink Biotechnologies | Cat# N3001 | |
| Chemical compound | 3'-Deoxyguanosine- 5'-Triphosphate | TriLink Biotechnologies | Cat# N3002 | |
| Chemical compound | 3'-Deoxycytidine-5'-Triphosphate | TriLink Biotechnologies | Cat# N3003 | |
| Chemical compound | 3'-Deoxyuridine-5'-Triphosphate | TriLink Biotechnologies | Cat# N3005 | |
| Chemical compound | Trioxsalen | Sigma-Aldirch | Cat# T6137 | |
| Chemical compound | [α−32P]-ATP | Perkin Elmer | Cat# BLU003H250UC | |
| Commercial assay or kit | T4 DNA ligase | NEB | Cat# 0202L | |
| Commercial assay or kit | E. coli DNA ligase | NEB | Cat# 0205L | |
| Commercial assay or kit | Phusion high-fidelity DNA polymerase | NEB | Cat# M0530S | |
| Commercial assay or kit | DraIII-HF | NEB | Cat# R3510S | |
| Commercial assay or kit | BsaI-HF | NEB | Cat# R3535L | |
| Commercial assay or kit | BglI | NEB | Cat# R0143S | |
| Commercial assay or kit | EagI-HF | NEB | Cat# R3505S | |
| Commercial assay or kit | SapI | NEB | Cat# R0569S | |
| Sequence-based reagent | Lambda DNA | NEB | Cat# N3011S | |
| Recombinant DNA reagent | Primers for making constructs and DNA templates | This paper | IDT custom order | Supplementary file 1 |
| Recombinant DNA reagent | pGEM-3z/601 | Addgene | Cat# 26656 | |
| Recombinant DNA reagent | pGM3z-8×repeat-2×BsaI | This paper | N/A | |
| Software, algorithm | LabVIEW VIs | Comstock et al. (2011) | RRID:SCR_014325 | |
| Software, algorithm | Matlab scripts for data analysis | This paper | RRID:SCR_001622 | |
| Software, algorithm | Python scripts for data analysis | This paper | RRID:SCR_008394 | |
| Software, algorithm | ImageJ | NIH (open source) | RRID:SCR_003070 | |
| Other | Pierce Streptavidin Magnetic Beads | Thermo Fisher | Cat# 88816 | |
| Other | BD 1 mL Insulin Syringe with Slip Tip | Fisher Scientific | Cat# 14-823-434 | |
| Other | Dual-trap time-shared high resolution optical tweezers | (Comstock et al., 2011) | N/A | |
| Other | Multi-channel optical tweezers chamber | This study | N/A | |
| Other | 1 µm carboxylated polystyrene beads | Bang Laboratories, Inc. | Cat# SVP-10–5 | |
| Other | 1.26 µm streptavidin polystyrene beads | Spherotech | Cat# PC04001-PC04N | |
| Other | Streptavidin Coated Magnetic Particles | Spherotech | Cat# SVM-08–10 | |
| Other | Hi-Trap Q HP columns 5 × 1 mL | Genesee Scientific | Cat# 17-1153-01 | |
| Other | Ni-NTA Agarose | Qiagen | Cat# 30210 | |
| Other | HisTrap HP 1 × 5 mL | Genesee Scientific | Cat# 84–208 | |
| Other | Amicon Ultra-0.5 Centrifugal Filter Unit, 100K | Millipore Sigma | Cat# UFC500324 | |
| Other | Amicon Ultra-0.5 Centrifugal Filter Unit, 3K | Millipore Sigma | Cat# UFC510024 | |
| Other | Zyppy Plasmid Maxiprep Kit | Zymo Research | Cat# D4028 | |
| Other | Typhoon imager | GE Healthcare | TRIO Variable Mode |
Additional files
-
Supplementary file 1
Oligos used in this study.
- https://doi.org/10.7554/eLife.48281.028
-
Supplementary file 2
Pol II backtrack analysis.
- https://doi.org/10.7554/eLife.48281.029
-
Transparent reporting form
- https://doi.org/10.7554/eLife.48281.030





