The role of premature evidence accumulation in making difficult perceptual decisions under temporal uncertainty
Figures
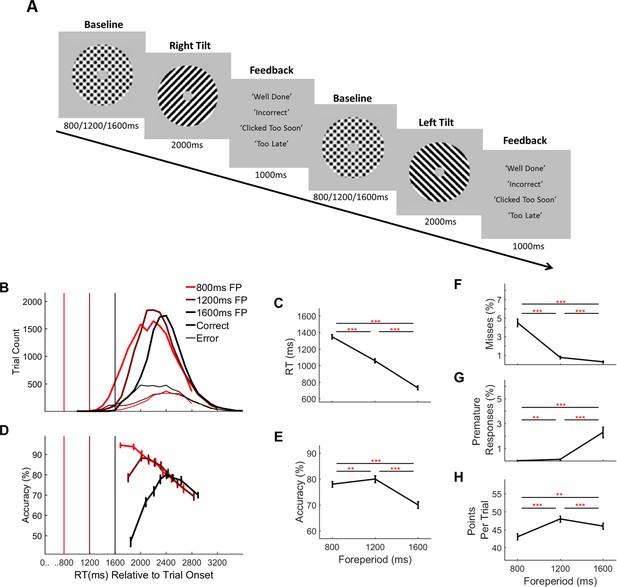
Two-alternative forced choice contrast discrimination task and behaviour separated by foreperiod (fp) duration.
(A) Task Schematic: Each trial commenced with the presentation of two overlaid left- and right-tilted gratings (45° relative to the vertical midline) within a circular aperture against a grey background. At baseline the gratings were each presented at 50% contrast. After an initial foreperiod, the duration of which varied unpredictably from trial-to-trial (800 ms/1200 ms/1600 ms), one grating stepped up in contrast by an individually predetermined amount (M = 5.5%, SD = 1.35%, range = 2–7%) while the other stepped down by a corresponding amount. Schematic depicts a right-tilted target followed by a left-tilted target with 50% contrast changes for illustration purposes only. Feedback was presented at the end of each trial. (B) RT distributions separated by foreperiod and response accuracy. Vertical line markers indicate the times of the contrast change. (C) Mean RT (corrects and errors pooled) separated by foreperiod duration. (D) Conditional accuracy functions showing accuracy as a function of RT separated by foreperiod. Vertical line markers indicate the times of the contrast change. Mean accuracy (E), missed response rate (F), premature response rate (G) and points earned per trial (H), separated by foreperiod duration. Error bars represent standard error of the mean. Asterisks’ indicate statistical significance of post-hoc comparisons: **=p < 0.017, ***=p < 0.001; Bonferroni corrected critical p-value=0.017.
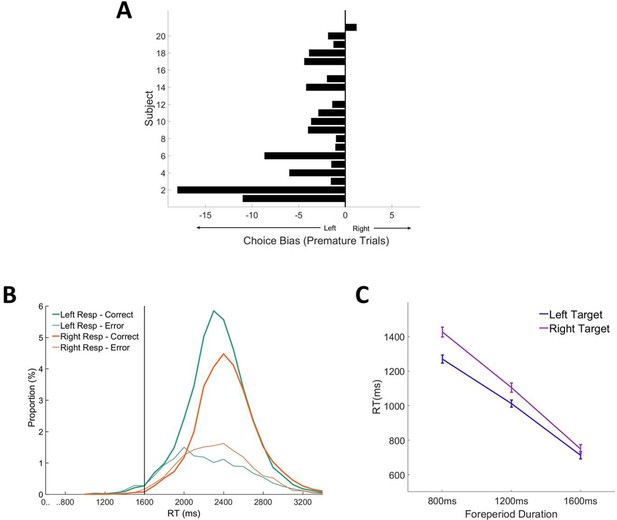
Choice biases in contrast discrimination task.
(A) Choice bias ratios for each subject (subjects with fewer than 10 premature trials were left blank). (B) RT distributions for long foreperiod (1600 ms) trials separated according to chosen response and response accuracy. (C) Mean RT (correct and error responses pooled) as a function of target direction and foreperiod duration.
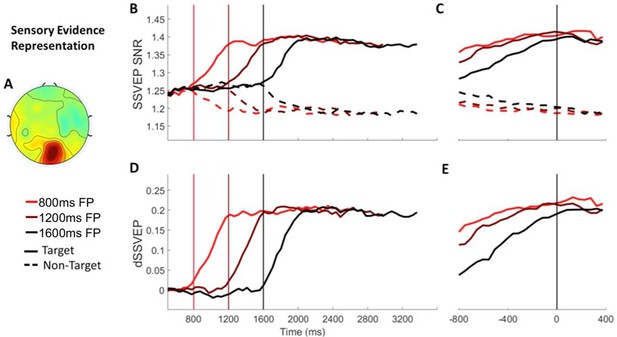
Sensory evidence representation (SSVEP) Signals separated by foreperiod (FP).
(A) The topography of the d-SSVEP measured prior to response was maximal over the occipital cortex (Oz). (B) Stimulus-aligned target and non-target SSVEP signals separated according to foreperiod duration, plotted relative to the onset of the stimulus. Vertical line markers at 800/1200/1600 ms indicate the times of the contrast change. (C) Response-aligned target and non-target SSVEP signals separated according to foreperiod duration. The vertical line marker at 0 ms denotes the time of response. (D) Stimulus-aligned d-SSVEP signal separated according to foreperiod duration, plotted relative to the onset of the stimulus. Vertical line markers at 800/1200/1600 ms indicate the times of the contrast change. (E) Response-aligned d-SSVEP signal separated according to foreperiod duration. The vertical line marker at 0 ms denotes the time of response.
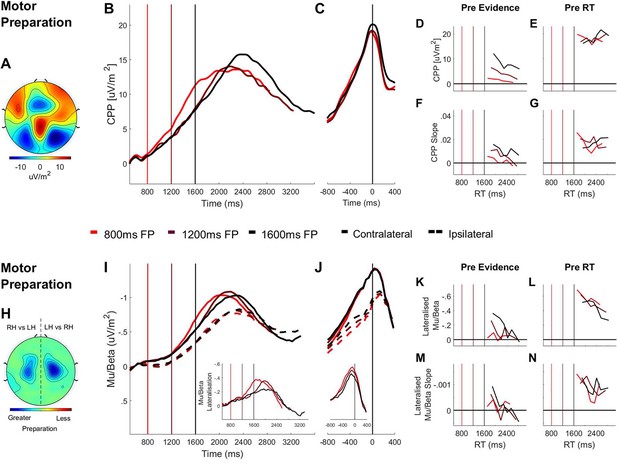
Domain-general (CPP) and effector-selective (Mu/Beta 10–30 Hz).
Decision Signals Separated by Foreperiod (FP) in the Contrast Discrimination Task. (A) Topography of the ERP signal measured prior to response (−150 ms to −50 ms) showing a positive going centroparietal component maximal over Pz. (B) Stimulus-aligned CPP separated by foreperiod duration, plotted relative to the onset of the overlaid gratings stimulus. Vertical line markers at 800/1200/1600 ms indicate the times of the contrast change across the three levels of foreperiod duration. (C) Response-aligned CPP separated by foreperiod duration. The vertical line marker at 0 ms denotes the time of response. (D) CPP amplitude measured at contrast change (−50 ms to 50 ms) and (E) at response (−150 ms to −50 ms) plotted as a function of RT separately for each foreperiod. (F) Pre-evidence CPP build-up rate (−250 ms to 50 ms) and (G) pre-response CPP build-up rate (−500 ms to −200 ms), plotted as a function of RT separately for each foreperiod. (H) Topography of lateralised Mu/Beta band (10–30 Hz) activity measured prior to response (−150 ms to −50 ms) calculated separately for each hemisphere by subtracting ipsilateral from contralateral hand responses (LH = left hand; RH = right hand).The topography shows stronger lateralisation over each hemisphere when preparing a contralateral response. (I) Stimulus-aligned contralateral and ipsilateral Mu/Beta waveforms, separated by foreperiod duration, plotted relative to the onset of the overlaid gratings. Vertical line markers at 800/1200/1600 ms denote the times of the contrast change across the three levels of foreperiod duration. Insert: stimulus-aligned Mu/Beta lateralisation (contralateral-ipsilateral) traces. (J) Response-aligned contralateral and ipsilateral Mu/Beta waveforms, separated by foreperiod duration with a vertical line marker at 0 ms denoting the time of response. Insert: response-aligned Mu/Beta lateralisation (contralateral-ipsilateral) traces. (K) Mu/beta lateralisation at contrast change (−50 to 50 ms) and (L) response (−150 ms to −50 ms), plotted as a function of RT separately for each foreperiod. (M) Pre-evidence Mu/Beta lateralisation slope (−250 ms to 50 ms) and (N) pre-response Mu/Beta lateralisation slope (−500 ms to −200 ms) plotted as a function of RT separately for each foreperiod.
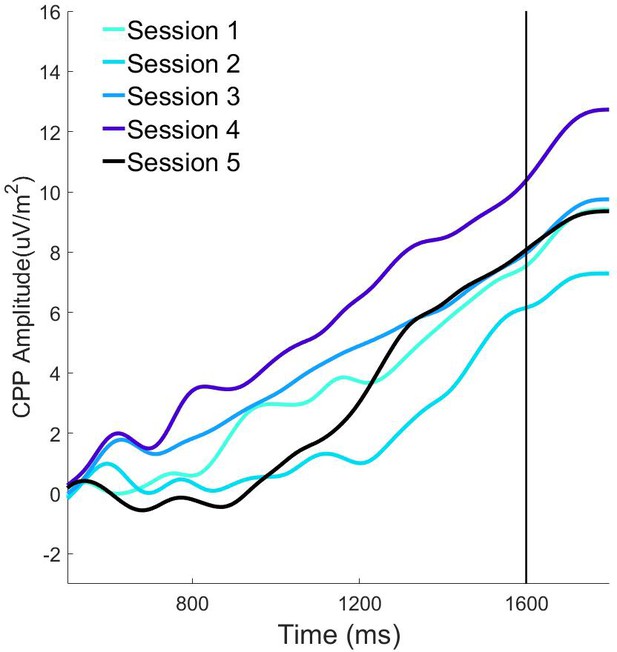
Pre-evidence stimulus-aligned CPP waveforms on long (1600ms).
Foreperiod trials as a function of training session.
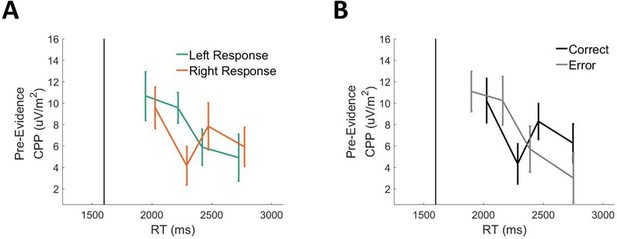
Pre-evidence cpp amplitude on long foreperiod (1600ms).
Trials as a function of rt separately for left versus right responses (A) and correct versus error responses (B).
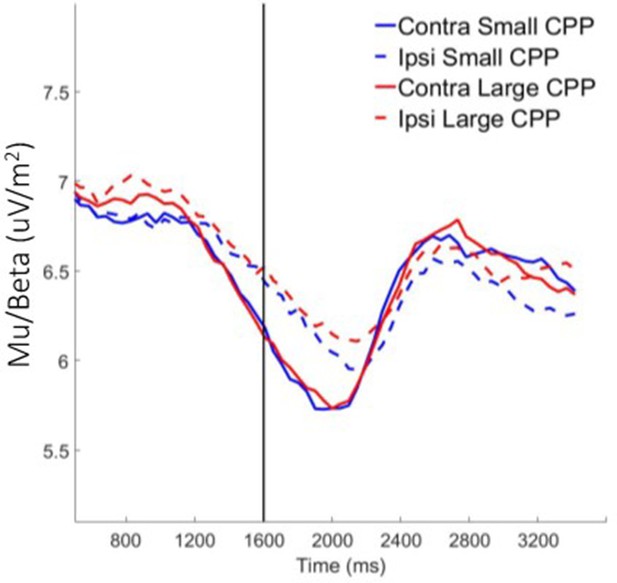
Contralateral and ipsilateral motor preparation as a function of pre-evidence CPP amplitude bin (large versus small based on median split).
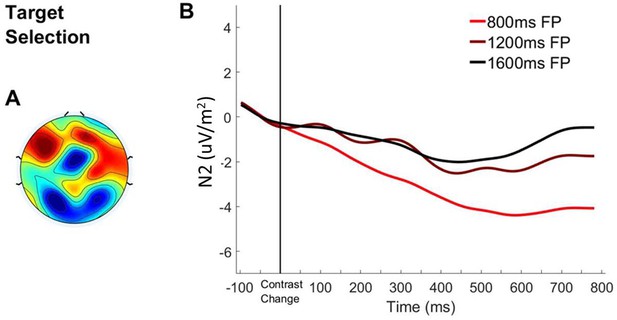
Bilateral Occipital ERP aligned to Contrast Change.
(A) Topography of the ERP signal measured 200–300 ms post-contrast change. (B) ERP waveform over bilateral occipital cortex aligned to the contrast change with waveforms separated according to foreperiod duration.
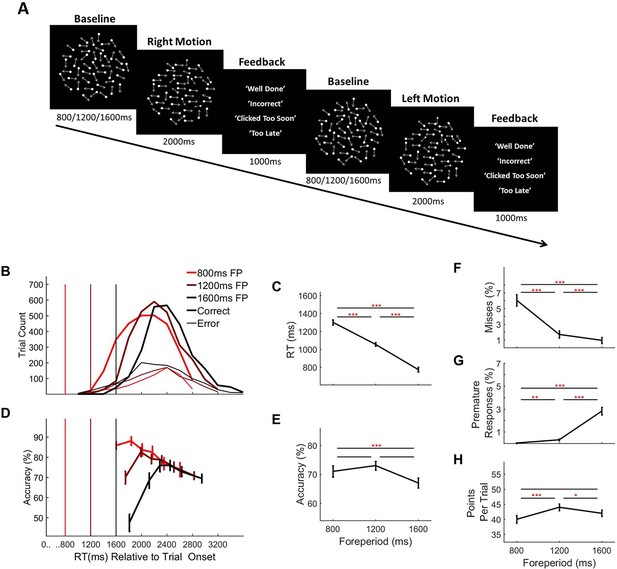
Random dot motion discrimination task and behaviour separated by forepriod (fp) duration.
(A) Task Schematic: Each trial commenced with the presentation of a cloud of moving dots within a circular aperture against a black background. At baseline, the dots were displaced at random from one trial to the next. After an initial foreperiod, the duration of which varied unpredictably from trial-to-trial (800 ms/1200 ms/1600 ms), a portion of the dots, determined separately for each individual subject (M = 7.42%, SD = 2.61%, range = 3–12%), began to move coherently in either a leftward or rightward direction. The schematic depicts a rightward motion trial followed by a leftward motion trial (Note: Arrows are used here to illustrate the direction of motion but were not a feature of the actual stimulus). Feedback was presented at the end of each trial. (B) RT distributions separated by foreperiod duration and response accuracy. Vertical line markers indicate the time of coherent motion onset. (C) Mean RT separated by foreperiod duration and (D) Conditional accuracy functions showing accuracy as a function of RT separated by foreperiod duration. Vertical line markers indicate the time of coherent motion onset. Mean accuracy (E), missed response rate (F), premature response rate (G) and points earned per trial (H), separated by foreperiod. Error bars represent standard error of the mean. Asterisks’ indicate statistical significance of post-hoc comparisons: *=<0.05, **=p < 0.017, ***=p < 0.001; Bonferroni corrected critical p-value=0.017.
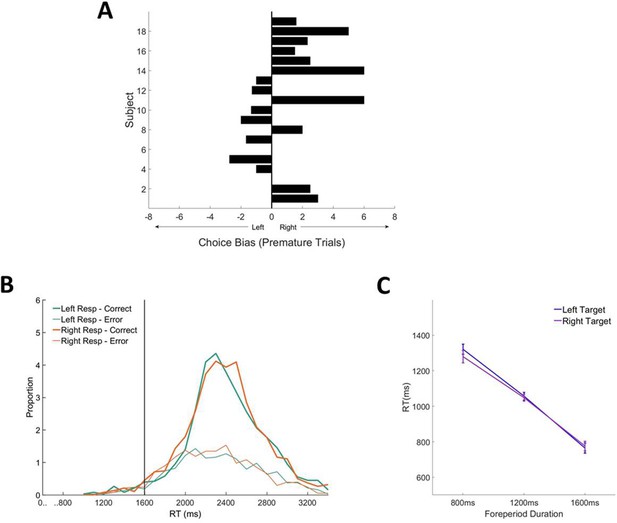
Choice biases in contrast discrimination task.
(A) Individual subject choice bias ratios (subjects with fewer than 10 premature trials were left blank). (B) RT distributions for long foreperiod (1600 ms) trials separated according to chosen response and response accuracy. (C) Mean RT (corrects and errors pooled) as a function of target direction and foreperiod duration.
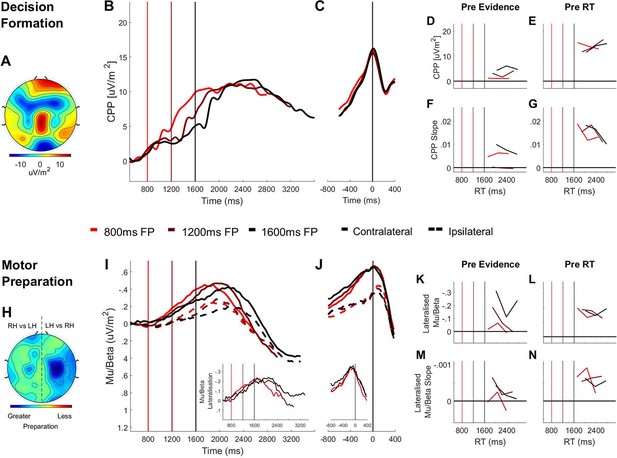
Domain-general (CPP) and effector-selective (Mu/Beta 10–30 Hz).
Decision signals separated by foreperiod (FP) in the random dot motion discrimination task. (A) Topography of the ERP signal measured prior to response (−150 ms to −50 ms) showing a positive going centroparietal component maximal over Pz. (B) Stimulus-aligned CPP separated by foreperiod duration, plotted relative to the onset of the dot motion stimulus. Vertical line markers at 800/1200/1600 ms indicate the times of coherent motion onset across the three levels of foreperiod duration. (C) Response-aligned CPP separated according to foreperiod duration. The vertical line marker at 0 ms denotes the time of response. (D) CPP amplitude measured at coherent motion onset (−50 ms to 50 ms) and E) at response (−150 ms to −50 ms) plotted as a function of RT separately for each foreperiod. (F) Pre-evidence CPP build-up rate (−250 ms to 50 ms) and G) pre-response CPP build-up rate (−500 ms to −200 ms), plotted as a function of RT separately for each foreperiod. (H) Topography of lateralised Mu/Beta band (10–30 Hz) activity measured prior to response (−150 ms to −50 ms) calculated separately for each hemisphere by subtracting ipsilateral from contralateral hand responses (LH = left hand; RH = right hand). (I) Stimulus-aligned contralateral and ipsilateral Mu/Beta waveforms separated by foreperiod duration, plotted relative to the onset of the dot motion stimulus. Vertical line markers at 800/1200/1600 ms denote the times of coherent motion onset across the three level of foreperiod duration. Insert: stimulus-aligned Mu/Beta lateralisation (contralateral-ipsilateral) traces. (J) Response-aligned contralateral and ipsilateral Mu/Beta waveforms, separated by foreperiod duration with a vertical line marker at 0 ms denoting the time of response. Insert: response-aligned Mu/Beta lateralisation (contralateral-ipsilateral) traces. (K) Mu/beta lateralisation at coherent motion onset (−50 to 50 ms) and L) response (−150 ms to −50 ms), plotted as a function of RT separately for each foreperiod. (M) Pre-evidence Mu/Beta lateralisation slope (−250 ms to 50 ms) and N) pre-response Mu/Beta lateralisation slope (−500 ms to −200 ms) plotted as a function of RT separately for each foreperiod.
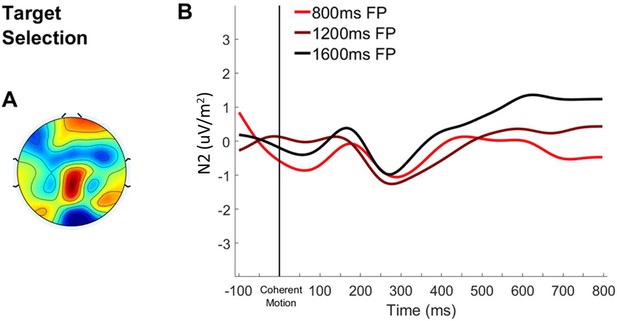
Bilateral occipital erp aligned to coherent motion.
(A) Topography of the ERP signal measured 200–300 ms post-coherent motion onset. (B) ERP waveform over bilateral occipital cortex aligned to coherent motion onset with waveforms separated according to foreperiod duration.
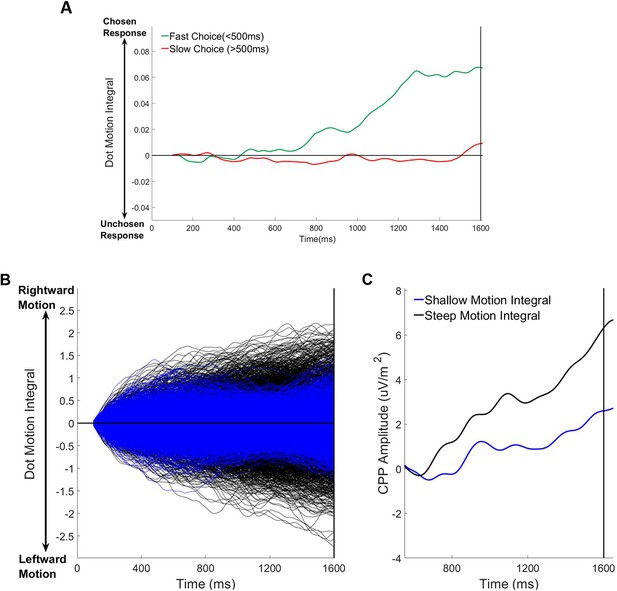
Relationship between pre-evidence cumulative dot motion energy, choice and pre-evidence cpp build-up (1600ms foreperiod trials only).
(A) Average cumulative dot motion integral (measured between 100–1600 ms) separated for fast (RT <500 ms) and slow (RT >500 ms) responses. (B) Single trial cumulative dot motion integral traces separated according to the slope of the pre-evidence dot motion integral (100–1600 ms). (C) Grand average stimulus-aligned CPP separated according to the slope of the the pre-evidence dot motion integral.
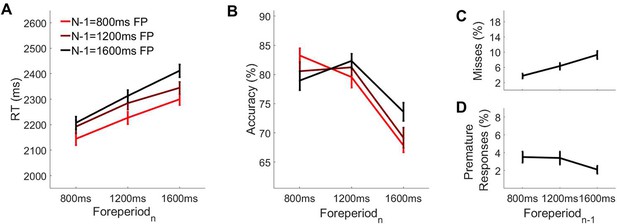
Behavioural performance as a function of foreperiodn-1 and foreperiodn.
Mean accuracy (A) and mean RT (B) as a function of foreperiodn-1 and foreperiodn. (C) Missed response rate on short foreperiod rials as a function of foreperiodn-1was long. (D) Premature response rate on long foreperiod trials as a function of foreperiodn-1.
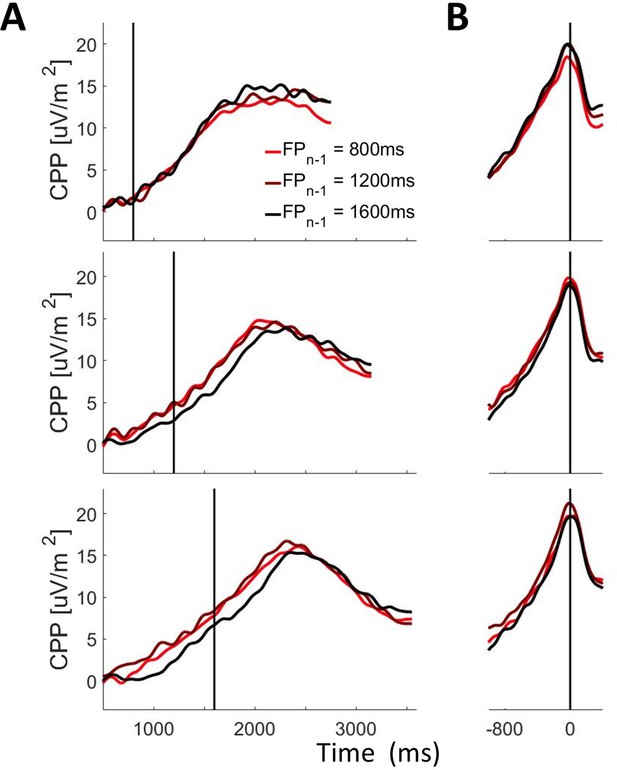
CPP as a function of foreperiodn and foreperiodn-1.
(A) Stimulus-aligned and (B) Response-aligned CPP waveforms separated according to foreperiodn (Top Panel: 800 ms; Middle Panel 1200 ms; Bottom Panel 1600ms) and foreperiodn-1. Shaded grey bars correspond to the pre-evidence (A) and pre-response (B) measurement windows. Solid black vertical lines mark the time of evidence onset (A) and response (B).





