Spatiotemporal constraints on optogenetic inactivation in cortical circuits
Figures
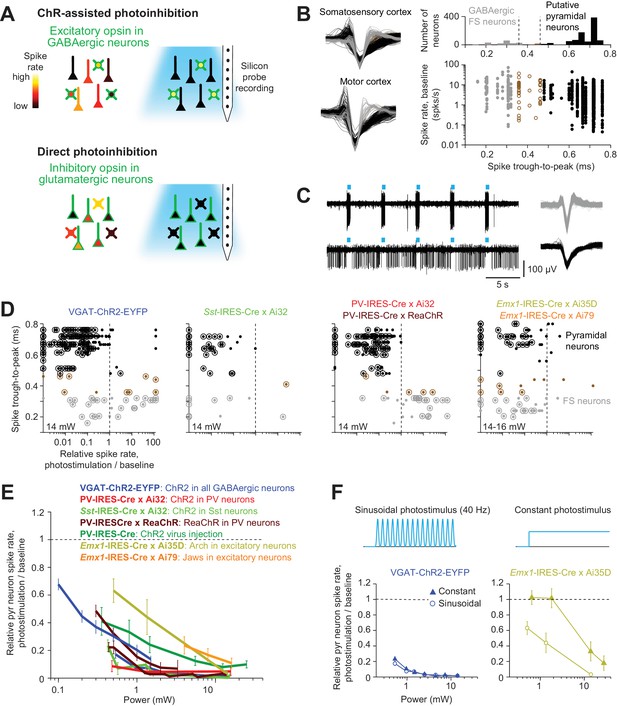
Optogenetic inactivation and cell-type specific recording.
(A) Summary of inactivation methods. ChR-assisted photoinhibition was induced by photostimulating excitatory opsins in various GABAergic interneurons. Direct photoinhibition was achieved by photostimulating inhibitory opsins in pyramidal neurons. (B) Cell-type classification based on spike waveform. Left, spike waveforms for putative FS neurons (gray) and putative pyramidal neurons (black) in two different cortical areas. Right, histogram of trough-to-peak spike durations (top) and relationship to baseline spike rate (bottom). Neurons were classified into putative GABAergic fast spiking (FS) neurons and pyramidal neurons based on spike width (Materials and methods). Neurons that could not be classified (brown) were excluded from analysis. (C) Silicon probe recordings during photostimulation in a VGAT-ChR2-EYFP mouse. Top, a putative FS neuron. Bottom, a putative pyramidal neuron. Right, corresponding spike waveforms. (D) Effects of photostimulation on cell types defined by spike waveform. Dots correspond to individual neurons. Spike rates of each neuron during photostimulation were normalized to its baseline (‘relative spike rate’, Materials and methods). Neurons with significant spike rate changes (p<0.05, two-tailed t-test) are highlighted by circles. Gray, putative FS neurons; black, putative pyramidal neurons; brown, neurons that could not be classified. (E) Relative spike rate as a function of laser power for different photoinhibition methods. Pyramidal neurons within 0.4 mm from laser center across all cortical depth. Mean ± s.e.m. across neurons, bootstrap (Materials and methods). Two datasets (lines) are shown for VGAT-ChR2-EYFP: the line spanning 0.5–10.5 mW shows data from barrel cortex, n = 153; the line spanning 0.1–1.5 mW shows data from ALM, n = 188; PV-IRES-Cre x Ai32, data from barrel cortex, n = 16; Sst-IRES-Cre x Ai32, data from barrel cortex, n = 65; two datasets (lines) are shown for PV-IRES-Cre x ReaChR: the line spanning 0.5–10.5 mW shows data from barrel cortex, n = 211; the line spanning 0.3–3 mW shows data from ALM, n = 55; PV-IRES-Cre, ChR2 virus injection, data from barrel cortex, n = 78; Emx1-IRES-Cre x Ai35D, data from barrel cortex, n = 26; Emx1-IRES-Cre x Camk2a-tTA x Ai79, data from barrel cortex, n = 176. (F) Effect of photostimulus profile on photoinhibition. Top, 40 Hz sinusoid photostimulus and constant photostimulus. Bottom, relative spike rate as a function of laser power for ChR-assisted photoinhibition (left) and direct photoinhibition mediated by Arch (right). Pyramidal neurons within 0.4 mm from laser center across all cortical depth. Mean ± s.e.m. across neurons, bootstrap (Materials and methods). 40 Hz sinusoid photostimulus is the standard photostimulus used in this study.
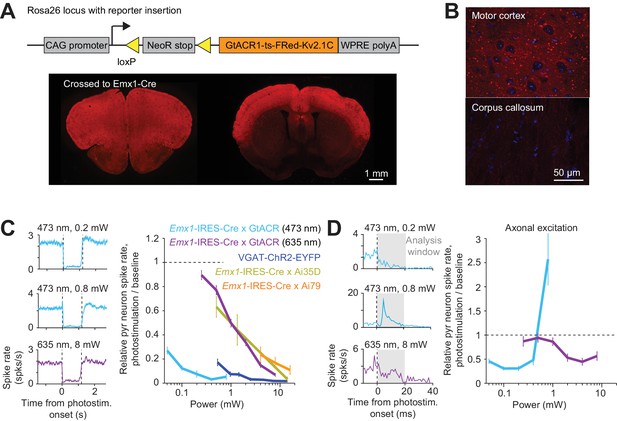
Direct photoinhibition with a GtACR reporter mouse.
(A) Top, generation of the Cre-dependent GtACR reporter line. Construct includes loxP sites, and GtACR1-ts-FRed-Kv2.1C with WPRE, driven by CAG promoter targeted to the Rosa26 locus. Bottom, cross to Emx1-IRES-Cre mice. GtACR1 is expressed in cortical excitatory neurons. (B) Confocal images showing dense expression of GtACR1 in cortex and low levels of expression in the corpus callosum, implying low trafficking of GtACR1 to axons. (C) Left, mean peristimulus time histogram (PSTH, 50 ms bin) for pyramidal neurons with blue and red photostimuli. Dashed lines, photostimulus onset and offset. Pyramidal neurons within 0.4 mm from laser center across all cortical depth. Blue light photostimulation, n = 335 neurons from ALM; Red light photostimulation, n = 285 neurons from ALM. Right, relative spike rate as a function of laser power. Mean ± s.e.m. across neurons, bootstrap (Materials and methods). VGAT-ChR2-EYFP, Emx1-IRES-Cre x Ai35D, Emx1-IRES-Cre x Camk2a-tTA x Ai79, data from Figure 1E are replotted here for reference. (D) Left, mean PSTH (1 ms bin) at the onset of the photostimulation. Same data as in (C). Axonal excitation was induced at 0.8 mW laser power for blue light photostimulation. Right, relative spike rate during the first 20 ms of photostimulation onset.
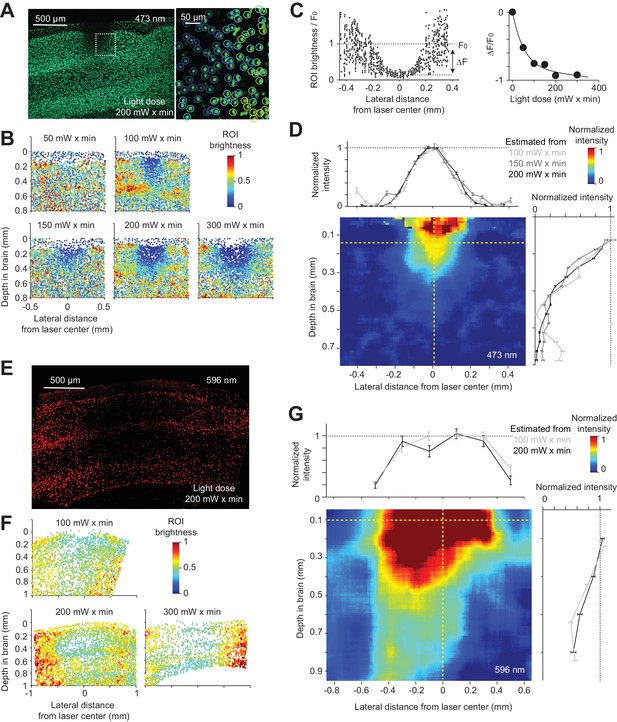
Spatial profile of light in the cortex.
(A) Photobleaching was induced in a transgenic mouse line expressing GFP in the nuclei of excitatory neurons (Rosa-LSL-H2B-GFP crossed to Emx1-IRES-Cre; Materials and methods). Left, coronal section showing an example photobleaching site. Light dose, 200 mW x min. The photostimulus was a laser beam with a Gaussian profile (diameter at 4σ, 0.4 mm) at the brain surface. Right, a confocal image showing a region highlighted by the dash line. GFP intensity was measured in regions of interest (ROI) around individual nuclei (circles). Color indicates ROI fluorescence intensity. (B) Photobleaching assay measuring the spread of blue light in the neocortex. Photobleaching was induced with different light doses (as indicated). Dots, individual nuclei. Color indicates ROI fluorescence intensity. (C) Left, ROI fluorescence intensity as a function of lateral distance from the laser center (data from light dose 200 mW x min, at 0.2 mm depth). F0, baseline ROI intensity, computed by averaging all ROIs far away from the laser center. ΔF, ROI fluorescence intensity change caused by photobleaching, computed by averaging all ROIs near the laser center and subtracting F0 (Materials and methods). Right, photobleaching (ΔF/ F0) at various light doses. Line, exponential fit to ΔF/ F0 as a function of light dose. (D) The estimated spatial profile of blue light in tissue. ΔF/ F0 was converted to light intensity using the exponential fit in (C) (see Material and methods). Light intensity is shown as a function of lateral distance (top) and cortical depth (right), along the yellow dashed lines. Mean ± s.e.m. across ROIs, bootstrap (Materials and methods). The color map shows the spatial profile estimated from the 200 mW x min light dose data. Estimates from other light doses produced similar spatial profiles. (E) - (G) Same as (A) - (D), but for orange light (594 nm) measured with photobleaching of mCherry (Material and methods).
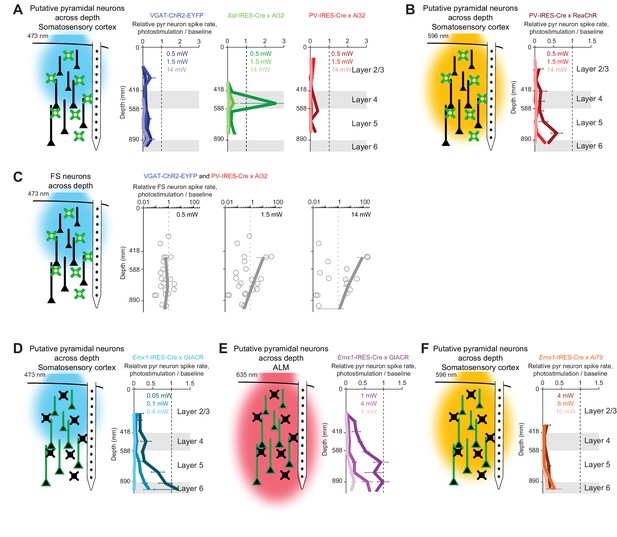
Photoinhibition across cortical layers.
(A) Left, schematics, ChR-assisted photoinhibition using blue light. Right, relative spike rate across cortical depth for three laser powers (0.5, 1.5, 14 mW). Pyramidal neurons within 0.2 mm from laser center. Data from barrel cortex. Mean ± s.e.m. across neurons, bootstrap (Materials and methods). VGAT-ChR2-EYFP, n = 170; Sst-IRES-Cre x Ai32, n = 33; PV-IRES-Cre x Ai32, n = 61. (B) Same as (A), but for orange light photostimulation of red-shifted ChR in PV neurons (PV-IRES-Cre x R26-CAG-LSL-ReaChR-mCitrine, n = 95). (C) FS neuron responses during ChR-assisted photoinhibition using blue light. Relative spike rate for putative FS neurons across cortical depth for three laser powers (0.5, 1.5, 14 mW). Putative FS neurons within 1 mm from laser center. Data from barrel cortex and motor cortex are combined. Data from VGAT-ChR2-EYFP and PV-IRES-Cre x Ai32 mice are combined (n = 22). Circles, individual neurons; mean ± s.e.m. over neurons. (D) Same as (A), but for blue light photostimulation of light-gated Cl- channel GtACR1 in pyramidal neurons (Emx1-IRES-Cre X GtACR, n = 198). (E) Same as (A), but for red light photostimulation of GtACR1 in pyramidal neurons (n = 236). (F) Same as (A), but for orange light photostimulation of light-gated Cl- pump Jaws in pyramidal neurons (Emx1-IRES-Cre X Camk2a-tTA X Ai79, n = 176).
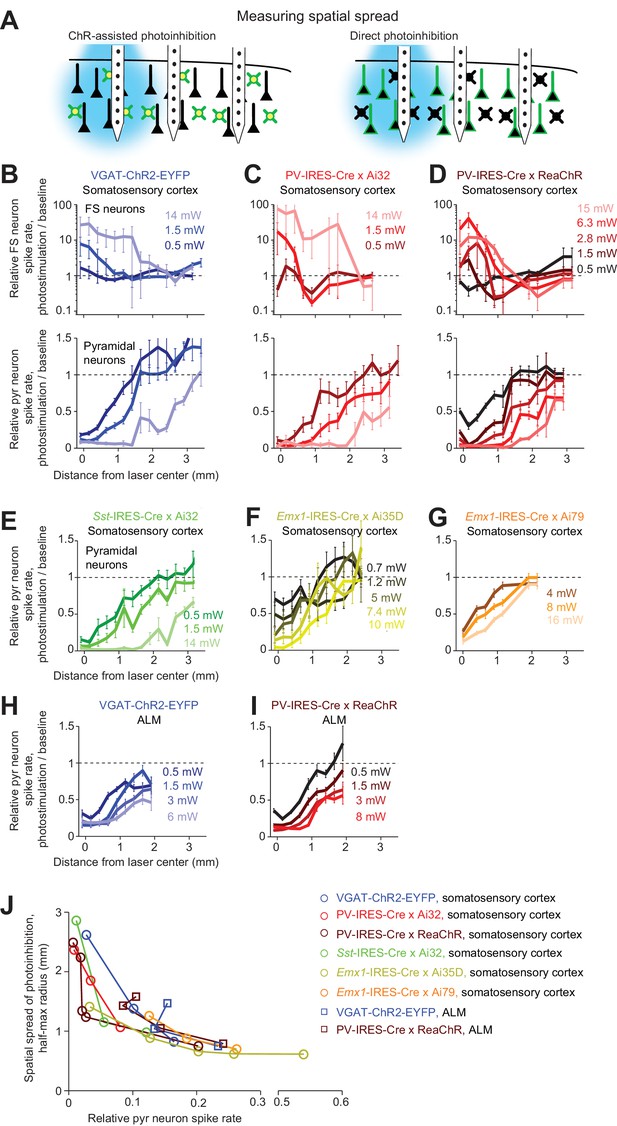
Spatial profile of photoinhibition.
(A) Silicon probe recording at different lateral distances from photostimulus. (B) Relative spike rate versus lateral distance from the photostimulus center for three laser powers (0.5, 1.5, 14 mW). Blue light photostimulation in barrel cortex of VGAT-ChR2-EYFP mice. Top, FS neurons (n = 18). Bottom, pyramidal neurons (n = 111). Neurons were pooled across cortical depths. Mean ± s.e.m. across neurons, bootstrap (Materials and methods). (C) Same as (B), but for blue light photostimulation in barrel cortex of PV-IRES-Cre x Ai32 mice (FS neurons, n = 5; pyramidal neurons, n = 16). (D) Same as (B), but for orange light photostimulation in barrel cortex of PV-IRES-Cre x R26-CAG-LSL-ReaChR-mCitrine mice (FS neurons, n = 10; pyramidal neurons, n = 82). (E) Same as (B), but for blue light photostimulation in barrel cortex of Sst-IRES-Cre x Ai32 mice. Pyramidal neurons only (n = 65). (F) Same as (E), but for orange light photostimulation in barrel cortex of Emx1-IRES-Cre x Ai35D mice (n = 26). (G) Same as (E), but for orange light photostimulation in barrel cortex of Emx1-IRES-Cre X Camk2a-tTA X Ai79 mice (n = 174). (H) Same as (E), but for blue light photostimulation in ALM of VGAT-ChR2-EYFP mice (n = 96). (I) Same as (E), but for orange light photostimulation in ALM of PV-IRES-Cre x R26-CAG-LSL-ReaChR-mCitrine mice (n = 129). (J) Photoinhibition strength versus spatial spread. Relative spike rate is the average across all pyramidal neurons near laser center (<0.4 mm, all cortical depths). Spatial spread is the distance at which photoinhibition strength is half of that at the laser center (‘radius, half-max’). Each circle represents data from one photostimulation power. Lines connect all circles of one method.
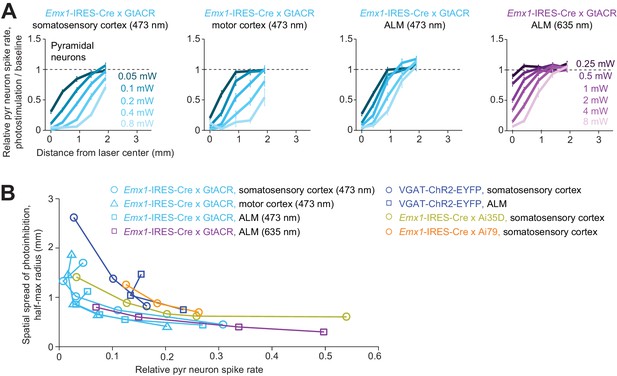
Spatial profile of direct photoinhibition in GtACR reporter mouse.
(A) Relative spike rate versus lateral distance from the photostimulus center for various laser powers. Pyramidal neurons only. Blue light (437 nm) photostimulation in barrel cortex, n = 198; blue light photostimulation in motor cortex, n = 236; blue light photostimulation in ALM, n = 335; red light (635 nm) photostimulation in ALM, n = 236. Neurons were pooled across cortical depths. Mean ± s.e.m., bootstrap across neurons. (B) Photoinhibition strength versus spatial spread. Relative spike rate is the average across all pyramidal neurons near laser center (<0.4 mm, all cortical depths). Spatial spread is the distance at which photoinhibition strength is half of that at the laser center (‘radius, half-max’). Each circle represents data from one photostimulation power. Lines connect all circles of one method. VGAT-ChR2-EYFP, Emx1-IRES-Cre x Ai35D, Emx1-IRES-Cre x Camk2a-tTA x Ai79, data from Figure 5J replotted here for reference.
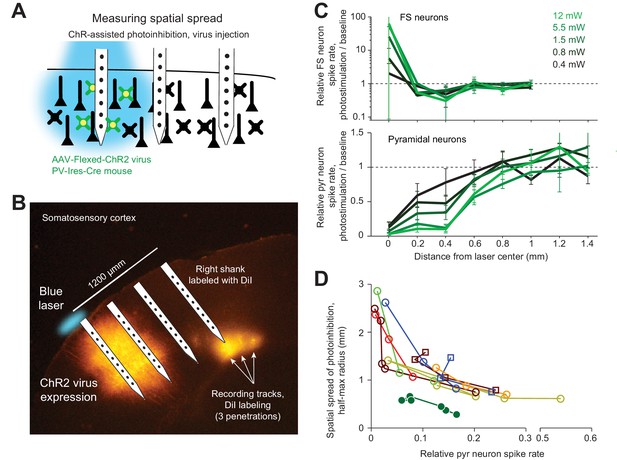
ChR-assisted photoinhibition using virus injection can achieve submillimeter spatial resolution.
(A) Schematics, confined ChR2 expression in PV neurons and silicon probe recording at different distances from the expression site. (B) Silicon probe recording in barrel cortex during photostimulation. The right shank of the silicon probe was painted with DiI to label the recording tracks. Coronal section showing viral expression of ChR2-tdTomato, electrode and photostimulus locations. The photostimulus was aligned to the virus injection site. (C) Relative spike rate versus lateral distance from the photostimulus center for different laser powers. Top, putative FS neurons (n = 14). Bottom, pyramidal neurons (n = 78). Neurons were pooled across cortical depths. (D) Same as Figure 5J, but with virus injection data added.
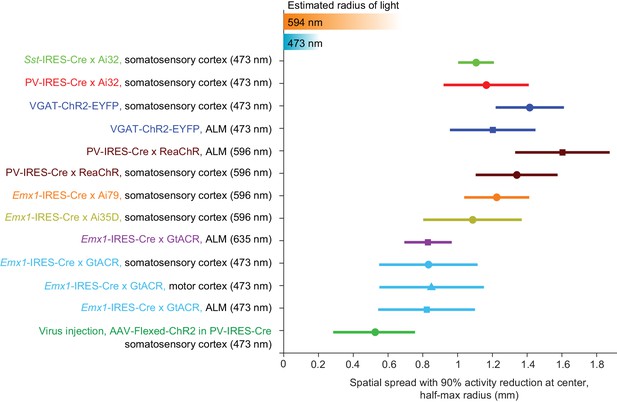
Summary of spatial resolution for all photoinhibition methods.
Half-max radius of photoinhibition when the activity reduction at laser center is 90%. Data based on Figures 5J, 6B and 7D when relative pyramidal neuron spike rate is 0.1. Error bars show 90% confidence interval, bootstrap (Materials and methods). Estimated radius of light is based on data in Figure 3D and G.
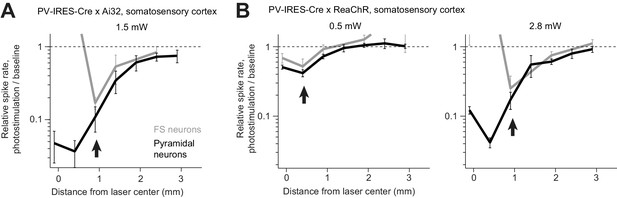
Proportional activity decrease in pyramidal and FS neurons during ChR-assisted photoinhibition.
(A) Relative spike rate versus lateral distance from the photostimulus center for PV-IRES-Cre x Ai32. Data from Figure 5C replotted with activity shown on a log scale. FS neurons (gray) and pyramidal neurons (black). The arrows point to regions in the photostimulus surround where activity of FS neurons and pyramidal neurons decrease in proportion (paradoxical effect). (B) Same as (A) but for PV-IRES-Cre x ReaChR. Data from Figure 5D.
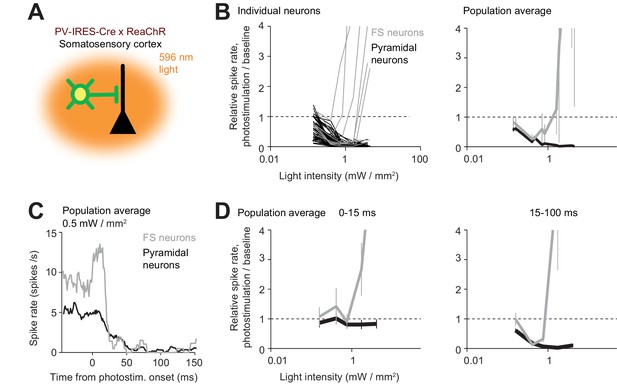
The paradoxical effect.
(A) Photostimulating PV neurons using orange light. Laser beam diameter, 2 mm (Materials and methods). (B) Relative spike rate as a function of light intensity (<0.4 mm from laser center, all cortical depths). FS neurons (gray) and pyramidal neurons (black). Left, individual neurons (lines). Right, mean ± s.e.m. across neurons, bootstrap. Laser power was divided by the illuminated area to obtain light intensity. FS neurons, n = 10, pyramidal neurons n = 82. (C) Mean peristimulus time histogram for FS neurons (gray) and pyramidal neurons (black). (D) Same as (B) but for relative spike rate at different epochs of photostimulation.
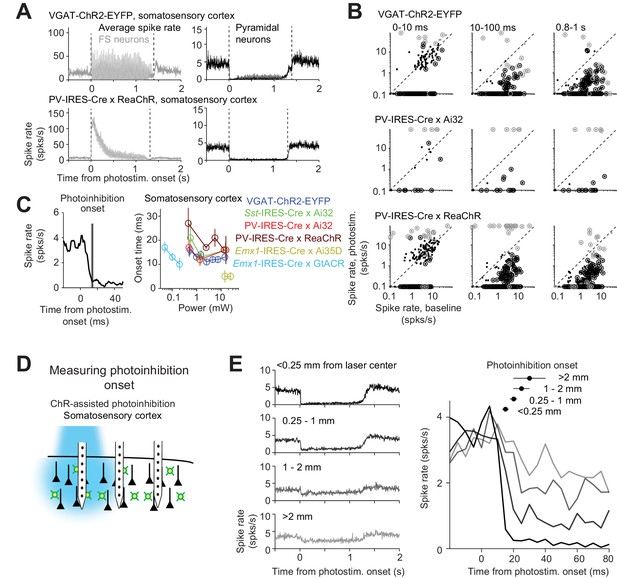
Time course of ChR2-assisted photoinhibition.
(A) Mean peristimulus time histogram (PSTH, 1 ms bin) for FS neurons (gray) and pyramidal neurons (black). All neurons within 0.4 mm from the laser center across all cortical depths were pooled. Laser power, 14–15 mW. VGAT-ChR2-EYFP, FS neurons, n = 12, pyramidal neurons, n = 152; PV-IRES-Cre x ReaChR, FS neurons, n = 23, pyramidal neurons, n = 207. (B) Spike rate of FS neurons (gray) and pyramidal neurons (black) at different epochs of photostimulation. Dots correspond to individual neurons. Neurons with significant firing rate changes relative to baseline (p<0.05, two-tailed t-test) are highlighted by circles. (C) Photoinhibition onset time. Left, mean PSTH of pyramidal neurons in VGAT-ChR2-EYFP mice (1.5 mW) and photoinhibition onset (mean ± s.e.m.). The photoinhibition onset is the time when spike rate reached 90% of the average spike rate reduction during the whole photostimulation period. Right, photoinhibition onset for different methods. The color scheme of photoinhibition methods is the same as in Figures 1E and 2C. Each circle represents data from one photostimulation power. Lines connect all circles of one method. (D) Schematic, measuring photoinhibition onset at different distances from the photostimulus. (E) Left, mean PSTH of pyramidal neurons at different distances from the photostimulus center. Right, spike rate at the onset of photostimulation (t = 0). Data from motor cortex and barrel cortex in VGAT-ChR2-EYFP, PV-IRES-Cre x Ai32, and PV-IRES-Cre x ReaChR mice are pooled (<0.25 mm, n = 301; 0.25–1 mm, n = 317; 1–2 mm, n = 262;>2 mm, n = 156). Laser power, 14–15 mW. Mean (± s.e.m. across neurons, bootstrap) photoinhibition onset latencies are shown on top.
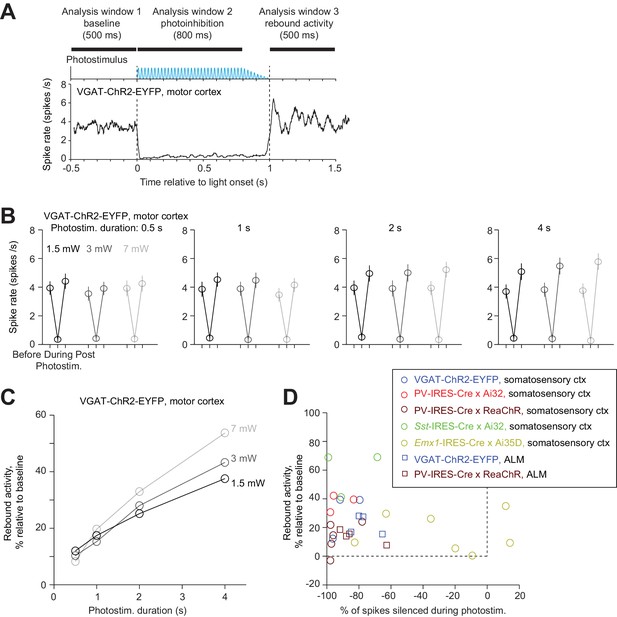
Rebound activity after photoinhibition.
(A) Mean PSTH of pyramidal neurons during photoinhibition in VGAT-ChR2-EYFP mice. Dashed lines, photostimulus onset and offset. All neurons within 0.4 mm from the laser center across all cortical depths (n = 78). Data from motor cortex. Laser power, 7 mW. Spike rates were analyzed in three time windows before, during, and after photostimulation. Photostimulus duration, 1 s. (B) Spike rate of pyramidal neurons before, during, and after photostimulation for different laser powers and photostimulation durations. Mean ± s.e.m. across neurons, bootstrap. (C) Rebound activity as a function of laser powers and photostimulation durations. Relative activity is the after-photostimulation spike rate increase from the baseline, normalized to the baseline spike rate (Materials and methods). (D) Rebound activity as a function of photoinhibition strength. Percent of spikes silenced was relative to the baseline spike rate. Data from all mouse lines. VGAT-ChR2-EYFP, barrel cortex, n = 111; PV-IRES-Cre x Ai32, barrel cortex, n = 16; PV-IRES-Cre x ReaChR, barrel cortex, n = 82; Sst-IRES-Cre x Ai32, barrel cortex, n = 65; Emx1-IRES-Cre x Ai35D, barrel cortex, n = 26; VGAT-ChR2-EYFP, ALM, n = 96; PV-IRES-Cre x ReaChR, ALM, n = 129.
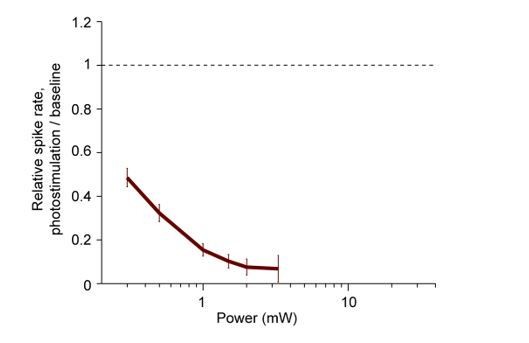
Normalized spike rate as a function of laser power.
Pyramidal neurons from ALM (< 0.4 mm from laser center, all cortical depths). Spike rates were normalized to baseline (dashed line, see Materials and methods). Mean ± s.e.m. across neurons, bootstrap. PV-IRES-Cre x ReaChR, 55 neurons.
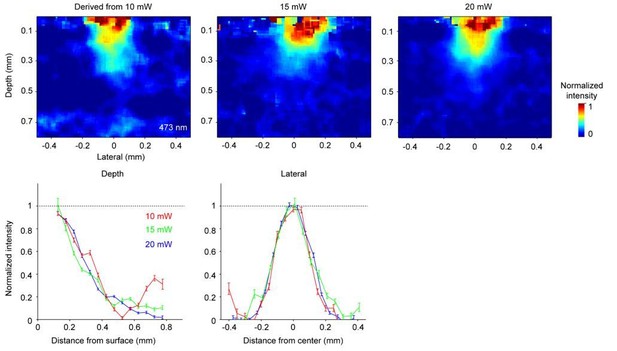
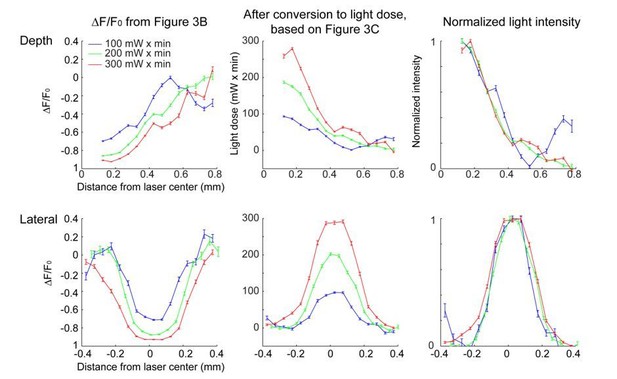
The estimated spatial profile of blue light in tissue.
Top, Light intensity profile derived from changes in fluorescence (ΔF/ F0) at three different light doses. Bottom, light intensity is shown as a function of cortical depth (left) and lateral distance (right) from the laser center. Profiles derived from photobleaching data at three different light doses are shown in different colors.
Tables
A list of photoinhibition methods tested in this study.
| Methods | Mouse (JAX #) | Reagents | Wavelength | Brain region |
|---|---|---|---|---|
| ChR-assisted photoinhibition | ||||
| ChR2 in all GABAergic neurons | VGAT-ChR2-EYFP or Slc32a1-COP4*H134R/EYFP (014548) | 473 nm | Somatosensory cortex, ALM | |
| ChR2 in PV expressing neurons | PV-IRES-Cre or Pvalb-IRES-Cre (008069) x Ai32 (012569) | 473 nm | Somatosensory cortex | |
| ChR2 in Sst neurons | Sst-IRES-Cre (013044) x Ai32 (012569) | 473 nm | Somatosensory cortex | |
| ReaChR in PV neurons | PV-IRES-Cre (008069) x R26-CAG-LSL-ReaChR-mCit (026294) | 594 nm | ALM | |
| ChR2 virally delivered to local PV neurons | PV-IRES-Cre (008069) | AAV2/1-CAG-FLEX-ChR2-tdTomato-WPRE (UPenn Viral Core, AV-1-ALL864) | 473 nm | Somatosensory cortex |
| Direct photoinhibition | ||||
| Arch in excitatory neurons | Emx1-IRES-Cre (005628) x Ai35D (012735) | 594 nm | Somatosensory cortex | |
| Jaws in excitatory neurons | Emx1-IRES-Cre (005628) x Ai79D (023529) x Camk2-tTA (003010) | 594 nm | Somatosensory cortex | |
| GtACR1 (somatic targeting) (Mahn et al., 2018) in excitatory neurons | Emx1-IRES-Cre (005628) x R26-CAG-LNL-GtACR1-ts-FRed-Kv2.1 (033089) | 473 nm, 635 nm | Somatosensory cortex, primary motor cortex, ALM | |