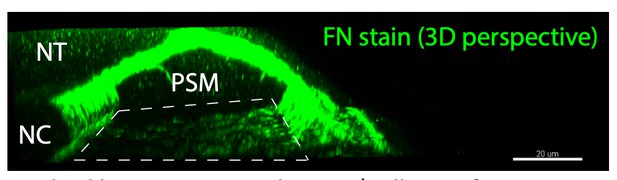
Fibronectin is a smart adhesive that both influences and responds to the mechanics of early spinal column development
Figures
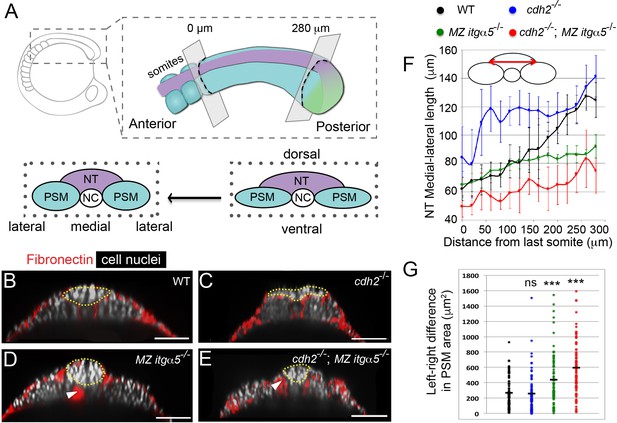
Reduction of Fibronectin matrix enhances neural tube convergence but abrogates bilaterally symmetric paraxial mesoderm morphogenesis.
(A) A schematic of the zebrafish tailbud and two transverse sections at the anterior and posterior ends of the presomitic mesoderm (PSM, cyan). The left and right PSM flank the neural tube (NT, magenta) and notochord (NC). The neural tube and PSMs converge along the medial-lateral axis, and the anterior tailbud is further converged than the less developed posterior tailbud. (B–E) Transverse sections 160 μm posterior to the last somite boundary at 12–14 somite stage in wt (B), cdh2-/- (C), MZ itgα5-/- (D), and cdh2-/-; MZ itgα5-/- (E). Sections were reconstructed at a distance of 160–180 μm from last somite boundary after wholemount labeling for fibronectin (red) and nuclei (grey). Yellow dotted lines delineate neural tube contours. White arrowheads indicate locations of tissue detachment (also see Figure 1—figure supplement 2E and F). Dorsal is to the top. Scale bars = 70 μm. (F) Quantification of the medial-lateral length of the neural tube (as indicated by red double arrow) along the anterior-posterior axis starting from the last somite boundary. Quantifications were performed on transverse sections spaced every 20 μm. Dots represent means and error bars represent SD. Sample size: n = 10 PSMs on five embryos for each genotype. (G) Quantification of left-right asymmetry in PSM area. Each dot denotes an absolute difference in left and right PSM areas at each transverse section. Sample size: n = 75 sections from five embryos for each genotype. ***p<0.0005, T-test. cdh2-/- vs WT, p=0.79; MZ itgα5-/- vs WT, p=2.51e-4; MZ itgα5-/-;cdh2-/- vs WT, p=3.34e-10.
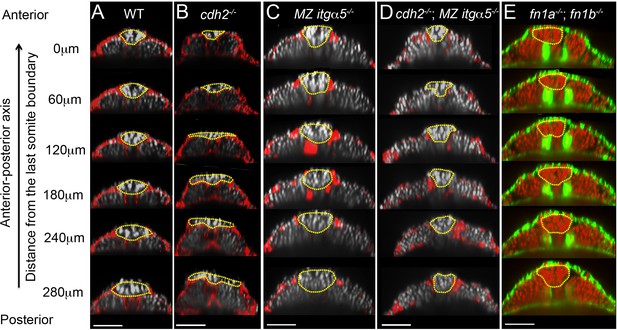
Reduction of cell-ECM interactions leads to precocious neural tube convergence and rescues cdh2 mutant neural tube convergence defects.
Each column shows a series of transverse sections in 12–14 somite stage embryos along the anterior-posterior axis of the tailbud starting from the last somite boundary (0 μm) in WT (A), cdh2-/- mutants (B), MZ itgα5-/- mutants (C), cdh2-/-; MZ itgα5-/- mutants (D), and fn1a-/; fn1b-/- mutants. (A–D) Immunostaining for FN (red) and nuclei labeling (grey). (E) F-actin labeling (green), and nuclei labeling (red). Yellow dotted lines delineate neural tube contours. Dorsal is to the top. Scale bars = 70 μm.
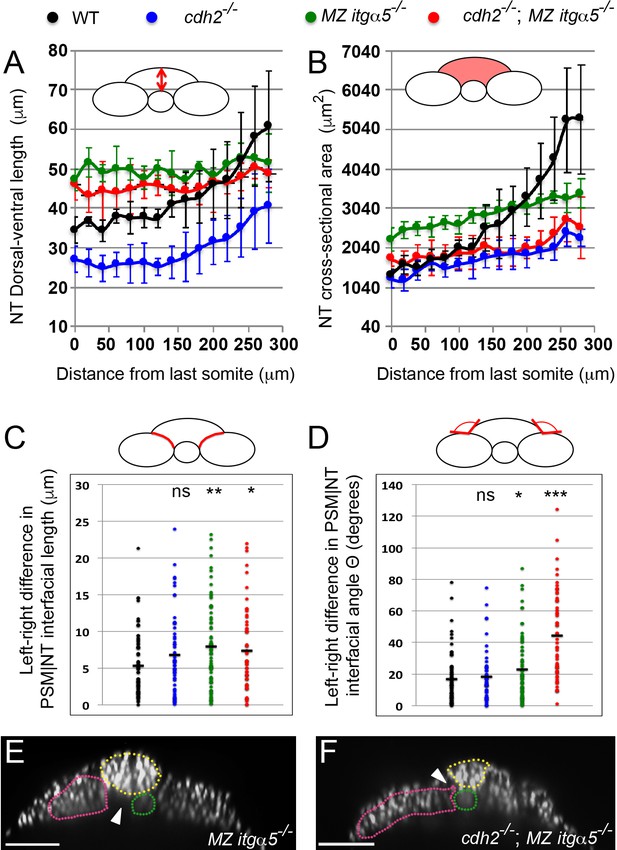
Reduction of cell matrix interactions provokes a precocious neural tube convergence and generates left-right asymmetries in the PSM|NT interfacial length and angle.
(A, B) Quantification of the dorsal-ventral length (A, as indicated by red double arrow) and the cross-sectional area (B, as indicated by the red area) of the neural tube along the anterior-posterior axis starting from the last somite boundary (0 μm). Measurements were performed on transverse sections taken every 20 μm. Dots represent means and error bars represent SD. Sample size n = 10 PSMs on five embryos for each genotype. (C, D) Quantification of left-right asymmetry in PSM|NT interfacial length (C) and PSM|NT interfacial angle (D). Each dot denotes an absolute difference in left and right PSM|NT interfacial length or angle in a transverse section. Sample size n = 75 sections from five embryos for each genotype. ***p<0.0005, **p<0.005, *p<0.05, via T-test. (C) cdh2-/- vs WT, p=0.095; MZ itgα5-/- vs WT, p=1.8e-3; MZ itgα5-/-; cdh2-/- vs WT, p=0.23. (D) cdh2-/- vs WT, p=0.57; MZ itgα5-/- vs WT, p=0.046; MZ itgα5-/-; cdh2-/- vs WT, p=1.56e-9. (E–F) Transverse sections for MZ itgα5-/- (E), and cdh2-/-; MZ itgα5-/- (F) identical to those presented in Figure 1D and Figure 1E but showing only the nuclei signal to highlight tissue detachments (arrowheads) between the notochord (green), the PSM (pink) and the neural tube(yellow).
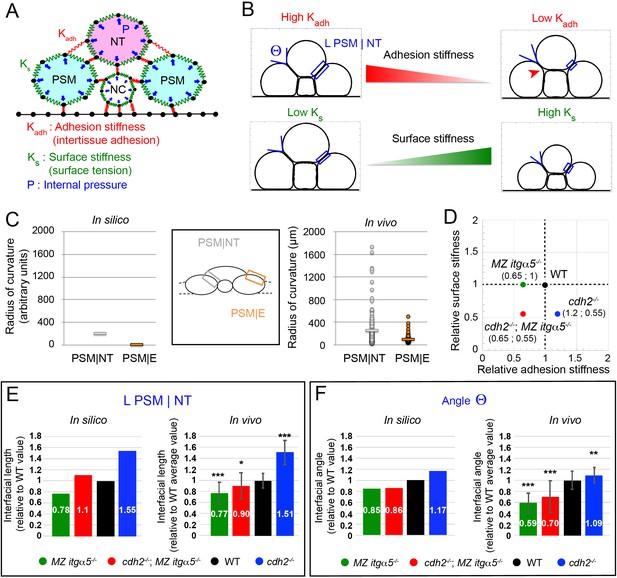
A computational model predicts tissue shapes across genotypes.
(A) A coarse-grained 2D model of a transverse section with three model parameters. Tissues are modeled as soft units with a fixed internal pressure (P, blue arrows), a surface stiffness (green springs along the tissue surfaces with individual spring constant Ks), and an adhesion stiffness (red springs connecting adjacent tissues with individual spring constant Kadh). Black dots represent material points subject to the forces. Black lines at the bottom model a rigid yolk surface. See Materials and methods for further details. (B) Variation of adhesion stiffness and surface stiffness in the model have opposing effects on the angle formed at the interface between PSM and neural tube (angle Θ) and on the length of the PSM|NT interface (L PSM|NT, blue box). Decreasing adhesion stiffness decreases interfacial angle and length, while decreasing surface stiffness increases angle and length. Reduction of adhesion stiffness also produces inter-tissue gaps showing local detachments (red arrowhead, top right panel). See Materials and methods for parameter values. (C) Radius of curvature for the PSM|NT interface (grey) and the PSM|E interface (orange) in silico (left panel) and in vivo (right panel). n = 150 on 75 sections from five embryos. (D) Different genotypes are arranged in a 2D parameter space according to their estimated levels of surface stiffness and adhesion stiffness relative to wild type. See Materials and methods for the details of the parameter values. (E–F) Comparison of Interfacial length (L PSM|NT) (E) and angle Θ (F) across genotypes relative to the average wild-type values, measured in silico and in vivo. Data represent mean with SD. Measurements in vivo were performed within the 140 μm posterior of last somite. n interfacial lengths = 150 on 75 sections on five embryos for WT and MZ itgα5-/-; 123 on 64 sections on five embryos for cdh2-/-; 129 on 73 sections on five embryos for cdh2-/-; MZ itgα5-/-. n angles = 145 on 75 sections on five embryos for WT; n = 115 on 63 sections on five embryos for cdh2-/-; 137 on 72 sections on five embryos for MZ itgα5-/-; 133 on 65 sections on five embryos for cdh2-/-; MZ itgα5-/-. T-tests were performed for the following comparisons: cdh2-/- vs WT, p=2.97E-25 (L PSM|NT) p=0.0007 (angle Θ); MZ itgα5-/- vs WT, p=1.16E-13 (L PSM|NT) p=5.37E-30(angle Θ); MZ itgα5-/-;cdh2-/- vs WT, p=0006 (L PSM|NT) p=4.34E-11 (angle Θ).
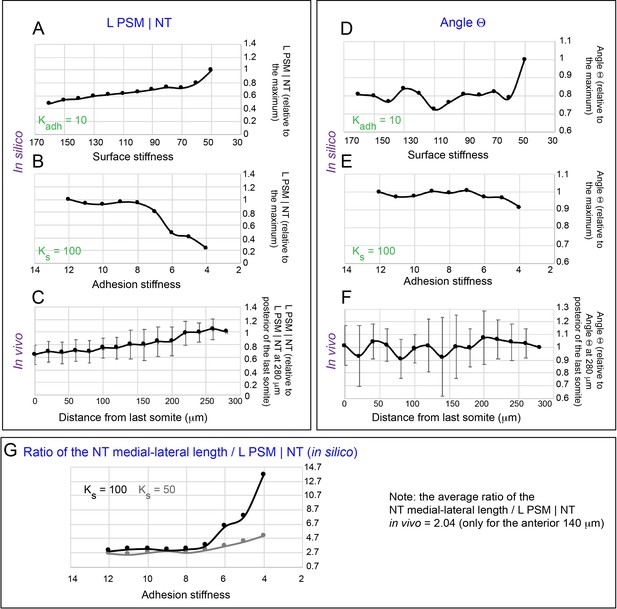
Parameter exploration of a simple 2D model of tissue morphology.
(A–C) Relative change in L PSM|NT in silico as a function of surface stiffness with a fixed Kadh = 10 (A), as a function of adhesion stiffness with a fixed Ks = 100 (B), or in vivo as a function of distance from the last somite (C). L PSM|NT decreases with surface stiffness and increases with adhesion stiffness in silico while L PSM|NT decreases from posterior to anterior in vivo as PSM solidifies and FN matrix content at the PSM|NT interface remains constant. (D–F) Relative change in Angle Θ in silico as a function of surface stiffness with a fixed Kadh = 10 (D), as a function of adhesion stiffness with a fixed Ks = 100 (E), or in vivo as a function of distance from the last somite (F). At higher surface stiffness (Ks >70) and adhesion stiffness (Kadh >7) regimes, the Angle Θ is not sensitive to changes in these parameters. The Angle Θ is only sensitive to changes in surface stiffness or adhesion stiffness at low regimes (Ks <70, Kadh <7), and it decreases with surface stiffness and increases with adhesion stiffness. In vivo, the Angle Θ is not varying significantly from posterior to anterior. (G) Ratio of medial-lateral length of NT to the length of interface between PSM and NT (NT ML length|L PSM|NT) in silico as a function of adhesion stiffness at a high (Ks = 100) or at a low (Ks = 50) surface stiffness. The NT ML length|L PSM|NT ratio was used to calibrate the Ks and Kadh values for the WT in our in silico model. The closest value of this ratio to the in vivo WT value (average value of 2.04 for the anterior 140 um) is obtained for Kadh between 8 and 12 for both surface stiffness conditions.
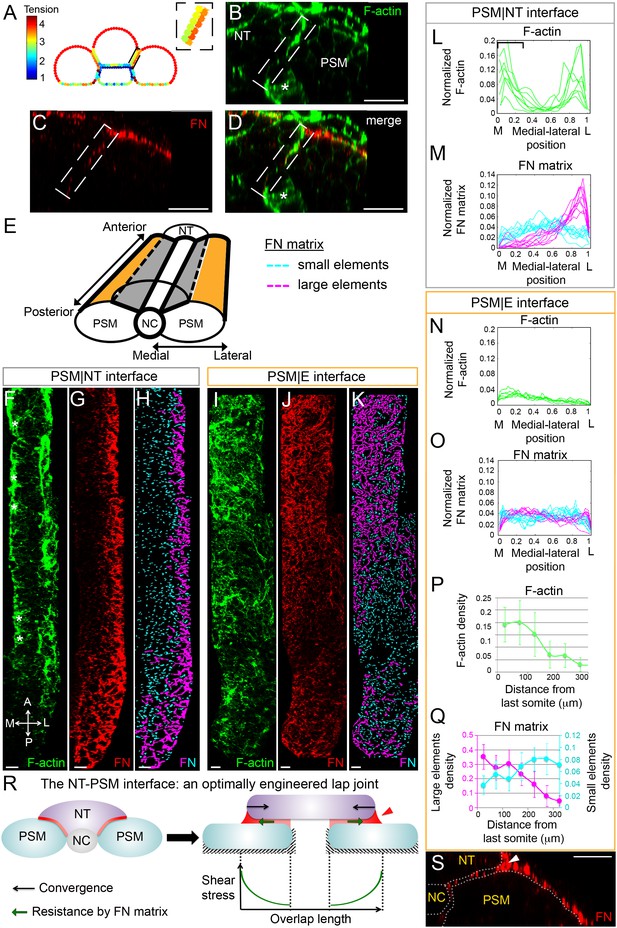
Gradients of Fibronectin matrix and F-actin correlate with in silico gradients of tension.
(A) Heat map of the tension distribution along tissue surfaces in silico. Warmer colors represent higher tension. Inset: magnification of the PSM|NT interface showing a medial-lateral tension gradient on the neural tube side of the interface. (B–D) Transverse sections taken 60 μm away from last somite boundary on a 12–14 s stage embryo co-labeled for Fibronectin (FN, red) and F-actin (green). There are colocalized medial-lateral gradients of both F-actin and Fibronectin matrix along the PSM|NT interface (dashed box). Star denotes differentiating myofibers that show high F-actin signal. Scale bars = 20 μm. (E) 3D schematic of the PSM|NT interface (grey) and the PSM|E interface (orange). To quantify matrix assembly along these interfaces, FN matrix signal is sorted into two categories based on size: small matrix elements (cyan) and large matrix elements (magenta). See SI for details. The F-actin signal (F, I), FN signal (G, J), and processed images of small and large matrix elements (H, K) are shown. The PSM|NT interface exhibits medial-lateral gradients of F-actin and Fibronectin (F–H), whereas the PSM|E interface shows an increase in F-actin and Fibronectin matrix assembly along the posterior to anterior axis (I–K). All images are projected dorsal views. A = anterior, P=posterior, M = medial, L = lateral. Scale bar = 10 μm. Quantification of the medial-lateral distributions of F-actin (L) and small and large FN matrix elements (M) along the PSM|NT interface. The bracket in (L) denotes the differentiating myofibers rich in F-actin. Quantification of the medial-lateral distributions of F-actin (N) and small and large FN matrix elements (O) along the PSM|E interface. Quantification the density of F-actin (P) and small and large matrix elements (Q) along the anterior-posterior axis of the PSM|E interface. Data represent means and SD. Sample sizes: L, n = 8 PSMs from six embryos; N, P, n = 7 PSMs from five embryos; M, O, Q, n = 10 PSMs from six embryos. (R) The NT-PSM interfaces represented as a lap-joint with a single sided strap. Neural tube (magenta) acts as a strap that is adhered to the two PSMs (cyan) via a graded adhesive (red) made of Fibronectin. Medial and ventral edges of PSM are attached to the notochord and yolk surface respectively (dashed region). Black arrows denote neural tube convergence and green arrows denote resistance to this convergence via the adhesive. Neural tube convergence with respect to the adhesive produces shear stress. Established theories of lap-joint predict a stress gradient with higher stress at the lateral edge of the PSM|NT interface. Extra adhesive, called a ‘spew fillet,’ in an arced shape at the lateral sides of the strap strengths the joint. (S) Transverse section on a 12–14 s stage embryo labeled for Fibronectin (FN, red) illustrating the spew fillet of FN matrix (arrowhead). Scale bar = 20 μm.
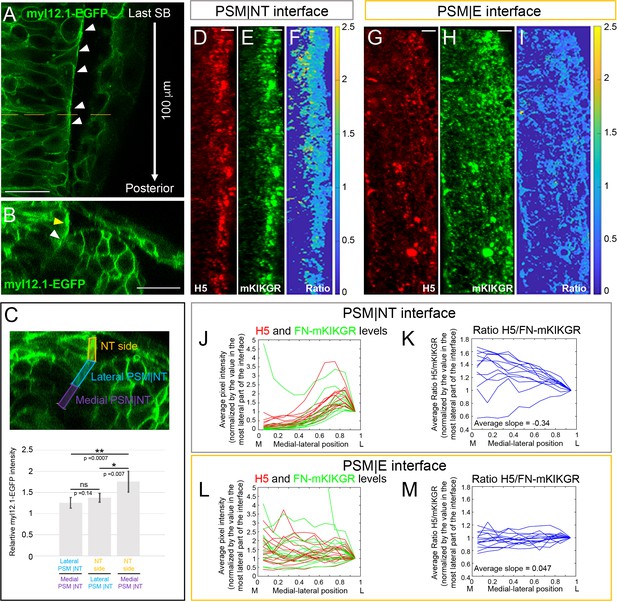
Medial-lateral gradients of Myosin II and Fibronectin matrix tension at the PSM|NT interface.
(A–C) Embryos were injected with myl12.1-EGFP mRNA to visualize distribution of Myosin-II (Araya et al., 2019; Behrndt et al., 2012). (A) A dorsal view of the neural tube of a live 12-somite stage embryo. The image shows the first 100 μm posterior of the last somite boundary in a single confocal plane roughly 10 μm underneath the dorsal surface of the neural tube. Local enrichment of Myosin-II can be seen in the lateral surface of the neural tube (white arrowheads). Anterior is is up. The dashed line indicates the position of the transverse section in B. Scale bar = 25 μm. (B) A transverse section 60 μm away from last somite where enrichment of Myosin-II can be seen both along the lateral side of the neural tube (yellow arrowhead) and the lateral part of the PSM|NT interface (white arrowhead). Scale bar = 25 μm. (C) Quantification of the relative Myosin II levels reveals a medial to lateral increase of Myosin-II levels from the medial part of PSM|NT interface to the lateral side of the neural tube. Transverse sections were generated every 5 μm along the first 100 μm posterior of the last somite boundary. For each section, the neural tube border was divided into three regions as indicated on the transverse section (top panel): the medial half of the PSM|NT interface (purple), the lateral half of the PSM|NT interface (blue), and the adjacent lateral side of the NT (orange). The mean myl12.1-EGFP intensity was measured in each region, and the relative intensity ratios (Lateral PSM|NT/Medial PSM|NT; NT side/Lateral PSM|NT; NT side/Medial PSM|NT) were calculated on each slice and averaged for each embryo. Average ratios across all embryos were then plotted (bottom panel). All ratios are above 1 according to the 95% confidence intervals (error bars). The difference between the ratios was evaluated via T-test. Sample size: n = 25 data points (each point is the average of 21 slices per embryo), from 13 embryos (two sides per embryo) from four experiments. (D–I) Immunostaining with the H5 antibody on 12 s stage embryos injected with FN1a-mKIKGR13.2-hsFNIII10-11 mRNA. The H5 signal (D, G), mKIKGR signal (E, H), and processed heatmaps of the H5/mKIKGR ratio (F, I) are shown. All images are projected dorsal views with anterior to the top and medial to the left. Scale bars = 10 μm. The PSM|NT interface (D–F) exhibits an increasing medial-lateral gradient of H5. By contrast, the H5/mKIK ratio shows an opposite gradient as the tension on individual fibers is lower laterally where stresses are distributed over more Fibronectin molecules. (G–I) The PSM|E interface does not exhibit any medial-lateral gradient. (J–M) Quantification of the medial-lateral distributions of H5 and mKIKGR levels (J, L) and the H5/mKIKGR ratio (K, M) along the PSM|NT (J, K) or PSM|E (L, M) interface. Sample size: n = 12 PSM|NT and 14 PSM|E interfaces from 10 and 9 embryos, respectively from three experiments. The average slopes of the H5/mKIKGR ratios for the PSM|NT and PSM|E interfaces differ (**, p=0.0046, T-test).
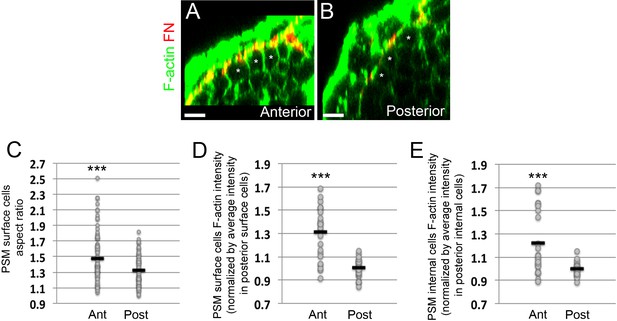
Epithelialization of PSM surface cells and increases in F-actin intensity in PSM cells as the PSM matures from posterior to anterior.
(A, B) Transverse sections taken in the anterior PSM (A, 0–100 μm away from last somite boundary) or posterior PSM (B, 200–300 μm away from last somite boundary) on 12–14 s stage embryos co-stained for Fibronectin (FN, red) and F-actin (green). Asterisks denote PSM surface cells. Scale bars = 10 μm. (C) Quantification of PSM surface cell aspect ratio in the anterior and posterior portions of the PSM. Sample size: n = 93 cells (anterior PSM) and 92 cells (posterior PSM) from three embryos. Anterior vs posterior, p=7.46e-5. (D–E) Quantification of the mean F-actin intensity of the PSM surface cells region (D) or PSM internal cells region (E) in the anterior and posterior PSM from three embryos. Each dot represents a transverse section for which the mean F-actin signal within the surface cells or internal cells was measured and normalized by the average intensity of the signal in the posterior sections of the same embryo. Sample size: n = 24 sections (anterior PSM) and 25 sections (posterior PSM). ***p<0.0005 using a T-test. (D) Anterior vs posterior, p=5.62e-7. (E) (D) Anterior vs posterior, p=7.7e-4.
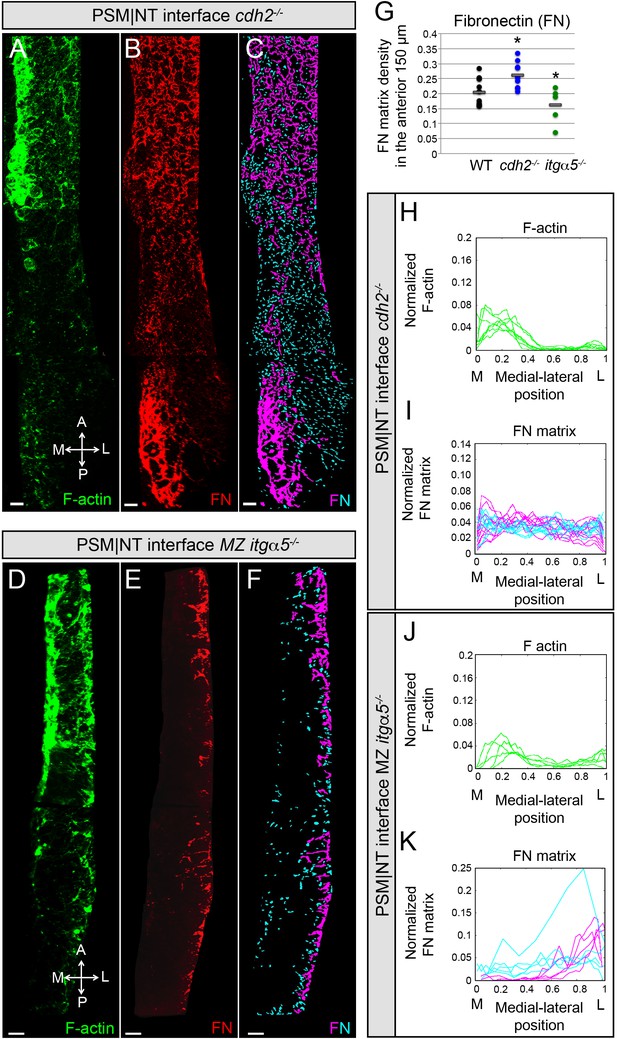
Reduction of cell-cell adhesion eliminates the medial-lateral gradients of Fibronectin matrix and F-actin.
PSM|NT interfaces of cdh2-/- (A, B, C) and MZ itgα5-/- (D, E, F) mutants at the 12–14 somite stage. F-actin (A, D), Fibronectin (B, E), and processed images of small and large Fibronectin matrix elements (C, F) are shown. All images are dorsal views. A = anterior, P = posterior, M = medial, L = lateral. Scale bars = 10 μm. (G) Quantification of Fibronectin density within the anterior 150 μm of the PSM|NT interface in wild type, cdh2-/- and MZ itgα5-/-. cdh2-/- vs WT, p=9.4e-3; MZ itgα5-/- vs WT, p=0.012. Quantification of the medial-lateral distributions of the F-actin (H, J) and small and large Fibronectin matrix elements (I, K) at the PSM|NT interface in cdh2-/- (H, I) and MZ itgα5-/- (J, K). Sample sizes: G, I, K, n = 10 PSMs from six embryos for WT, n = 10 PSM from five embryos for cdh2-/-, n = 5 PSM from five embryos for MZ itgα5-/-. Sample sizes: H, n = 7 PSM from four embryos; J, n = 5 PSM from five embryos.
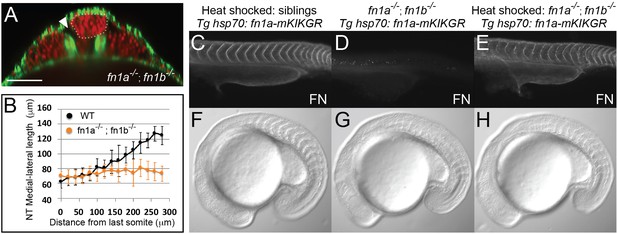
Fibronectin is required for inter-tissue adhesion and a hsp70: fn1a-mKIKGR transgene rescues fn1a -/-; fn1b-/- double mutants.
(A–B) fn1a -/-; fn1b-/- double mutants exhibit a precociously converged neural tube and tissue detachment similar to MZ itgα5-/- mutants. (A) Transverse section 160 μm from last somite boundary in 12–14 somite stage fn1a-/-; fn1b -/- embryos with labeled nuclei (red) and F-actin (green). Dotted line delineates the neural tube. Arrowhead indicates tissue detachment. Scale bar = 70 μm. (B) Quantification of the medial-lateral length of the neural tube along the anterior-posterior axis starting from the last somite boundary (0 μm). Data represent means and SD. Immunostaining for Fibronectin (FN) on a 24 hpf heat shocked sibling (C), a non-heat shocked fn1a -/-; fn1b -/-; Tg hsp70: fn1a-mKIKGR embryo (D) and a heat shocked fn1a -/-; fn1b -/-; Tg hsp70: fn1a-mKIKGR embryo (E). Lateral views of the trunk with anterior to the left. DIC images of a heat shocked sibling (F), a non-heat shocked fn1a -/-; fn1b-/-; Tg hsp70: fn1a-mKIKGR embryo (G) and a heat shocked fn1a -/-; fn1b -/-; Tg hsp70: fn1a-mKIKGR embryo (H).
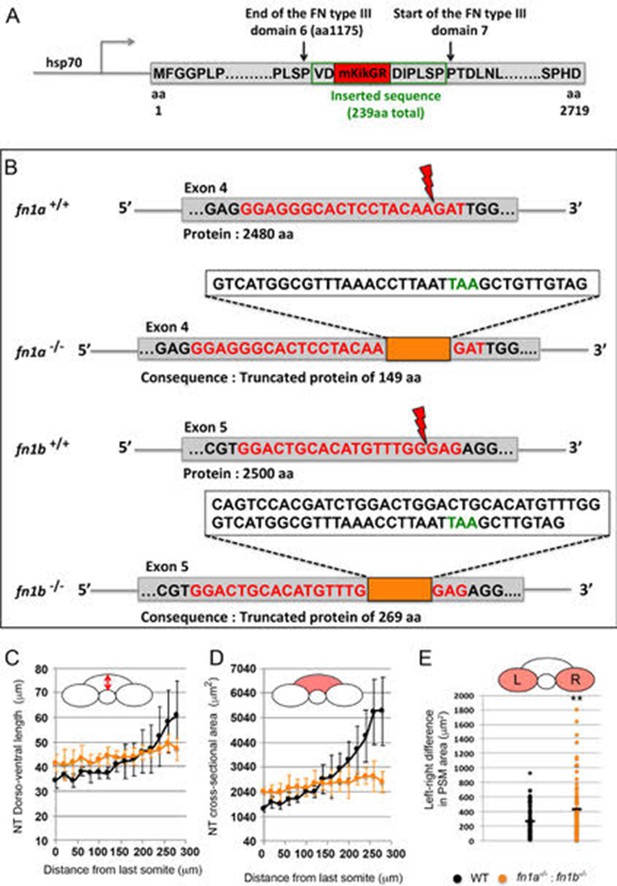
Generation of Tg hsp70: fn1a-mKIKGR transgenic zebrafish to study matrix remodeling in live embryos and generation of double fibronectin mutants which exhibit precocious neural tube convergence and left-right PSM asymmetries.
(A) Schematic of the hsp70: fn1a-mKIKGR transgene (protein sequence). Monomeric kikume (mKIKGR) was inserted in the coding sequence of fn1a between FN type III domains 6 and 7. Note that the last four amino acids of the FN type III domain 6 (PSPL) were added to the 5’ of the FN type III domain 7. (B) Generation of a double fibronectin mutant line using CRISPR. For each gene, the top sequence indicates the wild-type target site (red) that was recognized by Cas9 to create a double strand break (red lightning). A donor DNA containing a ‘stop cassette’ was co-injected to guarantee the introduction of a stop codon and to facilitate PCR genotyping. The bottom sequence indicates the resultant genomic sequence at the targeted locus with the inserted region (orange box) and the position of stop codon (green). (C–E) Double fibronectin mutants exhibit precocious neural tube convergence and disruption of bilateral symmetry. Quantification of the dorsal-ventral length (C, as indicated by red double arrow) and the cross-sectional area (D, as indicated by the red zone) of the neural tube along the anterior-posterior axis starting from the last somite boundary (0 μm). Measurements were performed on transverse sections taken every 20 μm. Dots represent means and error bars represent SD. Sample size n = 10 PSMs on five embryos for each genotype. (E) Quantification of left-right asymmetry in PSM area. Each dot denotes an absolute difference in left and right PSM areas at each transverse section. Sample size: n = 75 sections from five embryos for each genotype. **p<0.005, T-test. fn1a-/-;fn1b-/- vs WT, p=1.4e-3.
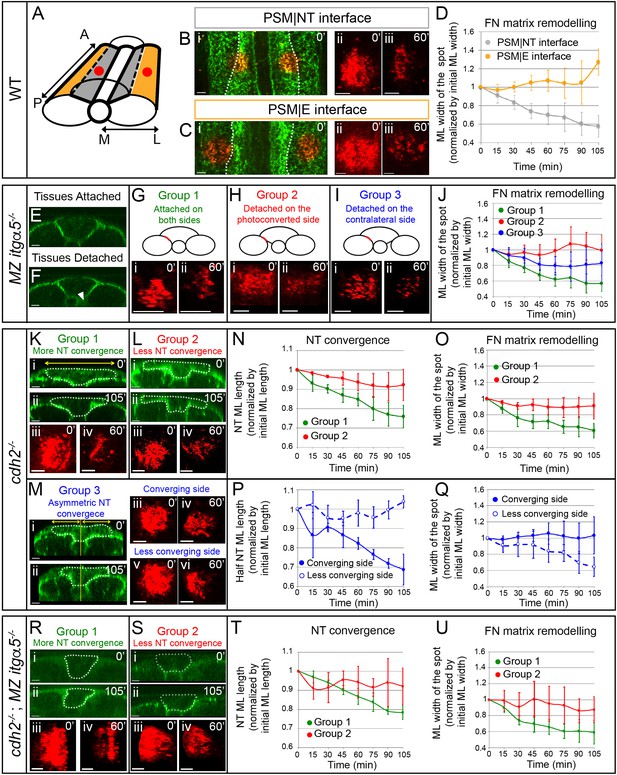
Anisotropic Fibronectin matrix remodeling dependent upon neural tube convergence and inter-tissue adhesion.
(A) 3D schematic indicating positions of the photoconverted regions (red dots) along the PSM|NT and PSM|E interfaces. A = anterior; P = posterior; M = medial; L = lateral. Spots of photoconverted Fibronectin matrix at PSM|NT interface (B) or at PSM|E interface (C). (i) Dorsal views of heat-shocked Tg hsp70:fn1a-mKIKGR embryos at 12 somite stage showing Fibronectin matrix (green) and Fibronectin spots immediately after photoconversion (red). Dotted lines delineate the boundary between the PSM|NT and PSM|E interfaces. Images of spots of Fibronectin at early (ii) and late (iii) timepoints. (D) Quantification of the medial-lateral width of the photoconverted spot over time. Sample size: n = 9 spots from four embryos for each interface. (E–J) Analysis of FN matrix remodeling at PSM|NT interface in MZ itgα5-/-. (E–F) Transverse sections of heat-shocked 12 somite stage MZ itgα5-/-; Tg hsp70:fn1a-mKIKGR embryos. MZ Itgα5 mutants have local tissue detachments (arrowhead in F). (G–I) Photoconversion experiments fall into three groups based on tissue detachment at the level of the photoconverted interface (red line in schematics). (i–ii) Images of the photoconverted spots at the beginning of the movie (i) and one hour later (ii). (J) Quantification of the medial-lateral width of the photoconverted spot over time. Sample size: n = 5 spots from three embryos for each of group 1 and 2; 7 spots from four embryos for group 3. (K–Q) Analysis of FN matrix remodeling at PSM|NT interface in cdh2-/-. (K–M) Cdh2 mutants show variability in neural tube convergence and fall into three groups based on the degree of neural tube convergence at the level of the photoconverted interface. Either the neural tube converged (Group 1 (K)); did not converge (Group 2 (L)); or converged asymmetrically (Group 3 (M)). (i–ii) Transverse sections of cdh2-/-; Tg hsp70:fn1a-mKIKGR heat-shocked embryos at the beginning (i, 12 somite stage) and at the end of the time lapse (ii). Dotted lines delineate the neural tube contour. Yellow lines indicate the midline of the embryo used as reference to divide the neural tube in left and right halves. Yellow double arrows indicate how the medial-lateral width of the total neural tube or half of the neural tube was quantified. (iii-vi) Images of the photoconverted spots at the beginning of the movie (iii, v) and one hour later (iv, vi). (N, P) Quantification of the medial-lateral length of the total neural tube (N) or of the half neural tube (P) over time. Sample size: group 1, four embryos; group 2, four embryos; group, three embryos. One transverse section per embryo. (O, Q) Quantification of the medial-lateral width of the photoconverted region over time. Sample size: group 1, n = 8 spots from four embryos; group 2, 10 spots from three embryos; group 3, 3 spots from three embryos. (R–U) Analysis of FN matrix remodeling at the PSM|NT interface in cdh2-/-; MZ itgα5-/- embryos. (R, S) Embryos were sorted into two categories based on a high (Group 1) or low (Group 2) degree of neural tube convergence at the level of the photoconverted interface. (i–ii) Transverse sections of cdh2-/-; MZ itgα5-/-; hsp70:fn1a-mKIKGR heat-shocked embryos at the beginning (i, 12 somite stage) and at the end of the time lapse (ii). Dotted lines delineate the neural tube contour. (iii-iv) Images of the photoconverted spots at the beginning of the movie (iii) and 1 hr later (iv). (T) Quantification of the medial-lateral length of the neural tube over time. Sample sizes: n = 3 neural tube sections on two different embryos for each of groups 1 and 2. (U) Quantification of medial-lateral width of the photoconverted region over time. Sample sizes: n = 8 spots from three embryos for group 1; 4 spots from two embryos for group 2. All images of photoconverted spots are projected dorsal views perpendicular to the interfaces with anterior to the top and medial to the left. Scale bars of all images = 15 μm. In all plots, dots represent means and error bars represent SD.
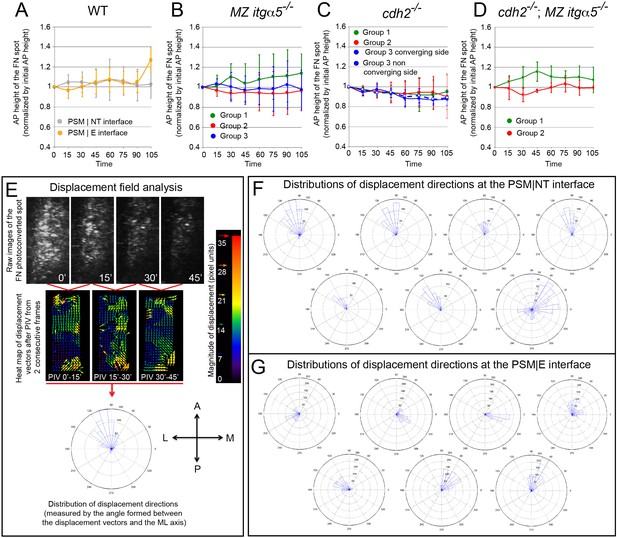
Medial-lateral ECM remodeling along the PSM|NT interface.
(A–D) Quantification of the anterior-posterior length of the photoconverted FN matrix spots over time in wild-type PSM|NT and PSM|E interfaces (A), and in the PSM|NT interfaces in MZ itgα5-/- (B), cdh2-/- (C), and cdh2-/-; MZ itgα5-/- (D). In all plots, dots represent means and error bars represent SD. Sample sizes are as indicated in Figure 6. (E) Displacement field analysis of the photoconverted spots by particle image velocimetry (PIV). A displacement fields is constructed using a PIV plugin in ImageJ (see Materials and methods) using two successive time-frames. A frame-to-frame correlation of signals was established, and all vectors were plotted in a rose plot. A = anterior P=posterior M=medial L=lateral. (F–G). Distribution of displacement directions of the photoconverted regions at the PSM|NT (F) and at the PSM|E (G) interfaces in wild-type embryos. Each rose plot represents the data for one photoconverted region obtained as described in (E). In F, there is a bias toward anterior-lateral direction in six out of seven samples while there is no consistent directional bias in G.
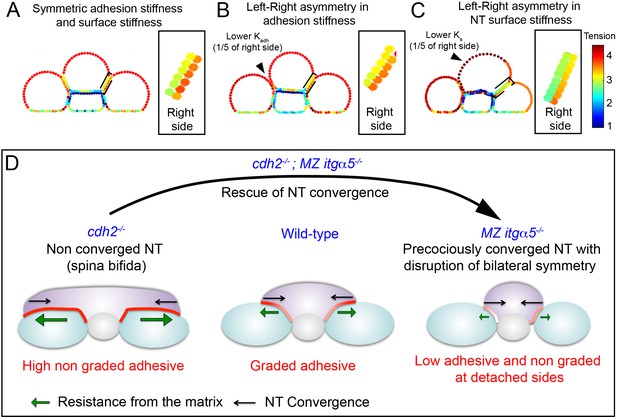
Fibronectin mediated inter-tissue adhesion ensures bilaterally symmetric morphogenesis but predisposes the neural tube to convergence defects.
(A–C) In silico heat maps of the tension distributions along the tissue surfaces with left-right variation either in adhesion stiffness (B) or in surface stiffness (C), compared to a symmetric condition (A). Warmer colors represent higher tension. Insets: magnification of the right PSM|NT interface, which is unperturbed in every condition, to show the contralateral effects in tension gradients. The gradients at the neural tube side of the interface become shallower in B and C compared to A. (D) The Fibronectin matrix mediates inter-tissue adhesion like a ‘smart glue’ that dynamically remodels in a graded fashion at the PSM|NT interface in response to neural tube convergence to have higher density at zones of higher inter-tissue stress. This inter-tissue adhesion mechanically couples left and right PSM to the neural tube like a ‘lap joint’ and maintain bilateral symmetry during elongation. The Fibronectin matrix also provides resistance (green arrows) against the neural tube convergence (black arrow). Thus, the ECM can act like a double-edged sword. Too much matrix deposition maintains the symmetry, but slows down the convergence due to high resistance, and predisposes the neural tube to spina bifida-like phenotype with an open neural tube (cdh2 mutant, left panel). Conversely, reduction in matrix deposition helps the neural tube convergence by reducing the resistance (as shown by the rescue of neural tube convergence in double cdh2; itgα5 mutants), but it produces local tissue detachment and breaks the mechanical coupling between the tissues generating left-right asymmetries (itgα5 mutants, right panel).
Videos
Changes in neural tube and PSM shapes over developmental time.
This movie is a series of transverse sections from posterior to anterior from a fixed embryo showing Fibronectin localization (green) and cell nuclei (magenta). The development of the vertebrate trunk and tail proceeds from anterior to posterior, thus this series illustrates the medial convergence and shape changes of the neural tube and PSM over time. The quantification of these shapes in different genotypes is detailed in Figures 1, 2 and 5, Figure 1—figure supplement 1, Figure 1—figure supplement 2, Figure 2—figure supplement 1 and Figure 5—figure supplement 1.
Tracking Fibronectin matrix dynamics at tissue interfaces.
Movies representing 105 min time-lapses (15 min interval) after local photoconversion (red spots) of Fn1a-mKikGR matrix (green) at 12 somite stage, either at the PSM|NT interface (top movie) or at the PSM|E interface (bottom movie). Dorsal views with anterior to the top.
Tables
Rescue experiments with a heat shock hsp70: fn1a-mKikGR at shield stage.
For each experiment, fn1a-/+; fn1b-/+;hsp70: fn1a-mKikGR adults were crossed. Three crosses include a parent homozygous for the transgene while one cross was from parents both hemizygous for the transgene. Embryos from each clutch were divided in half. 50% of embryos were controls that were not heat shocked, and 50% of embryos were heat shocked at the shield stage. The numbers shown correspond to the total number of embryos presenting each phenotype (either wild-type, fn1a-/- or fn1a-/-; fn1b-/-) based on the presence of somite border defects at the 14–18 somite stage.
| Embryos with no heat shock | Heat shocked embryos | ||
|---|---|---|---|
| Fluorescent | Non-fluorescent | ||
| Phenotypically wild-type | 301 | 351 | 26 |
| fn1a-/- | 91 | 0 | 3 |
| fn1a-/-; fn1b-/- | 20 | 0 | 4 |
Rescue experiments with heat shock fn1a-mKikGR at the 10–12 somite stage on pre-sorted fn1a-/-; fn1b-/- embryos.
For each experiment, fn1a-/+; fn1b-/+;hsp70: fn1a-mKikGR adults were crossed and embryos sorted for the fn1a-/-; fn1b-/- morphological phenotype were heat shocked at the 10–12 stage and assayed for somite border defects at 24 hpf. 1 or two embryos per experiment were not heat shocked as a control. Four experiments were performed and the numbers shown in the table represent the total number of embryos with each phenotype. * HS at the 12-somite stage will rescue body elongation and head development defects observed in the fn1a-/-; fn1b-/- embryos, however heat shock will not fully rescue border defects of the first 1–6 somites.
| Embryos with no heat shock | Heat shocked embryos | ||
|---|---|---|---|
| Fluorescent | Non-fluorescent | ||
| Rescued phenotype * | - | 13 | 0 |
| fn1a-/-; fn1b-/- | 5 | 0 | 1 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information | |
|---|---|---|---|---|---|
| Strain, strain background (Danio rerio male and female) | Wild-type strain TLAB | ZIRC | Crosses from AB strain (RRID:ZIRC_ZL1) with TL strain (RRID:ZIRC_ZL86) | ||
| Strain, strain background (Danio rerio male and female) | Wild-type strain TLF | ZIRC | RRID:ZIRC_ZL86 | ||
| Genetic reagent (Danio rerio) | strain cdh2 mutant tm101 | Lele et al., 2002 | RRID:ZFIN_ZDB-GENO-080110-3 | ||
| Genetic reagent (Danio rerio) | strain MZ itgα5 mutant thl30 | Jülich et al., 2005 | ZIRC ID: ZL2023 | ||
| Genetic reagent (Danio rerio) | Tg(hsp70:fn1a-mKIKGR) | This paper | Transgenic line expressing,, fibronectin 1a tagged with mKIKGR under the control of the heat-shock promoter hsp70. See Material and methods section. Available in Scott Holley laboratory (Yale University) | ||
| Genetic reagent (Danio rerio) | fn1a; fn1b double mutant | This paper | CRISPR/Cas9 generated mutant line where fn1a and fn1b genes have been knocked out by insertion of a stop cassette. See Material and methods section. Available in Scott Holley laboratory (Yale University) | ||
| Antibody | Anti-Fibronectin antibody (Rabbit polyclonal) | SIGMA | F3648 | 1/100 RRID:AB_476976 | |
| Antibody | Anti-V5 antibody (Goat polyclonal) | Abcam | Ab 9137 | 1/400 RRID:AB_307037 | |
| Antibody | H5-V5-tag antibody (recombinant purified scFv) | Cao et al., 2017 | 50 mg/ml | ||
| Antibody | Anti-goat Alexa 555 (Donkey polyclonal) | Thermofisher scientific | A32816 | 1/200 RRID:AB_2762839 | |
| Antibody | Anti-rabbit Alexa 555 (Donkey polyclonal) | Invitrogen | A31572 | 1/200 RRID:AB_162543 | |
| Sequence-based reagent | sgRNA fn1a-/-line | This paper | 5’ATTTAGGTGACACTATAGGAGGGCACTCCTACAAGATGTTTTAGAGCTAGAAATAGCAAG3’ | ||
| Sequence-based reagent | stop codon cassette oligonucleotide fn1a-/-line | This paper | 5’GAGGGAGGGCACTCCTACAAGTCATGGCGTTTAAACCTTAATTAAGCTGTTGTAGGATTGGAGACACATGGCAGA3’ | ||
| Sequence-based reagent | sgRNA fn1b-/-line | This paper | 5’ATTTAGGTGACACTATAGGACTGCACATGTTTGGGAGGTTTTAGAGCTAGAAATAGCAAG3’ | ||
| Sequence-based reagent | stop codon cassette oligonucleotide fn1b-/-line | This paper | 5’CGTGGACTGCACATGTTTGGGTCATGGCGTTTAAACCTTAATTAAGCTGTTGTAGGAGAGGGAAACGGACGCATC3’ | ||
| Sequence-based reagent | fn1a DiagA1 primer | This paper | 5’GACTGTACTTGCATTGGCTCTG3’ | ||
| Sequence-based reagent | fn1b DiagA1 primer | This paper | 5’GAGCGTTGCTATGATGACTCAC3’ | ||
| Sequence-based reagent | stop A primer | This paper | 5’GCTTAATTAAGGTTTAAACGCC3’ | ||
| Recombinant DNA reagent | mKikGR plasmid | Habuchi et al., 2008 | |||
| Recombinant DNA reagent | Tg(hsp70:fn1a-mKIKGR) plasmid | This paper | Plasmid designed to generate the Tg(hsp70:fn1a-mKIKGR) transgenic line. See Materials and methods section for detailed information about the sequence of this transgene. Available in Scott Holley laboratory (Yale University) | ||
| Recombinant DNA reagent | FN1a-mKIKGR13.2-hsFNIII10-11 plasmid | This paper | Plasmid designed to generate the mRNA coding for the human/zebrafish chimeric FN1a-mKIKGR13.2-hsFNIII10-11 protein. See Materials and methods section. Available in Scott Holley laboratory (Yale University) | ||
| Software, algorithm | Imaris | Bitplane | RRID:SCR_007370 | ||
| Software, algorithm | Matlab | Mathworks | RRID:SCR_001622 | ||
| Software, algorithm | Fiji | Opensource | RRID:SCR_002285 | ||
| Other | Alexa Fluor 488 Phalloidin | Life technologies | A12379 |