Optical dopamine monitoring with dLight1 reveals mesolimbic phenotypes in a mouse model of neurofibromatosis type 1
Figures
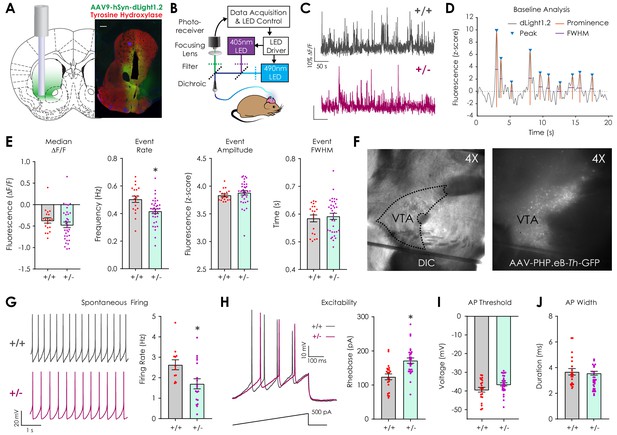
Assessment of basal dopaminergic function in vivo with dLight1.2 and ex vivo patch clamp electrophysiology.
(A) Illustration showing location of stereotaxic injection of the AAV9-hSyn-dLight1.2 viral vector and photometry fiber implantation (left). Representative histological image (right, scale: 300 μm) showing the fiber tip location and expression of dLight1.2 (stained for GFP, green) and dopaminergic terminal tyrosine hydroxylase (TH, Red). (B) Schematic of fiber photometry system used for dLight1.2 (490 nm) and isosbestic (405 nm; reference signal) excitation and emission signal detection in freely moving mice. (C) Representative dLight1.2 traces in Nf1+/+ and Nf1+/- mice. (D) Representative trace and analysis features for baseline peak detection. (E) Peak analysis of baseline dLight1.2 recordings revealed that Nf1+/- mice (n = 33) exhibit reduced transient frequency (unpaired t-test; t50 = 3.06, p=0.004) but not median fluorescence (unpaired t-test; t50 = 1.01, p=0.32), transient amplitude (unpaired t-test; t50 = 0.83, p=0.41), or full width at half maximal amplitude (FWHM; unpaired t-test; t50 = 0.43, p=0.67) when compared to Nf1+/+ littermates (n = 19). (F) 4X differential interference contrast (DIC) image (left) of an acute horizontal midbrain slice containing the ventral tegmental area (VTA) and 4X epifluorescence image (right) with GFP-labeled catecholaminergic neurons following systemic delivery of AAV-PHP.eB-Th-GFP (1 × 1011 v.g./mouse). (G) Representative traces showing spontaneous whole-cell firing of putative VTA dopaminergic neurons (left). Spontaneous firing rates (right) were lower (unpaired t-test; t28 = 2.58, p=0.0 w) in Nf1+/- putative dopaminergic neurons (n = 18) compared to Nf1+/+ neurons (n = 12). (H) Representative electrophysiological traces (left) showing evoked firing by a 1 pA/ms ramp current from −60 mV in Nf1+/+ and Nf1+/- putative dopaminergic neurons. Rheobase (right; unpaired t-test; t48 = 4.05, p<0.001) but not action potential threshold (I; t48 = 1.93, p=0.06) or width (J; t48 = 0.39, p=0.70) was increased in Nf1+/- (n = 29) putative dopaminergic neurons compared to Nf1+/+ (n = 21). *denotes p<0.05 vs Nf1+/+. Data presented as mean ± SEM.
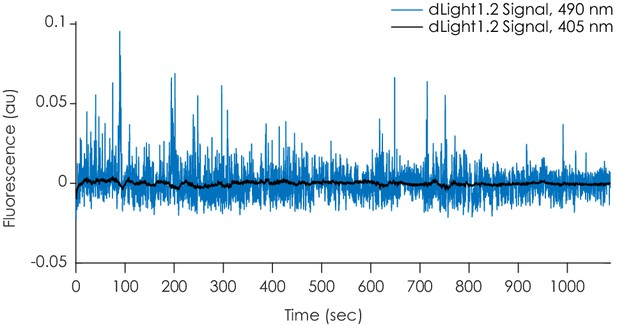
Raw fluorescent photometry signals.
Example traces showing dLight1.2 fluorescence produced by simultaneous isosbestic (405 nm, black) or dopamine-dependent (490 nm, blue) excitation that were used to calculate the ΔF/F values.
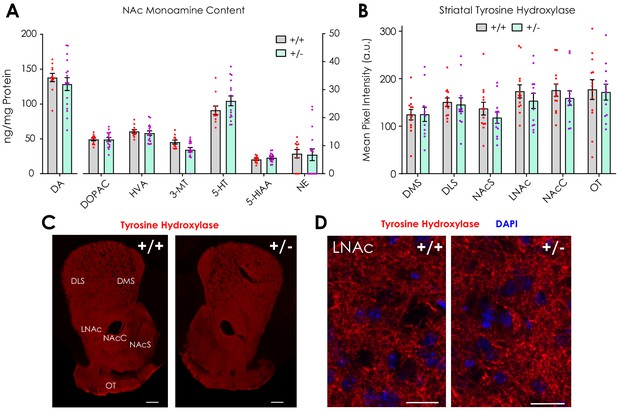
Striatal catecholamine content and tyrosine hydroxylase immunofluorescence.
(A) NAc monoamine and monoamine metabolite content determined by HPLC with electrochemical detection (multiple t-tests; n+/+ = 11; n+/- = 16): dopamine (DA; t25 = 0.77, q = 0.83), 3,4-dihydroxyphenylacetic acid (DOPAC; t25 = 0.076, q = 0.99), homovanillic acid (HVA; t25 = 0.52, q = 0.90), 3-methoxytyramine (3-MT; t25 = 2.44, q = 0.16), serotonin (5-HT; t25 = 1.35, q = 0.68), 5-hydroxyindoleacetic acid (5-HIAA; t25 = 1.11, q = 0.68), and norepinephrine (NE; t25 = 0.12, q = 0.99). (B) Quantification of TH expression (multiple t-tests; n+/+ = 13; n+/- = 12) in the dorsolateral (DLS; t23 = 0.31, q > 0.99) and dorsomedial striatum (DMS; t23 = 0.029, q > 0.99); lateral nucleus accumbens (LNAc; t23 = 0.96, q = 0.88); nucleus accumbens core (NAcC; t23 = 0.83, q = 0.88) and shell (NAcS; t23 = 1.06, q = 0.88); and the olfactory tubercle (OT; t23 = 0.21, q > 0.99). (C–D) Representative fluorescent images showing dopaminergic terminal TH expression in Nf1+/- and Nf1+/- mice (C scale: 300 μm; D scale: 20 μm). Multiple t-tests were corrected with the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 5%. Data presented as mean ± SEM.
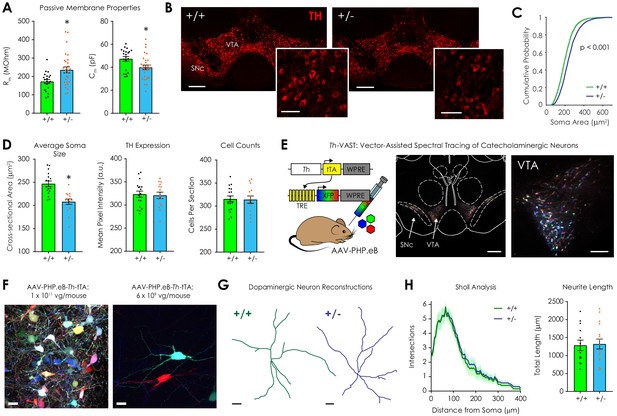
Morphological analysis of ventral tegmental dopaminergic neurons in Nf1+/+ and Nf1+/- mice.
(A) Whole-cell recordings revealed that Nf1+/- putative dopaminergic neurons (n = 29) had increased input resistance (Rm; left; unpaired t-test; t48 = 2.97, p=0.005) and decreased capacitance (Cm; right; t48 = 2.54, p=0.01) compared to Nf1+/+ neurons (n = 21). (B) Representative ventral midbrain images containing the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) stained for tyrosine hydroxylase (TH, scale: 300 μm); TH-positive neurons in the VTA (inset, scale: 100 μm). (C) The cumulative probability distribution of the cross sectional area of manually traced Nf1+/+ (n = 2344) and Nf1+/- (n = 2586) VTA dopaminergic neuron somata (two-sample Kolmogorov-Smirnov test; D = 0.18, p<0.001). (D) Average VTA dopaminergic soma area (left; n+/+ = 17, n+/- = 15; unpaired t-test; t30 = 4.65, p<0.001), TH immunofluorescence (middle; t30 = 0.25, p=0.90), and number of neurons/histological section (right; t30 = 0.15, p=0.88) per mouse. (E) Th-VAST (left) produced multicolor labeling of dopaminergic neurons in the VTA (middle, scale: 300 μm; right, scale: 100 μm). (F) Dense (left, scale: 20 μm) or sparse multi-color labeling (right, scale: 20 μm) was achieved via retro-orbital injection of either 1 × 1011 or 6 × 109 vg/mouse AAV-PHP.eB-Th-tTA, respectively, and 1 × 1012 total vg/mouse of the XFP cocktail (AAV-PHP.eB-TREx7-mRuby2, -mNeonGreen, or -mTurquoise2). (G) Representative dopaminergic neuron reconstructions following neurite tracing (scale: 20 μm). (H) Sholl analysis failed to detect a difference in dendritic complexity (left; two-way repeated measures ANOVA; F80,2160 = 0.052, pdistance x genotype >0.99; F80,2160 = 63.9, pdistance <0.001; F1,27 = 0.25, pgenotype = 0.63) or total neurite length (right; unpaired t-test; t27 = 0.18, p=0.86) between genotypes (n+/+ = 13, n+/- = 16 for +/- group). * denotes p<0.05 vs Nf1+/+. Data presented as mean ± SEM.
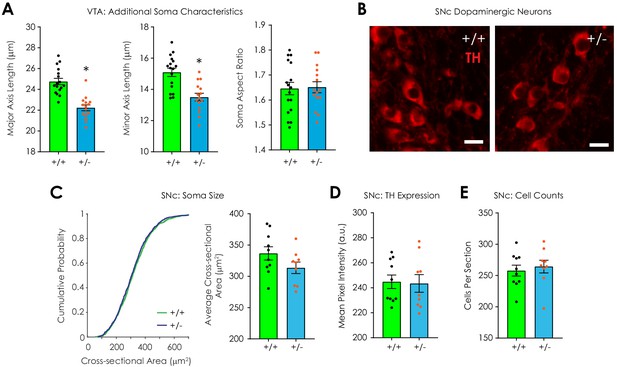
Additional data: histological analysis.
(A) Nf1+/- dopaminergic neurons (n = 15) have smaller major axis length (left; unpaired t-test; t30 = 6.06, p<0.001) and minor axis length (middle; t30 = 4.36, p<0.001) compared to Nf1+/+ dopaminergic neurons (n = 17) in the VTA; no difference in soma aspect ratio was observed (right; t30 = 0.16, p=0.87). (B) Representative fluorescent images of tyrosine hydroxylase (TH)-labeled dopaminergic neurons in the substantia nigra pars compacta (SNc; scale = 20 μm). (C) The cumulative probability distribution of the cross sectional area of manually traced Nf1+/+ (n = 1131) and Nf1+/- (n = 1099) SNc dopaminergic neuron somata (left; two-sample Kolmogorov-Smirnov test; D = 0.042, p=0.27). Average SNc dopaminergic soma area (right; n+/+ = 10; n+/- = 9; unpaired t-test; t17 = 1.63, p=0.12). (D) Average SNc dopaminergic TH immunofluorescence (n+/+ = 10; n+/- = 9; t17 = 0.15, p=0.88). (E) Average number of dopaminergic neurons/SNc histological section (n+/+ = 10; n+/- = 9; t17 = 0.49, p=0.63) per mouse. *denotes p<0.05. Data presented as mean ± SEM.
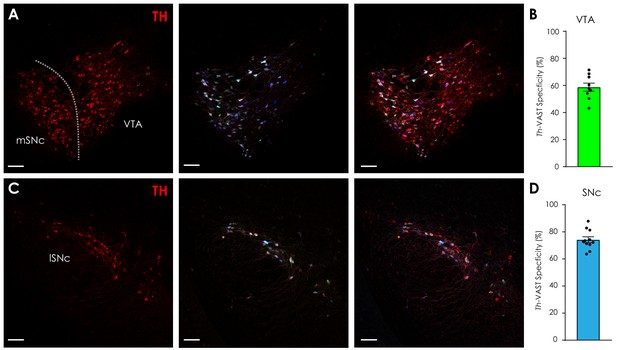
Additional data: Th-VAST.
(A) Representative confocal images of tyrosine hydroxylase (TH)-stained (left) and Th-VAST-labeled (middle; AAV-PHP.eB-TRE-XFP: 1 × 1012 total vg/mouse total, AAV-PHP.eB-Th-tTA: 1 × 1011 vg/mouse) dopaminergic neurons in coronal sections containing the medial substantia nigra pars compacta (mSNc) and ventral tegmental area (VTA, scale = 100 μm). (Right) Overlay. (B) Specificity of Th-VAST vectors in the VTA was 58.7 ± 3.0%. Each data point represents one histological section. (C) Representative confocal images of TH-stained (left) and Th-VAST-labeled (middle; AAV-PHP.eB-TRE-XFP: 1 × 1012 total vg/mouse total, AAV-PHP.eB- Th-tTA: 1 × 1011 vg/mouse) dopaminergic neurons in coronal sections containing the lateral substantia nigra pars compacta (lSNc, scale = 100 μm). (Right) Overlay. (D) Specificity of Th-VAST vectors in the SNc was 74.2 ± 2.2%. Each data point represents one histological section. Data presented as mean ± SEM.
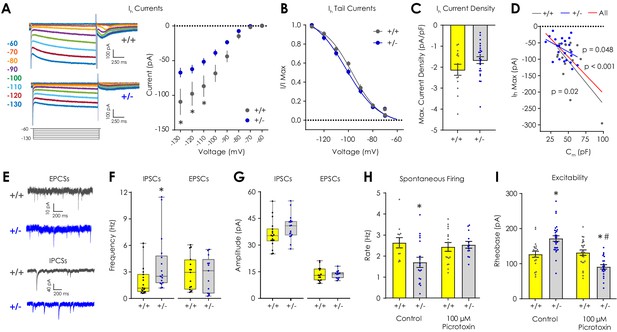
Electrophysiological characterization of Ih, inhibitory, and excitatory currents in VTA dopaminergic neurons ex vivo.
(A) Representative traces showing Ih currents during hyperpolarizing voltage steps from −60 to −130 mV. (B) Ih current magnitude was smaller (2-way repeated measures ANOVA with Bonferroni post hoc tests; F7,252 = 5.38, pgenotype x voltage <0.001) in Nf1+/- putative dopaminergic neurons (n = 24) compared to Nf1+/+ neurons (n = 14). (B) Tail current analysis showed no difference in the Ih voltage dependence between Nf1+/+ (n = 14, EV50 = −96.98 mV, 95% CI = −99.69 to −94.52 mV) and Nf1+/- putative dopaminergic neurons (n = 24, EV50 = −101.9 mV, 95% CI = −106.5 to −98.86 mV). (C) Maximum Ih current density did not differ between Nf1+/+ (n = 14) and Nf1+/- (n = 24) putative dopaminergic neurons (unpaired t-test; t36 = 1.56, p=0.13). (D) Ih magnitude was negatively correlated with Cm in Nf1+/+ (R2 = 0.39, p=0.02), Nf1+/- (R2 = 0.17, p=0.049), and across all putative dopaminergic neurons (R2 = 0.35, p<0.001). (E) Representative traces of spontaneous excitatory (sEPSC) and inhibitory (sIPSC) post-synaptic currents. (F) The frequency of sIPSCs (n+/+ = 18, n+/- = 17; Mann-Whitney U test; U = 74.5, p=0.009; unpaired t-test; t33 = 2.20, p=0.03) but not sEPSCs (n+/+ = 15, n+/- = 13; U = 87.0, p=0.65; t26 = 0.19, p=0.85) was lower in Nf1+/- putative dopaminergic neurons. (G) Amplitude of sIPSCs (n+/+ = 18, n+/- = 17; U = 96.5, p=0.06; t33 = 1.63, p=0.11) and sEPSCs (n+/+ = 15, n+/- = 13; U = 90.0, p=0.75; t26 = 0.07, p=0.94). (H) 100 μM picrotoxin rescued spontaneous firing of Nf1+/- putative dopaminergic neurons (n+/+ = 16, n+/- = 13; two-way ANOVA with Bonferroni post hoc tests; F1,55 = 5.18, pgenotype x drug = 0.03; control: p+/+ vs +/- = 0.03, picrotoxin: p+/+ vs +/- > 0.99) relative to control neurons (n+/+ = 12, n+/- = 18) and (I) lowered rheobase (n+/+ = 25, n+/- = 20) relative to control Nf1+/- neurons (n+/+ = 21, n+/- = 24; F1,91 = 30.0, pgenotype x drug <0.001; control: p+/+ vs +/- < 0.001, picrotoxin: p+/+ vs +/- = 0.003, Nf1+/-: pcontrol vs picrotoxin <0.001). * denotes p<0.05 vs Nf1+/+. # denotes p<0.05 vs control. Data presented as mean ± SEM, except box plots in F-G.
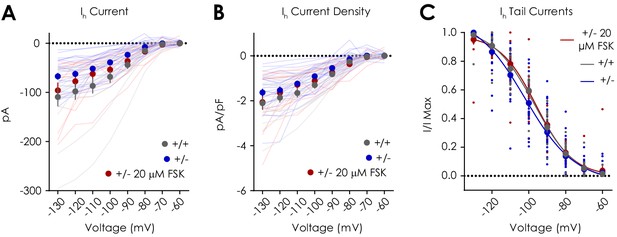
Effect of 20 μM forskolin (FSK) on putative dopaminergic neuron Ih currents.
(A) Hyperpolarizing voltage steps from −60 mV to −130 mV evoked voltage-dependent increases in Ih current density that were genotype dependent (n+/+ = 14, n+/- = 24, n+/- FSK = 14; two-way repeated measures ANOVA with Bonferroni post hoc tests; F14,343 = 2.79, pvoltage x treatment group <0.001; F7,343 = 130.2, pvoltage <0.001; F2,49 = 3.44, ptreatment group = 0.04; p+/+ vs. +/- = 0.04; p+/+ vs. +/- FSK = 0.92; p+/- vs. +/- FSK = 0.49). (B) Voltage-dependent increases in Ih current density (F7,343 = 150.2, pvoltage <0.001) did not depend on treatment condition (F14,343 = 1.12, pvoltage x treatment group = 0.33; F2,49 = 1.83, ptreatment group = 0.17; p+/+ vs. +/- = 0.21; p+/+ vs. +/- FSK > 0.99; p+/- vs. +/- FSK = 0.79). (C) 20 μM forskolin did not affect the voltage dependence of Ih in Nf1+/- (n = 14; EV50 = −95.96 mV; 95% CI = −98.93 to −93.11 mV) when compared to control Nf1+/+ (n = 14; EV50 = −96.98 mV; 95% CI = −99.69 to −94.52 mV) and Nf1+/- (n = 24; EV50 = −101.9 mV; 95% CI = −106.5 to −98.86 mV) putative dopaminergic neurons. Data presented as mean ± SEM.
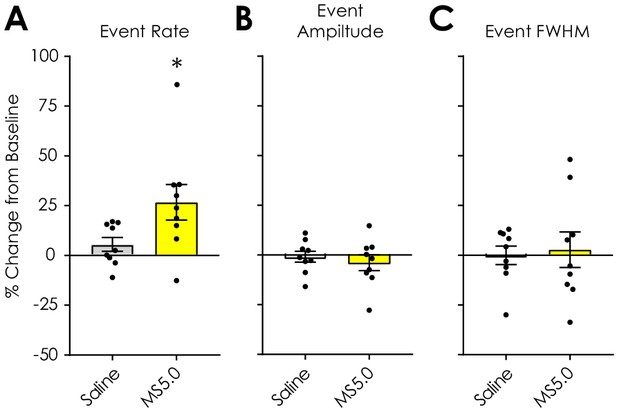
Effect of 5 mg/kg morphine sulfate on spontaneous dLight1.2 transients in Nf1+/- mice.
(A) Pre-treatment with 5.0 mg/kg (s.c.) morphine sulfate (MS5.0) increased dLight1.2 transient rate versus saline pre-treatment (paired t-test; t8 = 2.65, p=0.03) in Nf1+/- mice (n = 9). (B) Morphine had no effect on dLight1.2 transient amplitude (t8 = 0.82, p=0.44) or (C) full width at half maximal amplitude (FWHM; t8 = 0.40, p=0.70). Data presented as mean ± SEM.
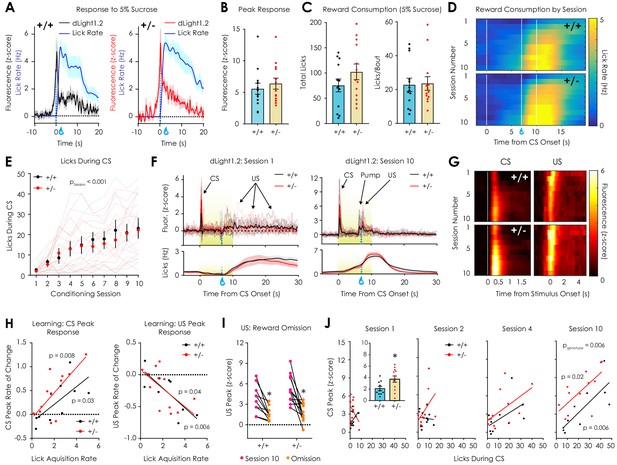
In vivo optical monitoring of dopamine dynamics during reward consumption and Pavlovian conditioning.
(A) Consumption of 5% sucrose evoked robust, time-locked fluorescent dopamine transients in both Nf1+/+ (left) and Nf1+/- mice (right). (B) Peak dLight1.2 responses to the onset sucrose consumption (n+/+ = 13, n+/- = 13; unpaired t-test; t24 = 0.66, p=0.51). (C) No difference in total number of licks (left; t24 = 1.33, p=0.20) or licks per bout (right; t24 = 0.14, p=0.89) were observed between genotypes. (D) Average session-by-session reward seeking during Pavlovian conditioning; the unconditioned stimulus (US, 5% sucrose) was delivered 7 s after the onset of a reward-predictive 10 s conditioned stimulus (CS, 5 kHz tone with house light illumination). (E) Nf1+/+ (n = 10) and Nf1+/- (n = 12) mice displayed learned licking during the CS that was not dependent on genotype (two-way repeated measures ANOVA; F9,180 = 0.48, pgenotype x session = 0.89; F9,180 = 21.36, psession <0.001; F1,20 = 0.09, pgenotype = 0.77). (F) Individual averaged dLight1.2 traces before (left, Session 1) and after (right, Session 10) learning showing CS, US, and pump responses. (G) Heatmap showing average dLight1.2 responses to the CS (left; two-way repeated measures ANOVA; peak response: F9,180 = 0.81, pgenotype x session = 0.61) or US (right; peak response: F9,180 = 0.49, pgenotype x session = 0.88) across training sessions. (H) Across sessions, the rate of acquisition of licking during the CS was correlated with the rate of change of the CS (Nf1+/+: R2 = 0.48, p=0.03; Nf1+/-: R2 = 0.52, p=0.008) and US peak (Nf1+/+: R2 = 0.63, p=0.006; Nf1+/-: R2 = 0.36, p=0.03) in both genotypes. (I) Unexpected omission resulted in a significant reduction in US magnitude in both Nf1+/+ (n = 10; paired t-test; t9 = 4.03, p=0.003) and Nf1+/+ mice (n = 12; paired t-test; t11 = 4.50, p<0.001). (J) Correlation between CS peak response and CS licking during session 1 (Nf1+/+: R2 = 0.25, p=0.14; Nf1+/-: R2 = 0.16, p=0.21; pgenotype = 0.04; inset: average peak; unpaired t-test; t20 = 2.34, p=0.03), session 2 (Nf1+/+: R2 = 0.008, p=0.80; Nf1+/-: R2 = 0.22, p=0.12; pgenotype = 0.14), session 4 (Nf1+/+: R2 = 0.28, p=0.12; Nf1+/-: R2 = 0.30, p=0.07; pgenotype = 0.26), and session 10 (Nf1+/+: R2 = 0.63, p=0.006; Nf1+/-: R2 = 0.46, p=0.02; pgenotype = 0.006). * denotes p<0.05. Data presented as mean ± SEM.
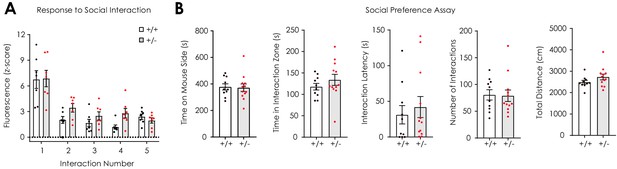
dLight1.2 responses to social interaction and measurement of social preference.
(A) dLight1.2 responses to the onset of social interaction decremented from bout to bout (n+/+ = 7; n+/- = 7; 2-way repeated measures ANOVA; F4,48 = 24.3, ptrial <0.001) and did not depend on genotype (F4,48 = 1.08, pgenotype x trial = 0.38; F1,12 = 4.21, pgenotype = 0.06). (B) No differences in time on the mouse-paired side (unpaired t-test; t20 = 0.16, p=0.87), time in the interaction zone (t20 = 1.01, p=0.33), interaction latency (t20 = 0.54, p=0.60), number of social interactions (t20 = 0.12, p=0.91), or total distance traveled (t20 = 1.44, p=0.17) was observed during a social preference assay between Nf1+/+ (n = 10) and Nf1+/- (n = 12) mice. Data presented as mean ± SEM.
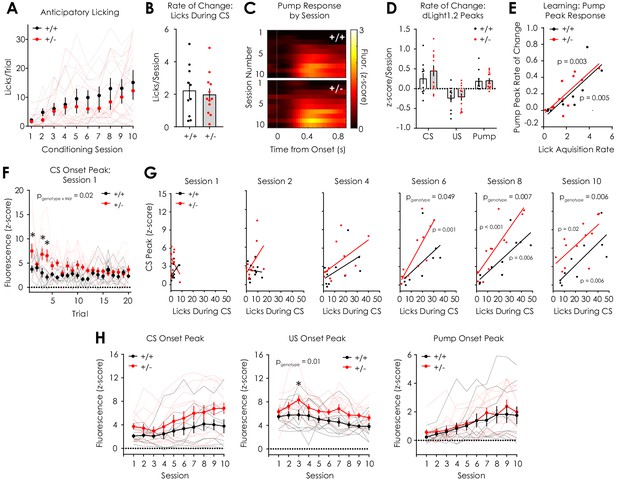
Additional data: Pavlovian conditioning.
(A) Anticipatory licking across conditioning sessions (F9,180 = 0.39, pgenotype x session = 0.94; F9,180 = 9.06, psession <0.001; F1,20 = 1.02, pgenotype = 0.32) in Nf1+/+ and Nf1+/- mice (n+/+ = 10, n+/- = 12). (B) No difference in the slope of the linear fit of CS licking across sessions (i.e. the lick acquisition rate) was observed between genotypes during the Pavlovian conditioning task (n+/+ = 10, n+/- = 12; unpaired t-test; t20 = 0.23, p=0.82). (C) Heatmap showing dLight1.2 responses to the sound of the sucrose delivery pump by session. (D) No difference between genotypes was observed in the rate of change of the CS (multiple t-tests; t20 = 1.00, q = 0.93), US (t20 = 0.24, q = 0.93), and pump (t20 = 0.14, q = 0.93) peak responses. (E) The rate of change of peak pump responses was correlated with lick acquisition rate in both Nf1+/+ (R2 = 0.64, p=0.005) and Nf1+/- (R2 = 0.61, p=0.003) mice independent of genotype (pgenotype = 0.47). (F) Peak dLight1.2 responses to CS onset during session one decreased on a trial-by-trial basis (n+/+ = 10, n+/- = 12; 2-way repeated measures ANOVA with Bonferroni post hoc tests; F19,380 = 3.07, ptrial <0.001) and was greater in Nf1+/- mice (F19, 380 = 2.24, pgenotype x trial = 0.002; F1,20 = 6.24, pgenotype = 0.02). (G) Correlation between CS peak response and CS licking during session 1 (Nf1+/+: R2 = 0.25, p=0.14; Nf1+/-: R2 = 0.16, p=0.21; pgenotype = 0.04), session 2 (Nf1+/+: R2 = 0.008, p=0.80; Nf1+/-: R2 = 0.22, p=0.12; pgenotype = 0.14), session 4 (Nf1+/+: R2 = 0.28, p=0.12; Nf1+/-: R2 = 0.29, p=0.07; pgenotype = 0.27), session 6 (Nf1+/+: R2 = 0.32, p=0.09; Nf1+/-: R2 = 0.68, p=0.001; pgenotype = 0.049), session 8 (Nf1+/+: R2 = 0.63, p=0.006; Nf1+/-: R2 = 0.74, p<0.001; pgenotype = 0.007), and session 10 (Nf1+/+: R2 = 0.63, p=0.006; Nf1+/-: R2 = 0.46, p=0.02; pgenotype = 0.006). (H) Peak responses to CS (two-way repeated measures ANOVA with Bonferroni post hoc tests; F9,180 = 0.81, pgenotype x session = 0.61; F9,180 = 8.47, psession <0.001; F1,20 = 2.55, pgenotype = 0.13), US (F9,180 = 0.50, pgenotype x session = 0.87; F9,180 = 5.28, psession <0.001; F1,20 = 7.23, pgenotype = 0.01), and the sucrose delivery pump (F9,180 = 0.30, pgenotype x session = 0.97; F9,180 = 11.73, psession <0.001; F1,20 = 0.28, pgenotype = 0.28) in Nf1+/+ and Nf1+/- mice (n+/+ = 10, n+/- = 12). *denotes p<0.05. Multiple t-tests were corrected with the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli with a false discovery rate of 5%. Data presented as mean ± SEM.
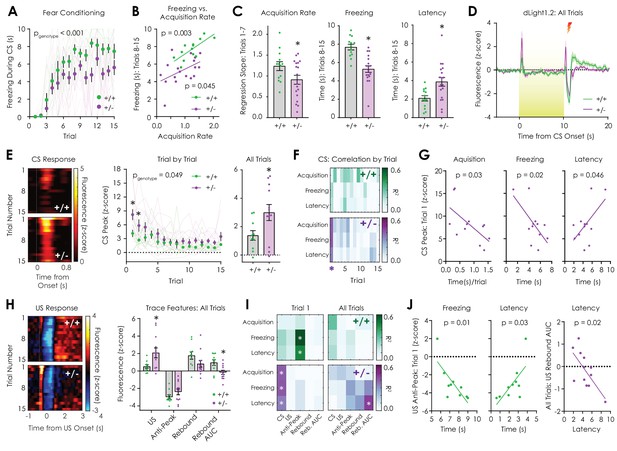
In vivo optical monitoring of dopamine dynamics during cued fear conditioning.
(A) During the cued fear conditioning assay, mice displayed a trial-by-trial increase in freezing that was greater in Nf1+/+ mice but not dependent on genotype (n+/+ = 13, n+/- = 18; 2-way repeated measures ANOVA; F14,406 = 1.321, pgenotype x trial = 0.19; F14,406 = 28.56, ptrial <0.001; F1,29 = 18.54, pgenotype <0.001). (B) The freezing acquisition rate during trials 1–7 was correlated with freezing during trials 8–15 in both Nf1+/+ (n = 13; R2 = 0.56, p=0.003) and Nf1+/- mice (n = 18; R2 = 0.23, p=0.045). (C) The freezing acquisition rate (left; unpaired t-test; t29 = 2.08, p=0.046) and average freezing during trials 8–15 (middle; t29 = 4.79, p<0.001) were decreased in Nf1+/- mice due to increased latency to freeze (right; t29 = 2.90, p=0.007). (D) Averaged dLight1.2 traces showing responses to CS (10 s, 3 kHz tone with house light illumination) presentation and US (1 s, 0.4 mA shock) delivery. (E) Heatmaps showing trial-by-trial changes in dLight1.2 signal in response to the CS (left). Nf1+/- mice (n = 12) displayed increased CS responses across trials (middle; two-way repeated measures ANOVA; F14,280 = 1.662, pgenotype x trial = 0.06; F14,280 = 9.30, ptrial <0.001; F1,20 = 4.37, pgenotype = 0.049) and when traces were averaged (right; unpaired t-test; t20 = 2.324, p=0.03) compared to Nf1+/+ mice (n = 10). (F) Correlation matrix showing trial-by-trial correlation strength between behavioral measures and CS peak response. (G) In Nf1+/- mice, there were significant correlations between the CS peak in trial one and the freezing acquisition rate (R2 = 0.40, p=0.03), time spent freezing (R2 = 0.41, p=0.02), and the latency to freeze (R2 = 0.34, p=0.046). (H) Heatmaps showing trial-by-trial changes in dLight1.2 signal in response to US delivery (left). Nf1+/- mice (n = 12) exhibited increased average peak responses (right) to US onset (t20 = 2.50, q = 0.04) and decreased integrated post-US rebound (area under the curve or AUC; t20 = 2.85, q = 0.03) compared to Nf1+/+ mice (n = 10). (I) Correlation matrices displaying strength of US feature peak-behavior correlations during trial one and across trials. (J) There were significant correlations between the US anti-peak magnitude in trial one and freezing (R2 = 0.58, p=0.01) or the latency to freeze (R2 = 0.46, p=0.03) in Nf1+/+ mice and the integrated post-US rebound across all trials and the latency to freeze (R2 = 0.45, p=0.02) in Nf1+/- mice. *denotes p<0.05. Multiple t-tests were corrected with the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli with a false discovery rate of 5%. Data presented as mean ± SEM.
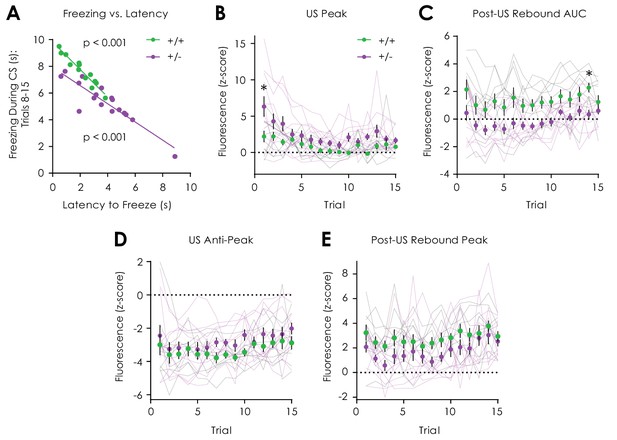
Additional data: Cued fear conditioning.
(A) The latency to freeze during trials was correlated with freezing during trials 8–15 in both Nf1+/+ (n = 13; R2 = 0.93, p<0.001) and Nf1+/- mice (n = 18; R2 = 0.82, p<0.001). (B) Nf1+/- mice (n = 12) displayed increased US responses across trials (two-way repeated measures ANOVA with Bonferroni post hoc tests; F14,280 = 1.83, pgenotype x trial = 0.03; F14,280 = 8.23, ptrial <0.001; F1,20 = 6.59, pgenotype = 0.02) when compared to Nf1+/- littermates. (C) The post-US rebound area under the curve (AUC) was greater in Nf1+/- mice (F1,20 = 8.98, pgenotype = 0.007) independent of trial (F14,280 = 0.59, pgenotype x trial = 0.87; F14,280 = 4.63, ptrial <0.001) when compared to Nf1+/- littermates. (D) No differences in the US anti-peak (F14,280 = 0.71, pgenotype x trial = 0.77; F14,280 = 3.78, ptrial <0.001; F1,20 = 1.50, pgenotype = 0.24) or (E) post-US rebound peak (F14,280 = 0.50, pgenotype x trial = 0.93; F14,280 = 4.50, ptrial <0.001; F1,20 = 3.51, pgenotype = 0.08) were observed between genotypes. *denotes p<0.05. Data presented as mean ± SEM.
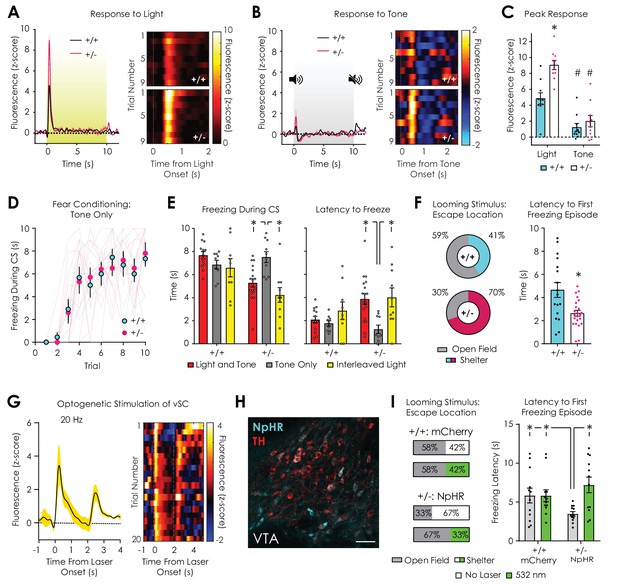
Dopaminergic and behavioral responses to salient visual stimuli.
(A) Average (left) and trial-by-trial (right) fluorescent dopamine response to a 10 s overhead light stimulus. (B) Average (left) and trial-by-trial (right) fluorescent dopamine response to a 10 s auditory stimulus (5 kHz tone). (C) Nf1+/- mice had greater peak responses to light (p<0.001) but not tone onset (p>0.99) compared to Nf1+/+ mice (n+/+ = 10, n+/- = 10; two-way ANOVA with Bonferroni post hoc tests; F1,36 = 7.27, pgenotype x condition = 0.01; F1,36 = 15.48, pgenotype <0.001). In both genotypes, responses to light were greater than responses to tone (F1,36 = 71.02, pstimulus <0.001; p+/+ = 0.002, p+/- < 0.001). (D) No difference in cued fear conditioning was observed when a tone-only CS was used (n+/+ = 9, n+/- = 10; two-way repeated measures ANOVA; F9,153 = 0.26, pgenotype x trial = 0.98). (E) Nf1+/- mice exhibited increased freezing (left) and decreased latency to freeze (right) in tone-only CS trials (n = 10) compared to light and tone (n = 18; unpaired t-test; freezing: t26 = 3.75, p<0.001; latency: t26 = 3.75, p<0.00) or interleaved light trials (n = 10; paired t-test; freezing: t9 = 5.30, p<0.001; latency: t9 = 3.48, p=0.007). No differences in freezing or latency to freeze was observed between tone-only CS trials (n = 9) and light and tone (n = 13; unpaired t-test; freezing: t20 = 1.66, p=0.11; latency: t20 = 0.81, p=0.43) or interleaved light trials (n = 10; paired t-test; freezing: t8 = 0.42, p=0.69; latency: t8 = 1.49, p=0.19) in Nf1+/+ mice. (F) Nf1+/+ (n = 17) and Nf1+/- mice (n = 23) had similar reaction times to a looming stimulus (left; t38 = 0.79, p=0.43), yet Nf1+/+ mice were more likely to escape to the shelter after stimulus presentation (left) and exhibited shorter latency to the first freezing episode after looming onset than Nf1+/- mice (t38 = 3.24, p=0.003). (G) Optogenetic stimulation of the ventral superior colliculus (vSC) produced time-locked dopamine release in the LNAc (n = 3 mice; average trace, left; trial-by-trial response, right). (H) Representative confocal image showing tyrosine hydroxylase (TH)-positive dopaminergic and Th-Off-NpHR-eYFP neurons in the VTA (scale: 50 μm). (I) In the absence of photoinhibition, VTATh-Off-NpHR-eYFP Nf1+/- mice (n = 12) were more likely to escape to the shelter (left) and had shorter latency to the first freezing episode (right; unpaired t-test; t22 = 2.36, p=0.03) compared with VTATh-Off-mCherry Nf1+/+ mice (n = 12). Optogenetic inhibition of VTAnon-Th neurons with 532 nm light (5 mW, 30 Hz, 20 ms pulse width) decreased the probability of escape to the shelter (left) and increased the latency to the first freezing episode (right; paired t-test; t11 = 3.82, p=0.003) in VTATh-Off-NpHR-eYFP Nf1+/- mice to levels that were similar to VTATh-Off-mCherry Nf1+/+ mice (unpaired t-test; Nf1+/- Laser On vs Nf1+/+ Laser Off: t22 = 0.98, p=0.34; Nf1+/- Laser On vs Nf1+/+ Laser On: t22 = 1.09, p=0.29). No difference was observed in VTATh-Off-mCherry Nf1+/+ mice between stimulation conditions (paired t-test; t11 = 0.02, p=0.99). *denotes p<0.05. # denotes p<0.05 vs light stimulus (panel C). Data presented as mean ± SEM.
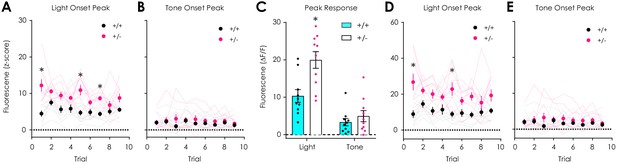
Additional data: dLight1.2 responses to auditory and visual stimuli.
(A) dLight1.2 peak responses (z-scored) to overhead light onset were greater in Nf1+/- mice (n = 10) versus Nf1+/+ littermates (n = 10) across trials (n+/+ = 10, n+/- = 10; two-way repeated measures ANOVA with Bonferroni post hoc tests; F8,144 = 2.78, pgenotype x trial = 0.007). (B) No differences in dLight1.2 responses (z-scored) to 60 dB tone were observed across trials (F8,144 = 0.92, pgenotype x trial = 0.50). (C) Nf1+/- mice (n = 10) had greater peak responses (ΔF/F) to overhead light (multiple t-tests; t18 = 3.41, q = 0.003) but not tone onset (t18 = 0.90, q = 0.19) compared to Nf1+/+ mice (n = 10). (D) dLight1.2 peak responses (ΔF/F) to overhead light onset were greater in Nf1+/- mice (n = 10) versus Nf1+/+ littermates (n = 10) across trials (n+/+ = 10, n+/- = 10; two-way repeated measures ANOVA with Bonferroni post hoc tests; F8,144 = 2.45, pgenotype x trial = 0.02). (E) No differences in dLight1.2 responses (ΔF/F) to 60 dB tone were observed across trials (F8,144 = 1.15, pgenotype x trial = 0.33). Multiple t-tests were corrected with the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli with a false discovery rate of 5%. *denotes p<0.05. Data presented as mean ± SEM.
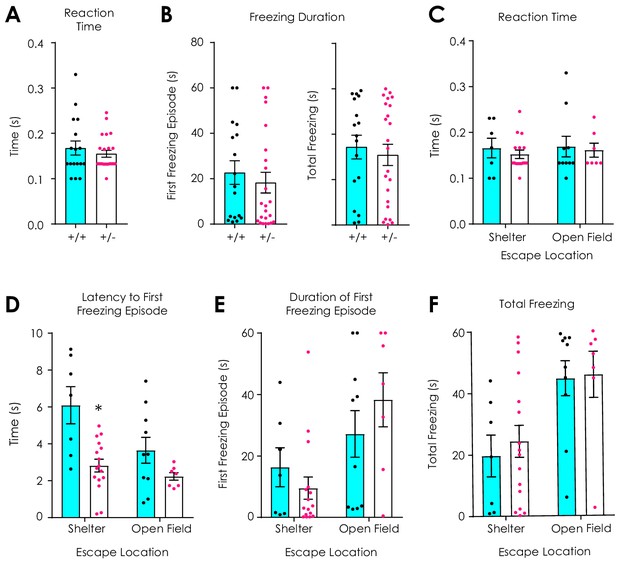
Additional data: Looming stimulus assay.
(A) Nf1+/+ (n = 17) and Nf1+/- mice (n = 23) have similar reaction times to a looming stimulus (t38 = 0.79, p=0.43). (B) No difference in the duration of the first freezing episode (left; t38 = 0.64, p=0.53) or total freezing during the first minute after looming (right; t38 = 0.49, p=0.62) was observed. (C) Reaction time to a looming stimulus did not depend on escape location (n+/+ shelter = 7, n+/+ open field = 10, n+/- shelter = 16, n+/- open field=7; two-way ANOVA with Bonferroni post hoc tests; F1,36 = 0.03, pgenotype x location = 0.87; F1,36 = 0.13, plocation = 0.72; F1,36 = 0.38, pgenotype = 0.54). (D) The latency to the first freezing episode was shorter in Nf1+/- mice (F1,36 = 15.0, pgenotype <0.001) and independent of freezing location (F1,36 = 2.35, pgenotype x location = 0.13), although a significant main effect of location was observed (F1,36 = 6.22, plocation = 0.02). (E) The duration of first freezing episode (F1,36 = 1.95, pgenotype x location = 0.17; F1,36 = 9.51, plocation = 0.004; F1,36 = 0.11, pgenotype = 0.74) and (F) the total time spent freezing during the first minute after looming (F1,36 = 0.07, pgenotype x location = 0.79; F1,36 = 12.68, plocation = 0.001; F1,36 = 0.20, pgenotype = 0.66) were significantly influenced by freezing location independent of genotype. *denotes p<0.05. Data presented as mean ± SEM.
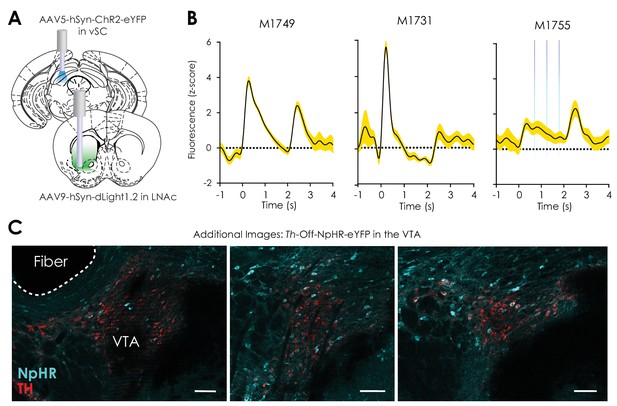
Additional data: Optogenetic control of the vSC and VTAnon-Th neurons.
(A) Illustration showing location of stereotaxic injection of the AAV5-hSyn-ChR2-eYFP viral vector and optical fiber in the ventral superior colliculus (vSC), as well as the location of the stereotaxic injection of the AAV9-hSyn-dLight1.2 viral vector and photometry fiber implantation in the LNAc. (B) Average fluorescence trace from individual mice showing optical LNAc dopamine signals evoked by activation of ChR2 in the ipsilateral vSC via two seconds of 20 Hz, 5 mW, 5 ms pulse-width, 473 nm laser stimulation. (C) Additional images showing Th-Off-NpHR-eYFP (cyan) and tyrosine hydroxylase (red)-labeled neurons in the VTA (scale = 50 μm).
Videos
Behavioral response of Nf1+/- mouse to CS presentation during fear conditioning.
https://doi.org/10.7554/eLife.48983.019Behavioral response of Nf1+/+ mouse to CS presentation during fear conditioning.
https://doi.org/10.7554/eLife.48983.020Behavioral response of Nf1+/- mouse to CS presentation during tone-only and interleaved light trials.
https://doi.org/10.7554/eLife.48983.025Behavioral response to optogenetic stimulation of the superior colliculus.
https://doi.org/10.7554/eLife.48983.026Behavioral response to looming stimulus in Nf1+/- mouse with or without optogenetic inhibition of VTAnon-Th neurons.
https://doi.org/10.7554/eLife.48983.027Tables
Action potential features across patch clamp electrophysiology experiments.
https://doi.org/10.7554/eLife.48983.006| Property | Experiment | p | +/+: Mean ± SEM, n | +/-: Mean ± SEM, n |
|---|---|---|---|---|
| Rheobase | Baseline characterization | <0.001 | 124.1 ± 8.65 pA, n = 21 | 171.7 ± 7.779 pA, n = 29 |
| AP Threshold | Baseline characterization | 0.059 | −39.32 ± 1.266 mV, n = 21 | −36.45 ± 0.8708 mV, n = 29 |
| AP Duration | Baseline characterization | 0.695 | 3.671 ± 0.2525 ms, n = 21 | 3.562 ± 0.1499 ms, n = 29 |
| AP Height | Baseline characterization | 0.555 | 60.89 ± 1.607 mV, n = 21 | 59.42 ± 1.749 mV, n = 29 |
| AP AHP | Baseline characterization | 0.897 | −15.43 ± 1.19 mV, n = 21 | −14.88 ± 1.046 mV, n = 29 |
| Firing Rate | Baseline characterization | 0.016 | 2.633 ± 0.2464 Hz, n = 12 | 1.703 ± 0.244 Hz, n = 18 |
| Rheobase | Picrotoxin rescue | <0.001 | 131.5 ± 7.537 pA, n = 25 | 89.14 ± 6.413 pA, n = 20 |
| AP Threshold | Picrotoxin rescue | 0.456 | −36.92 ± 1.193 mV, n = 25 | −38.33 ± 1.472 mV, n = 20 |
| AP Duration | Picrotoxin rescue | 0.610 | 4.156 ± 0.1589 ms, n = 25 | 4.03 ± 0.1891 ms, n = 20 |
| AP Height | Picrotoxin rescue | 0.946 | 56.16 ± 2.021 mV, n = 25 | 55.99 ± 1.151 mV, n = 20 |
| AP AHP | Picrotoxin rescue | 0.168 | −13.84 ± 1.125 mV, n = 25 | −11.78 ± 0.844 mV, n = 20 |
| Firing Rate | Picrotoxin rescue | 0.714 | 2.434 ± 0.208 Hz, n = 16 | 2.535 ± 0.1596 Hz, n = 13 |
Passive membrane properties across patch clamp electrophysiology experiments.
https://doi.org/10.7554/eLife.48983.010| Property | Experiment | p | +/+: Mean ± SEM, n | +/-: Mean ± SEM, n |
|---|---|---|---|---|
| Cm | Baseline characterization | 0.014 | 47.35 ± 2.032 pF, n = 21 | 41.27 ± 2.026 pF, n = 29 |
| Rm | Baseline characterization | 0.005 | 172.4 ± 10.94 MΩ, n = 21 | 235.7 ± 16.32 MΩ, n = 29 |
| Rs | Baseline characterization | 0.966 | 17.86 ± 1.73 pF MΩ, n = 21 | 17.95 ± 1.257 MΩ, n = 29 |
| Holding | Baseline characterization | 0.658 | −74.26 ± 10.95 pA, n = 21 | −81.01 ± 10.18 pA, n = 29 |
| Cm | Ihmeasurement | 0.047 | 51.94 ± 4.45 pF, n = 14 | 42.53 ± 2.351 pF, n = 24 |
| Rm | Ihmeasurement | 0.009 | 170.8 ± 11.96 MΩ, n = 14 | 222.4 ± 12.49 MΩ, n = 24 |
| Rs | Ihmeasurement | 0.528 | 17.78 ± 1.478 MΩ, n = 14 | 19.1 ± 1.334 MΩ, n = 24 |
| Holding | Ihmeasurement | 0.457 | −61.15 ± 8.657 pA, n = 14 | −52.11 ± 7.642 pA, n = 24 |
| Cm | Picrotoxin rescue | 0.004 | 47.74 ± 2.276 pF, n = 29 | 36.62 ± 2.956 pF, n = 20 |
| Rm | Picrotoxin rescue | 0.001 | 181 ± 8.464 MΩ, n = 29 | 239 ± 14.94 MΩ, n = 20 |
| Rs | Picrotoxin rescue | 0.670 | 17.23 ± 1.054 MΩ, n = 29 | 18.04 ± 1.648 MΩ, n = 20 |
| Holding | Picrotoxin rescue | 0.611 | −56.77 ± 5.88 pA, n = 29 | −61.64 ± 7.639 pA, n = 20 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti-tyrosine hydroxylase (Rabbit polyclonal) | EMD Millipore | Cat#: AB152 RRID:AB_390204 | IHC (1:1000) |
| Antibody | Anti-tyrosine hydroxylase (mouse monoclonal) | ImmunoStar | Cat#: 22941 RRID:AB_572268 | IHC (1:1000) |
| Antibody | Anti-GFP (mouse polyclonal) | Aves | Cat#: GFP-1020 RRID:AB_10000240 | IHC (1:1000) |
| Antibody | Alexa Fluor 488-conjugated donkey anti-chicken IgY F(ab’)two fragment | Jackson ImmunoResearch | Cat#: 703-546-155 RRID:AB_2340376 | IHC (1:1000) |
| Antibody | Alexa Fluor 647-conjugated donkey anti-mouse IgG Fab fragment | Jackson ImmunoResearch | Cat#: 711-607-003 RRID:AB_2340626 | IHC (1:1000) |
| Recombinant DNA reagent | pAAV-hSyn-dLight1.2 | Addgene | Plasmid#: 111068 RRID:Addgene_111068 | Gift on Lin Tian; produced by UC Davis Vector Core |
| Recombinant DNA reagent | pAAV-hSyn-hChR2(H134R)-EYFP | Addgene | Plasmid#: 26973 RRID:Addgene_26973 | Gift of Karl Deisseroth; produced by UNC Vector Core |
| Recombinant DNA reagent | AAV9-Th-PI-Cre-SV40 | Addgene | Plasmid#: 107788 RRID:Addgene_107788 | Addgene viral prep#: 107788-AAV9; gift of James M. Wilson |
| Recombinant DNA reagent | pAAV-DJ-Ef1α-DO-eNpHR3.0-eYFP-WPRE-pA | Addgene | Plasmid#: 37087 RRID:Addgene_37087 | Gift of Bernardo Sabatini |
| Recombinant DNA reagent | pAAV-DJ-Ef1α-DO-mCherry-WPRE-pA | Addgene | Plasmid#: 37119 RRID:Addgene_37119 | Gift of Bernardo Sabatini |
| Recombinant DNA reagent | pAAV-ihSyn1-tTA-WPRE | Addgene | Plasmid#: 99120 RRID:Addgene_99120 | |
| Recombinant DNA reagent | pAAV-Th-tTA-WPRE | Addgene | Plasmid#: 133268 RRID:Addgene_133268 | |
| Recombinant DNA reagent | pAAV-TRE-mRuby-WPRE | Addgene | Plasmid#: 99114 RRID:Addgene_99114 | |
| Recombinant DNA reagent | pAAV-TRE-mNeonGreen-WPRE | (Chan et al., 2017) | ||
| Recombinant DNA reagent | pAAV-TRE-mTurquoise-WPRE | Addgene | Plasmid#: 99113 RRID:Addgene_99113 | |
| Recombinant DNA reagent | pAAV-Th-GFP-WPRE | Addgene | Plasmid#: 99128 RRID:Addgene_99128 | |
| Recombinant DNA reagent | pUCmini-iCAP-PHP.eB | Addgene | Plasmid#: 103005 RRID:Addgene_103005 | |
| Recombinant DNA reagent | pAAV-DJ-Rep-Cap | Cell Biolabs, Inc | Cat#: VPK-420-DK | |
| Software, Algorithm | Matlab | Mathworks, Inc | RRID:SCR_001622 | |
| Software, Algorithm | GraphPad Prism 7 | GraphPad Software, Inc | RRID:SCR_002798 | |
| Software, Algorithm | ABET II Software for Operant Control | Lafayette Instrument Company | Model 89501 | |
| Software, Algorithm | Fiber Photometry Trace Processing | Gradinaru Lab | FP_Session_Processing_2 .m | https://github.com/GradinaruLab/dLight1/blob/master/FP_Session_Processing2.m |
| Other | ProLong Diamond Antifade Mountant | ThermoFisher Scientific | Cat#: P36965 | |
| Other | Refractive Index Matching Solution | (Yang et al., 2014) | RefractiveIndex = 1.46; protocol available in Treweek et al. (2015) | |
| Other | Mono Fiber-Optic Cannula | Doric Lenses, Inc | Cat#: MFC_400/430–0.48_5 mm_ZF1.25_FLT | OD: 400 μm, Length: 5 mm |
| Other | Mono Fiber-Optic Cannula | Doric Lenses, Inc | Cat#: MFC_300/330–0.48_3 mm_ZF1.25_FLT | OD: 300 μm, Length: 3 mm |
| Other | Mono Fiber-Optic Cannula | Doric Lenses, Inc | Cat#: MFC_300/330–0.48_5 mm_ZF1.25_FLT | OD: 300 μm, Length: 5 mm |
| Other | Mono Fiber-Optic Patch Cable | Doric Lenses, Inc | Cat#: MFP_400/430/LWMJ-0.48_2 m_FC-ZF1.25, Doric Lenses Inc | OD: 400 μm, Length: 2 m |
| Other | Mono Fiber-Optic Patch Cable | Doric Lenses, Inc | Cat#: MFP_300/330/LWMJ-0.48_1 m_FC-ZF1.25, Doric Lenses Inc | OD: 300 μm, Length: 1 m |
Additional files
-
Source data 1
Summary of statistical analysis.
- https://doi.org/10.7554/eLife.48983.028
-
Supplementary file 1
Trial-by-trial correlations between peak dLight1.2 responses to CS presentation and behavioral measures.
- https://doi.org/10.7554/eLife.48983.029
-
Supplementary file 2
Correlations between dLight1.2 responses and behavioral measures.
- https://doi.org/10.7554/eLife.48983.030
-
Transparent reporting form
- https://doi.org/10.7554/eLife.48983.031





