Phage integration alters the respiratory strategy of its host
Figures
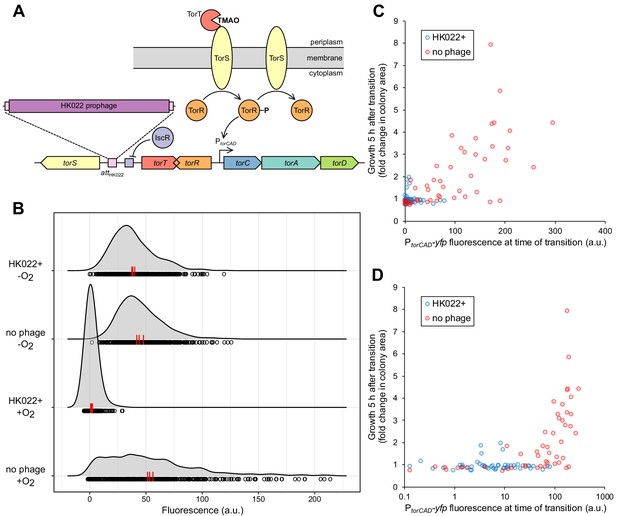
Bacteriophage HK022 integrates between the signaling genes torS and torT, disrupting regulation of torCAD and a metabolic bet-hedging strategy.
(A) HK022 integrates as a prophage at an integration site (attBHK022) between torS and torT, separating torS from the IscR binding site that represses its transcription. TorS regulates torCAD by phosphorylating and dephosphorylating the transcription factor TorR, which in its phosphorylated state activates transcription from the torCAD promoter. To phosphorylate TorR, TorS must interact with TMAO-bound TorT; in the absence of this interaction, TorS dephosphorylates TorR. When oxygen is present, transcription of torS and torT is repressed to an extremely low level by IscR, and stochasticity in the ratio of TorS to TorT leads to noisy torCAD transcription (Carey et al., 2018). (B) The HK022 prophage shuts off aerobic transcription of torCAD but leaves anaerobic expression intact. Distributions of single-cell fluorescence are shown for strains carrying a fluorescent reporter of torCAD transcription. Data are shown for an HK022 lysogen (DFE12) and a non-lysogen (MMR8) grown in the presence or absence of oxygen. Each circle represents a fluorescence measurement made in an individual cell. To facilitate qualitative comparisons between distributions, density curves (shown in gray) were generated from single-cell measurements (see Materials and methods). Data are pooled from three independent experiments, with the vertical red lines indicating the population mean fluorescence for each experiment. a.u., arbitrary units. (C,D) Most cells carrying the HK022 prophage fail to grow following rapid oxygen depletion. Each circle represents an individual cell monitored for growth following an aerobic-to-anaerobic transition. The same data are presented on a linear scale (C) for easier comparison with (B) and on a log scale (D) for clearer resolution of individual points. The HK022 lysogen (JNC173) constitutively expresses CFP to distinguish it from the non-lysogen (JNC174), which constitutively expresses mCherry. Both strains carry the YFP reporter of torCAD transcription and lack fhuA, the gene encoding the HK022 receptor. Growth is quantified as the ratio of microcolony area approximately 5 hr after oxygen depletion to the area of the parent cell at the time of depletion. Data are shown for a single representative experiment.
-
Figure 1—source data 1
Fluorescence measurements for Figure 1B.
- https://doi.org/10.7554/eLife.49081.004
-
Figure 1—source data 2
Fluorescence and growth measurements for Figure 1C, D.
- https://doi.org/10.7554/eLife.49081.005
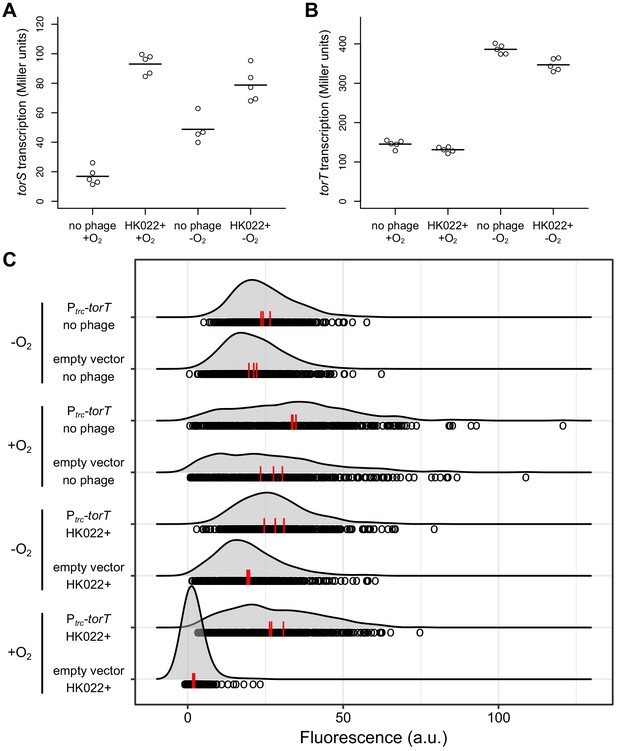
The HK022 prophage increases torS transcription and has no effect on torT transcription.
Aerobic and anaerobic transcription of torS (A) and torT (B) was measured by β-galactosidase assays in strains carrying torS-lacZ or torT-lacZ operon fusions, with or without the HK022 prophage (strains JNC166, JNC169, JNC163, and JNC168). Each circle represents a measurement obtained from an independent experiment, and the horizontal lines indicate average values. (C) Overexpression of torT restores aerobic torCAD expression in an HK022 lysogen. The distributions of single-cell fluorescence are shown for strains carrying a fluorescent reporter of torCAD transcription. The strains are an HK022 lysogen (DFE12) and a non-lysogen (MMR8) containing a plasmid for torT overexpression (pMR26) or an empty vector control (pDSW206), grown in the presence or absence of oxygen. Expression of torT from the plasmid is driven by a weakened trc promoter without added inducer. Each circle represents a fluorescence measurement made in an individual cell. To facilitate qualitative comparisons between distributions, density curves (shown in gray) were generated from single-cell measurements (see Materials and methods). Data are pooled from three independent experiments, with the vertical red lines indicating the population mean fluorescence for each experiment. a.u., arbitrary units.
-
Figure 2—source data 1
β-Galactosidase measurements for Figure 2A.
- https://doi.org/10.7554/eLife.49081.007
-
Figure 2—source data 2
β-Galactosidase measurements for Figure 2B.
- https://doi.org/10.7554/eLife.49081.008
-
Figure 2—source data 3
Fluorescence measurements for Figure 2C.
- https://doi.org/10.7554/eLife.49081.009
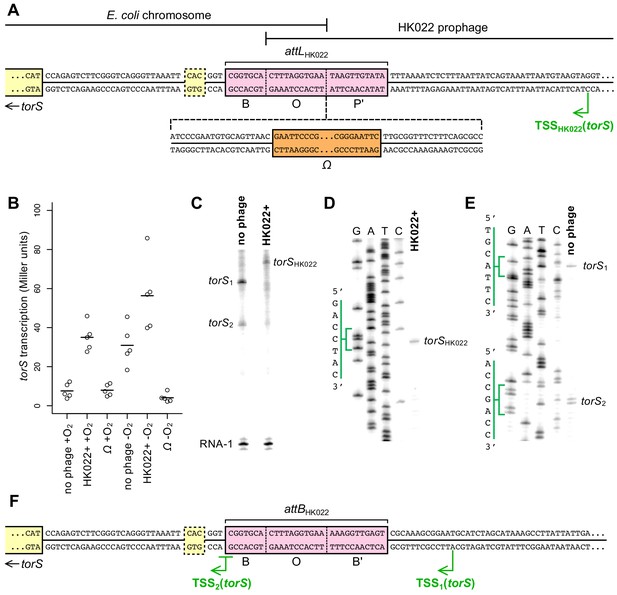
Transcription of torS in an HK022 lysogen originates from within the prophage.
(A) Sequence of the torS-adjacent HK022 integration site (attLHK022) in an HK022 lysogen. B, O, and P’ indicate the bacterial, overlap, and phage segments of the integration site, respectively (Campbell, 1992; Yagil et al., 1989). The location of the Ω element terminator insertion in strain JNC175 is indicated. In this strain, transcription reading toward torS from within the HK022 prophage is blocked by the Ω element. The transcription start site, TSSHK022, was mapped by in vitro transcription and primer extension, shown in (C) and (D). The previously inferred torS GTG start codon is outlined, and the experimentally confirmed ATG start codon is indicated as the start of the torS coding sequence. (B) Aerobic and anaerobic transcription of torS was measured by β-galactosidase assays in strains carrying a torS-lacZ operon fusion. Strains contained the wild-type HK022 prophage (JNC169), the prophage with an Ω element (JNC175), or had no prophage at the integration site (JNC166). Each circle represents a measurement obtained from an independent experiment, and the horizontal lines indicate average values. (C) In vitro transcription from plasmids containing sequence upstream of torS from the HK022 lysogen (‘HK022+’, pPK13256) or the non-lysogen (‘no phage’, pPK12669) shows that different transcripts are produced when the prophage is present or absent. Transcription using non-lysogen sequence produces two distinct transcripts, suggesting two transcription start sites for torS. RNA-1 is a control transcript for in vitro transcription and gel loading that is generated from a σ70-regulated promoter in pPK13256 or pPK12669. (D) Primer extension was performed to map the transcription start site of the in vitro ‘HK022+’ transcript shown in (C). The position of the start site is indicated in (A). (E) Primer extension was performed to map the transcription start sites of the in vitro ‘no phage’ transcripts shown in (C). The positions of the start sites are indicated in (F). (F) Sequence upstream of torS in a non-lysogen, with the torS transcription start sites depicted. Transcripts originating from TSS2 can begin at the underlined G or A position, as indicated by the adjacent bands in (E).
-
Figure 3—source data 1
β-Galactosidase measurements for Figure 3B.
- https://doi.org/10.7554/eLife.49081.012
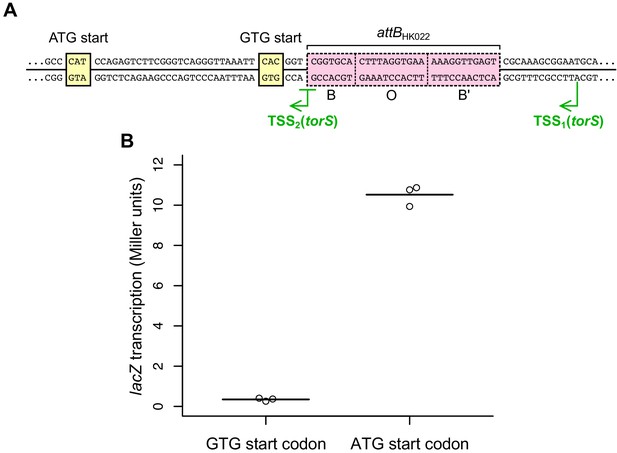
Identification of the torS start codon.
(A) Two start codons have been proposed for the torS gene: an upstream GTG start (UniProt Consortium, 2019) and a downstream ATG start (Jourlin et al., 1996). The GTG start codon appears to be too close to the 5’ end of the TSS2 transcript to permit ribosome binding and translation. (B) To determine which of the proposed start codons is used in vivo, we constructed strains containing chromosomally encoded lacZ translational fusions to each codon (PK13196 and PK13199). β-Galactosidase activity was only detected for the lacZ fusion to the ATG start codon, suggesting that this codon is the primary, or perhaps exclusive, start codon for torS. Each circle represents a measurement obtained from an independent experiment, and the horizontal lines indicate average values.
-
Figure 3—figure supplement 1—source data 1
β-Galactosidase measurements.
- https://doi.org/10.7554/eLife.49081.013
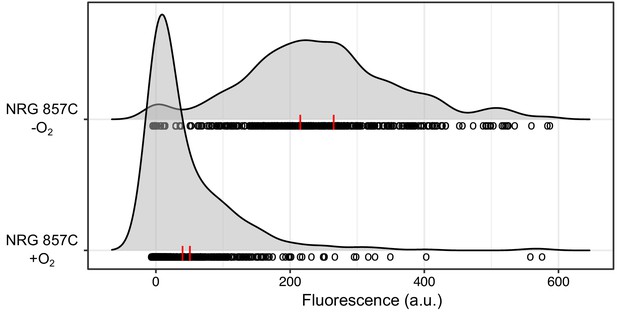
A wild E. coli strain carrying a prophage at attBHK022 shows an oxygen-dependent torCAD expression pattern similar to that of the HK022-infected laboratory strain.
A fluorescent reporter of torCAD transcription was introduced into the Crohn’s disease-associated E. coli strain NRG 857C and used to measure expression during aerobic and anaerobic growth. NRG 857C naturally carries a prophage at the HK022 integration site and displays a qualitatively similar pattern of torCAD expression as HK022-infected MG1655 (see Figure 1B). Distributions of single-cell fluorescence are shown for the NRG 857C PtorCAD-yfp strain (DFE34), with each circle representing a fluorescence measurement made in an individual cell. To facilitate qualitative comparisons between distributions, density curves (shown in gray) were generated from single-cell measurements (see Materials and methods). Data are pooled from two independent experiments, with the vertical red lines indicating the population mean fluorescence for each experiment. a.u., arbitrary units.
-
Figure 4—source data 1
Fluorescence measurements.
- https://doi.org/10.7554/eLife.49081.015
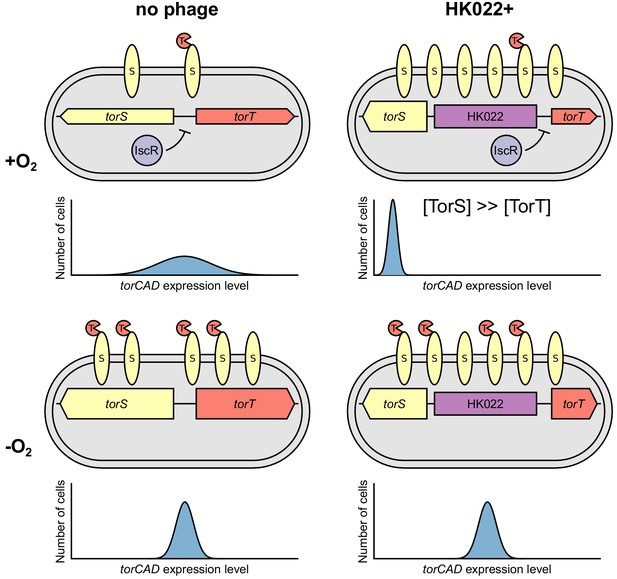
Model of how bacteriophage HK022 reprograms the regulation of torCAD expression during lysogeny.
In cells lacking the HK022 prophage, IscR repression of torS and torT during aerobic growth leads to very low TorS and TorT abundance. High variability in the ratio of TorS to TorT results in noisy torCAD transcription (top left). In the absence of oxygen, IscR repression of torS and torT is relieved, decreasing variability in the TorS-to-TorT ratio and noise in torCAD transcription (bottom left) (Carey et al., 2018). In HK022 lysogens, a prophage-encoded promoter drives high torS expression. IscR still represses torT during aerobic growth, and the resulting excess of TorS relative to TorT shuts down torCAD transcription (top right) (see Figure 1A). In the absence of oxygen, IscR repression of torT is relieved, and torCAD transcription is restored (bottom right).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Escherichia coli) | torS | NA | EcoCyc:G6514; UniProt:P39453 | |
| Strain, strain background (Escherichia virus HK022) | HK022 | PMID: 4569213 | RefSeq:NC_002166 | Dr. Max E. Gottesman (Columbia University) |
| Strain, strain background (E. coli) | DFE12 | this paper | MG1655 attBλ::(cat PtorCAD-yfp) ompA-cfp (HK022)n | |
| Strain, strain background (E. coli) | DFE34 | this paper | NRG 857C ΔlacIZY::PtorCAD-yfp-FRT-kan-FRT | |
| Strain, strain background (E. coli) | JNC151 | this paper | MG1655 (HK022)n | |
| Strain, strain background (E. coli) | JNC163 | PMID: 29502970 | MG1655 ΔlacZYA::FRT-cat-FRT torT-lacZ-FRT-kan-FRT ΔtorR | |
| Strain, strain background (E. coli) | JNC166 | PMID: 29502970 | MG1655 ΔlacZYA::FRT torS-lacZ-FRT-kan-FRT | |
| Strain, strain background (E. coli) | JNC168 | this paper | MG1655 ΔlacZYA::FRT-cat-FRT (HK022)n torT-lacZ-FRT-kan-FRT ΔtorR | |
| Strain, strain background (E. coli) | JNC169 | this paper | MG1655 ΔlacZYA::FRT torS-lacZ-FRT-kan-FRT (HK022)n | |
| Strain, strain background (E. coli) | JNC173 | this paper | MG1655 ΔfhuA::FRT-kan-FRT attBλ::(cat PtorCAD-yfp) ompA-cfp (HK022)n | |
| Strain, strain background (E. coli) | JNC174 | this paper | MG1655 ΔfhuA::FRT-kan-FRT attBλ::(cat PtorCAD-yfp) ΔxylAFG::PtetA-mcherry-FRT | |
| Strain, strain background (E. coli) | JNC175 | this paper | MG1655 ΔlacZYA::FRT torS-lacZ-FRT-kan-FRT (HK022)n attLHK022::Ω | |
| Strain, strain background (E. coli) | MG1655 | Coli Genetic Stock Center | CGSC:7740; RefSeq:NC_000913 | |
| Strain, strain background (E. coli) | MMR8 | PMID: 25825431 | MG1655 attBλ::(cat PtorCAD-yfp) ompA-cfp | |
| Strain, strain background (E. coli) | NRG 857C | PMID: 21108814 | RefSeq:NC_017634 | Dr. Alfredo G. Torres (UTMB) |
| Strain, strain background (E. coli) | PK13196 | this paper | MG1655 lacZ::kan-PtorS-(GTG)lacZ ΔiscR::FRT | |
| Strain, strain background (E. coli) | PK13199 | this paper | MG1655 lacZ::kan-PtorS-(ATG)lacZ ΔiscR::FRT | |
| Recombinant DNA reagent | pDSW206 | PMID: 9882665 | ori(pBR322) lacIq amp Ptrc attenuated promoter. Dr. Jon Beckwith (Harvard University) | |
| Recombinant DNA reagent | pMR26 | PMID: 25825431 | pDSW206 torT | |
| Recombinant DNA reagent | pPK7179 | PMID: 15659690 | ori(pBR322) ter(spf) amp RNA-1 | |
| Recombinant DNA reagent | pPK12669 | this paper | pPK7179 with −152 to +28 bp relative to the torS ATG start codon from MG1655 in XhoI/BamHI sites | |
| Recombinant DNA reagent | pPK13256 | this paper | pPK7179 with −231 to +28 bp relative to the torS ATG start codon from JNC151 in XhoI/BamHI sites | |
| Sequence-based reagent | native torS | this paper | 32P-labeled DNA oligonucleotide: 5’-TTAACAGCGCCATCAG-3’ | |
| Sequence-based reagent | HK022/torS | this paper | 32P-labeled DNA oligonucleotide: 5’-GGGTCAGGGTTAAATTCACGG-3’ | |
| Peptide, recombinant protein | E. coli σ70 RNA polymerase holoenzyme | New England Biolabs | NEB:M0551S | |
| Commercial assay or kit | HiSpeed Plasmid Maxi Kit | Qiagen | Qiagen:12662 | |
| Commercial assay or kit | MMLV Reverse Transcriptase 1st-Strand cDNA Synthesis Kit | Lucigen | Lucigen:MM070150 | |
| Commercial assay or kit | Sequenase Version 2.0 DNA Sequencing Kit | USB | USB:70770 | |
| Software, algorithm | BLAST | PMID: 23609542 | RRID:SCR_004870 | |
| Software, algorithm | ClermonTyping | PMID: 29916797 | v. 1.4.0 | |
| Software, algorithm | ggridges | Comprehensive R Archive Network | RRID:SCR_003005 | v. 0.5.0 |
| Software, algorithm | Mauve | PMID: 20593022 | RRID:SCR_012852 | v. 2015-02-25 |
| Software, algorithm | MUSCLE | PMID: 15034147 | v. 3.8.1551 | |
| Software, algorithm | R | R Foundation for Statistical Computing | RRID:SCR_001905 | v. 3.4.4 |
| Software, algorithm | SnapGene | GSL Biotech | RRID:SCR_015052 | v. 5.0b3 |
Additional files
-
Source data 1
FASTA sequence alignment file.
Source data for Supplementary file 2.
- https://doi.org/10.7554/eLife.49081.017
-
Supplementary file 1
Fully sequenced E. coli isolates carrying prophages at the HK022 integration site.
- https://doi.org/10.7554/eLife.49081.018
-
Supplementary file 2
Sequence alignment of the genomic region around the prophage-encoded torS transcription start site from all fully sequenced E. coli strains carrying a prophage at the HK022 integration site.
- https://doi.org/10.7554/eLife.49081.019
-
Supplementary file 3
Strains used in this study.
- https://doi.org/10.7554/eLife.49081.020
-
Supplementary file 4
Plasmids used in this study.
- https://doi.org/10.7554/eLife.49081.021
-
Transparent reporting form
- https://doi.org/10.7554/eLife.49081.022





