Taste bud formation depends on taste nerves
Figures
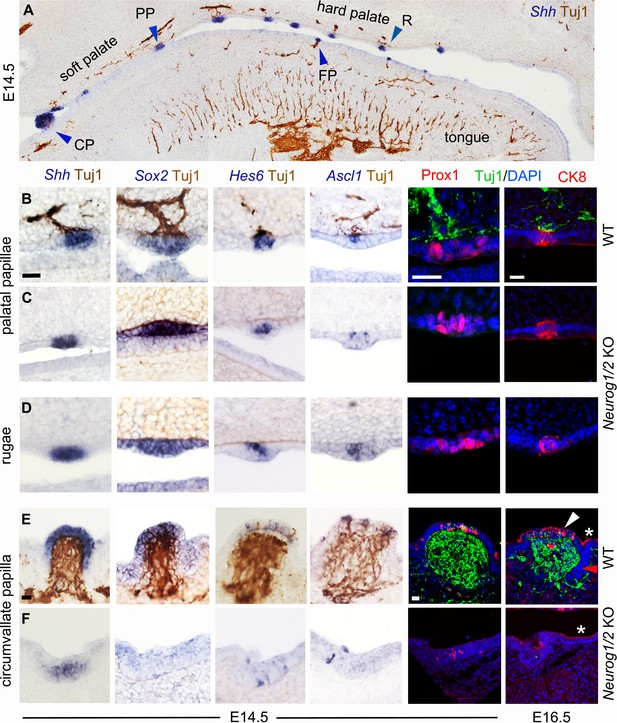
Soft palate taste papillae as well as hard palate rugae, but not the tongue circumvallate papilla form without innervation.
(A–F) Combined immunohistochemistry for β-III tubulin (Tuj1, brown) and in situ hybridization (blue) for the indicated probes (top panel and left four columns), or immunofluorescence for Prox1 or CK8, combined with immunofluorescence for β-III tubulin and a counterstain with DAPI (two right columns) in wild type (A,B,E) and double Neurog1/2 KO (C,D,F) at E14.5 or E16.5 as indicated. In the circumvallate papilla, markers are expressed on the dorsal surface (white arrowhead for CK8) but not at the lower part of the trenches (red arrowhead in the right column), where taste buds will develop after birth. CK8 is also expressed in flattened cells of the periderm (asterisks). For every probe two animals were examined. CP : circumvallate papilla; FP : fungiform papilla ; PP : palatal papilla ; R : ruga. Scale bars: 20 μm.
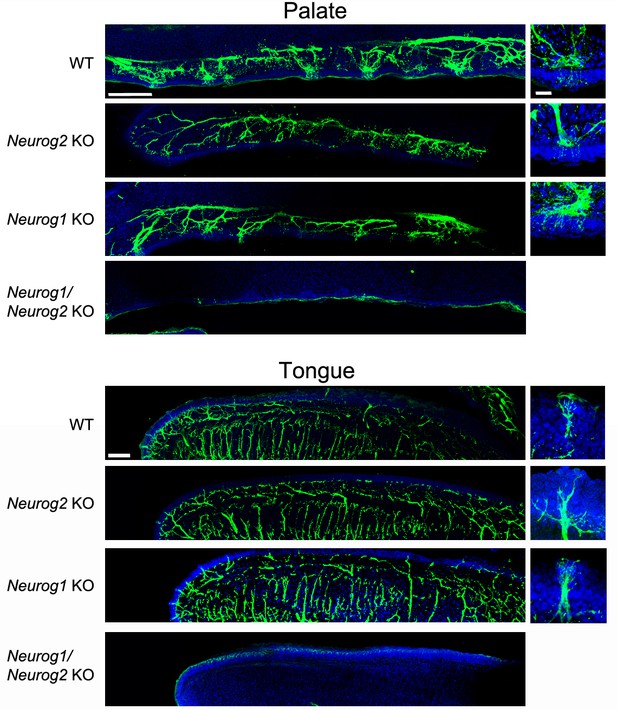
Sensory innervation of the oral cavity in Neurog1, Neurog2 and double Neurog1/Neurog2 knockouts.
Anti β-III tubulin immunofluorescence on the palate (top panels) and tongue (bottom panels) of E16.5 mouse embryos of the indicated genotypes. Single Neurog1 or Neurog2 knockouts maintain innervation of the palatal or lingual placodes. Since Neurog2 KO have lost the geniculate, but keep the trigeminal ganglion, the residual innervation of taste organs in these mutants correspond to the somatic (touch and pain) fibers. All innervation, visceral and somatic are lost in double knockouts. Scale bars: 150μm (left column), 20μm (right column).
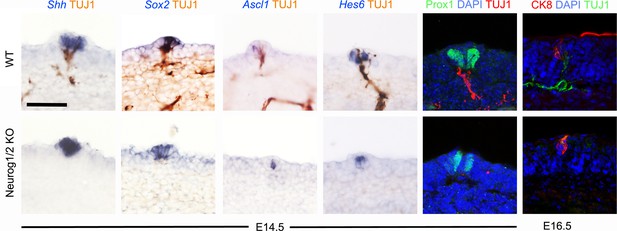
Placodal/papillary gene expression is nerve-independent in fungiform papillae.
Immunohistochemistry for β-III-tubulin combined with in situ hybridization for the indicated genes (left panels), and double immunofluorescence against Prox1 or CK8 and β-III-tubulin counterstained with DAPI (right panel), in the indicated genotypes at E14.5 or E16.5, as indicated. Scale bar, 50 μm.
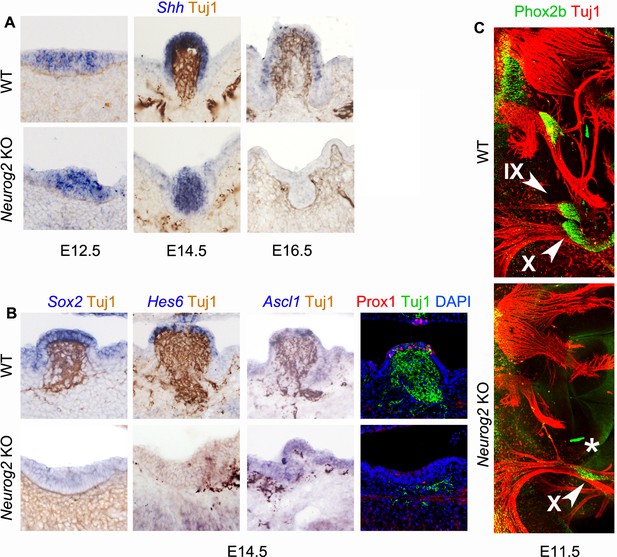
The circumvallate papilla does not form in the absence of visceral innervation.
(A,B) Combined immunohistochemistry and in situ hybridization or immunofluorescence for the indicated markers on coronal (A) or sagittal (B) sections through the circumvallate papilla. (C) Wholemount immunofluorescence on E11.5 embryos showing the absence (asterisk) of the petrosal ganglion (IX) in the Neurog2 KO. X: nodose ganglion.
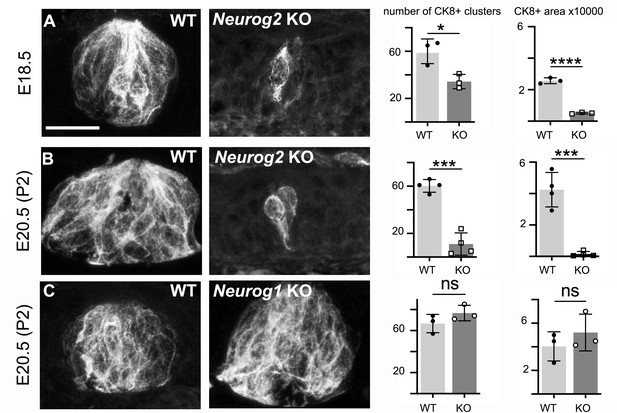
Formation of palatal taste buds requires visceral but not somatic innervation.
Typical examples of CK8+ cell clusters detected by immunofluorescence in wild type and Neurog2 or Neurog1 KO, at E18.5 (A) or E20.5 (B,C), and quantification of the number of clusters throughout the soft palate on alternate sections, and overall surface (in μm2) occupied by CK8+ cells (in alternate sections) throughout the soft palate. *: p<0.05, ***: p<0.001, ****: p<0.0001 and ns: p>0.05; error bars presented as mean ± SD (n = 3 for A, C and n = 4 for B). The individual results for each animal are represented by dots. Scale bar: 20 μm.
-
Figure 2—source data 1
Cross-sectional area (in μm2) occupied per taste bud.
- https://doi.org/10.7554/eLife.49226.009
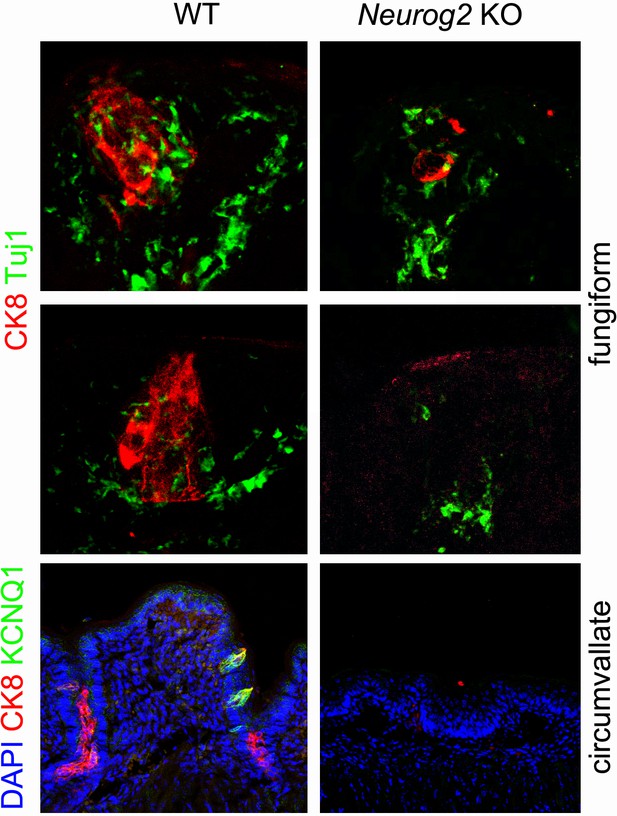
CK8+ cell clusters are atrophic in fungiform papillae and absent in the circumvallate papilla of Neurog2 KO.
Double immunofluorescence against the indicated markers on two representative fungiform papillae (2 upper panels) and the circumvallate papilla (lower panel) of WT and Neurog2 KO at E20.5.
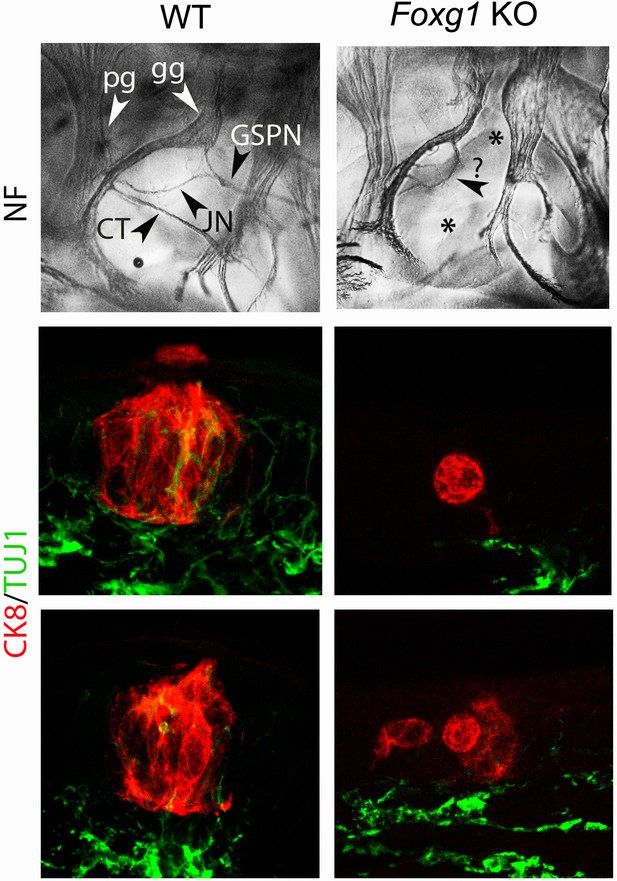
Taste buds do not form in the soft palate of Foxg1 knockouts.
(Top panels) Anti-neurofilament (NF) immunohistochemistry on whole mounts of E11.5 wild type (left) and Foxg1 KO (right) embryos. The mutant embryo has a conspicuously atrophic geniculate ganglion (gg), no greater superficial petrosal nerve (GSPN) or chorda tympani (CT) (asterisks), or Jacobson’s nerve (JN), replaced by an aberrant nerve (question mark). The deletion of the GSPN is fully penetrant (n = 5), the other defects only partially so (not shown). (Bottom panels) Combined immunofluorescence against CK8 and β-III-tubulin in the soft palate on two representative CK8+ cell clusters in wild type and Foxg1 KO mice, at E18.5.
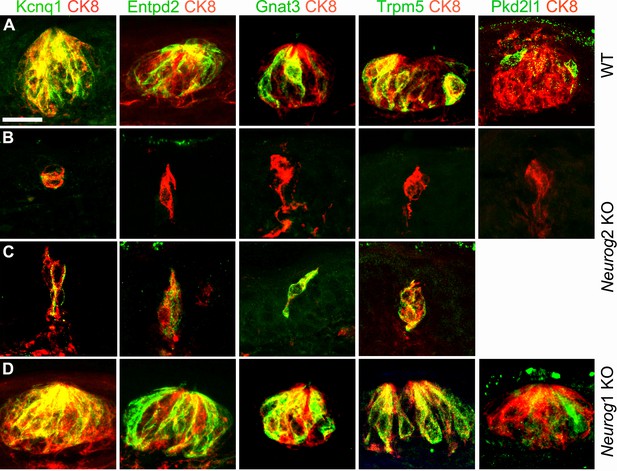
Taste bud cell differentiation is impeded, but not always, in the absence of visceral innervation.
Sections through taste buds in the soft palate of wild types (A), Neurog2 KO (B,C) and Neurog1 KO (D) at E20.5 (P2), immunostained with the indicated antibodies. Kcnq1, Entpd2, Gnat3 and Trpm5 are occasionally detected in residual CK8+ cells of Neurog2 KO embryos. In Neurog1 KOs, taste buds contained the normal complement of markers. Scale bar: 20 μm.
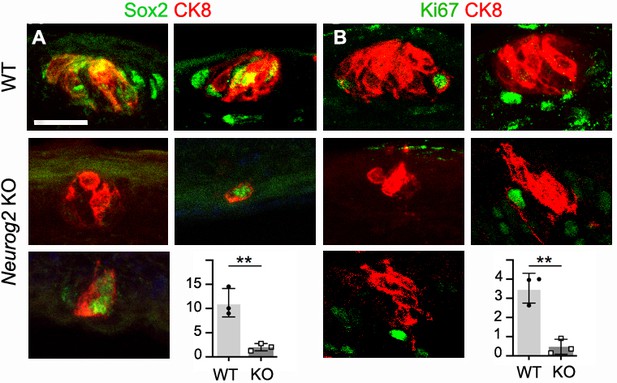
Taste nerves are required for Sox2 expression and cell proliferation around taste bud anlagen.
(A,B) Immunofluorescence against CK8 and Sox2, or CK8 and the proliferation marker Ki67 on representative CK8+ cell clusters in the soft palate from wild type, and Neurog2 KO, at E18.5. The graphs show the counts of Sox2High and Ki67+ cells inside or within two cell diameters of CK8+ cell clusters. **: p<0.01; error bars presented as mean ± SD (for Sox2High cells, a sample of 72 wild type and all (n = 100) mutant clusters were counted, on three animals; for Ki67+ cells, a sample of 71 wild type and all (n = 101) mutant clusters were counted on three animals). The individual results for each animal are represented by dots. Scale bar: 20 μm.
-
Figure 4—source data 1
Number of Sox2+ and number of Ki67+ cells for each K8+ cell cluster at E18.5 in wild type and Neurog2 KO pups.
- https://doi.org/10.7554/eLife.49226.012
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti- Neurofilament (mouse monoclonal) | Hybridoma Bank | Hybridoma Bank (#2H3)RRID:AB_531793 | one in 500 |
| Antibody | Anti-Gustducin (Gnat3) (goat polycolonal) | MyBioSource | MyBioSource (#MBS421805) RRID:AB_10889192 | one in 500 |
| Antibody | Anti-Entpd2 (rabbit polycolonal) | http://ectonucleotidases-ab.com/(Bartel et al., 2006) | RRID:AB_2314986 | one in 500 |
| Antibody | Anti-Pkd2l1 (rabbit polycolonal) | Millipore | Millipore (#AB9084) RRID:AB_571091 | one in 1000 |
| Antibody | Anti-Prox1 (rabbit polyclonal) | Millipore | Millipore (#AB5475) RRID:AB_177485 | one in 1000 |
| Antibody | Cytokeratin8 (Troma-I) (rat monoclonal) | Developmental Studies Hybridoma Bank (DSHB) | RRID:AB_531826 | one in 400 |
| Antibody | Anti-Kcnq1 (rabbit polyclonal) | Millipore | Millipore (#AB5932) RRID:AB_92147 | one in 1000 |
| Antibody | Anti-Ki67 (rabbite polycolonal) | abcam | abcam (#ab15580) RRID:AB_443209 | one in 200 |
| Antibody | Anti-Sox2 (goat polyclonal) | R and D Systems | R and D Systems (#AF2018) RRID:AB_355110 | one in 500 |
| Antibody | Anti-Trpm5 (guinea pig polyclonal) | Obtained from ER Liman’s lab, USC | one in 500 | |
| Antibody | Anti-βIII Tubulin (Tuj1) (mouse monoclonal) | Covance | Covance (#MMS-435P) RRID:AB_2313773 | one in 500 |
| Antibody | Anti-Phox2b (Rabbit polyclonal) | Pattyn et al., Development, 124,4065–4075 (1997) | one in 500 | |
| Antibody | Donkey anti-goat A488 | Thermo Fisher | Thermo Fisher (#A-11055) RRID:AB_2534102 | one in 500 |
| Antibody | Donkey anti- guinea pig A488 | Jackson Immunoresearch Laboratories | Jackson Immunoresearch Laboratories (#706-545-148) | one in 500 |
| Antibody | Donkey anti- mouse A488 | Invitrogen | Invitrogen (#A-21202) RRID:AB_141607 | one in 500 |
| Antibody | Donkey anti- rabbit A488 | Jackson Immunoresearch Laboratories | Jackson Immunoresearch Laboratories (#711-545-152) | one in 500 |
| Antibody | Donkey anti-rat Cy3 | Jackson Immunoresearch Laboratories | Jackson Immunoresearch Laboratories (#712-165-153) | one in 500 |
| Commercial kit | Vectastain Elite ABC Kits | Vector Laboratories | Vector Laboratories, PK-6101 and PK-6012 | |
| Chemical compound | DAB (3,3’-Diaminobenzidine) | Sigma | Sigma (#SLBP9645V) | |
| Commercial reagent | Proligesterone (Delvesterone) | MSD Animal Health | Delvosteron: NaCl (0.9%)=1:1 |
Additional files
-
Source data 1
Average width of K8+ cell clusters in wild type and Neurog2KO at E18.5 and E20.5.
- https://doi.org/10.7554/eLife.49226.013
-
Transparent reporting form
- https://doi.org/10.7554/eLife.49226.014





