Myogenic vasoconstriction requires G12/G13 and LARG to maintain local and systemic vascular resistance
Figures
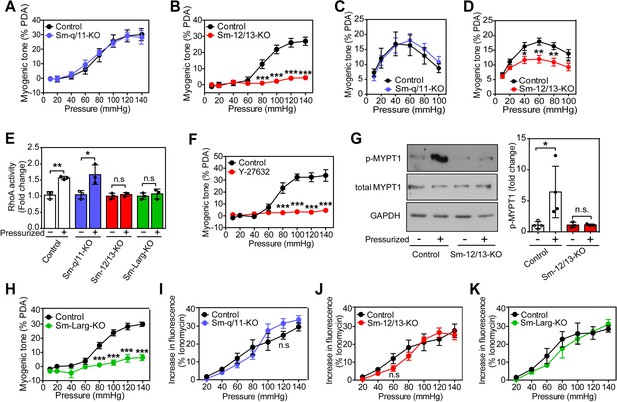
Role of G12/G13, ARHGEF12 (LARG) and RhoA signaling in myogenic contraction.
(A–D) First order mesenteric arteries (A,B) and posterior cerebral arteries (C,D) were isolated from control (black circles), Sm-q/11-KO (blue circles) and Sm-12/13-KO (red circles) mice and were pressurized with the indicated pressure steps. The myogenic tone (% of passive diameter (PDA)) was determined using MyoView four software (A,B) or with Acknowledge software (C,D) (n = 7 mice (control groups and Sm-q/11-KO) and eight mice (Sm-12/13-KO) in A and B and n = 5 mice (control group for Sm-q/11-KO), nine mice (Sm-q/11-KO), 27 mice (control group for Sm-12/13-KO) and 21 mice (Sm-12/13-KO) in C and D). (E) RhoA activity was determined in the pressurized (80 mmHg for 10 min, ‘+') and non-pressurized (‘-') mesenteric arterial segments (6–8 per animal) from control (open bars), Sm-q/11-KO (blue bars), Sm-12/13-KO (red bars) and Sm-Larg-KO (green bars) mice (n = 3 mice in each group). (F) Myogenic tone responses of first order mesenteric arteries incubated for 30 min in the presence (red circles) or absence (black circles) of Rho-kinase inhibitor (Y27632, 10 µM) (n = 3 mice for both groups). (G) Mouse MYPT1 phosphorylation (P-MYPT1) was determined in pressurized (80 mmHg ‘+') and non-pressurized (‘-') mesenteric arteries from control and Sm-12/13-KO mice after homogenization by immunoblotting. Total MYPT1 and GAPDH immunoblots served as controls. The bar diagram shows a statistical analysis of immunoblots (n = 8 mice in four independent experiments (control) and n = 6 mice in three independent experiments (Sm-12/13-KO)). (H) Myogenic tone responses of first order mesenteric arteries in control (black circles) and Sm-Larg-KO (green circles) mice (n = 6 mice (control) and five mice (Sm-Larg-KO)). (I–K) Determination of intracellular [Ca2+] during myogenic tone induction in first order mesenteric arteries isolated from control (black circles), Sm-q/11-KO, (blue circles), Sm-12/13-KO (red circles), and Sm-Larg-KO mice (green circles). All arteries were loaded with Fura-2-AM (12.5 µM) for determination of free intracellular [Ca2+] (n = 5 mice (control groups, Sm-q/11-KO and Sm-12/13-KO) and n = 4 mice (Sm-Larg-KO)). All values are mean values ± SEM. *, p≤0.05; **, p≤0.01; ***, p≤0.001; n.s. = non significant (2-way ANOVA and Bonferroni’s post-hoc test (in A-D and F-K); unpaired t-test (in E)).
-
Figure 1—source data 1
Analysis of pressure-induced changes in myogenic tone, RhoA-activation, Rock inhibition and intracellular calcium release in smooth muscle-specific Gαq/Gα111, Gα12/Gα13, and ARHGEF12 (LARG) deficient mice.
- https://doi.org/10.7554/eLife.49374.005
-
Figure 1—source data 2
Western blot pictures (uncut) of pressure-inducedP-MYPT1 phosphorylation in smooth muscle-specificGα12/Gα13 deficient mice.
- https://doi.org/10.7554/eLife.49374.006
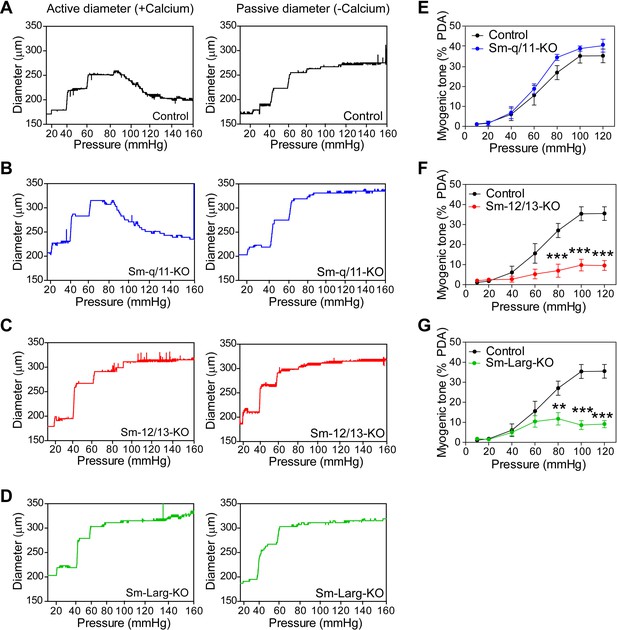
Effect of vascular smooth muscle-specific Gαq/Gα11, Gα12/Gα13, and ARHGEF12 (LARG) deficiency on myogenic tone responses.
(A-D) Representative traces of first order mesenteric arteries isolated from control (A, black), Sm-q/11-KO (B, blue), Sm-12/13-KO (C, red) and Sm-Larg-KO (D, green) mice showing changes in the luminal diameter over a range of increased perfusion pressure (10–140 mmHg) in the presence (active diameter, left panels) and absence of calcium (passive diameter, right panels).(E–G) Third order mesenteric arteries were isolated from control (black circles), Sm-q/11-KO (blue circles) and Sm-12/13-KO (red circles) mice and were pressurized with the indicated pressure steps. The myogenic tone was determined as described above (n = 7 mice (control), n = 5 mice (Sm-12/13-KO and Sm-Larg-KO)). All values are mean values ± SEM. **, p≤0.01; ***, p≤0.001 (2-way ANOVA and Bonferroni’s post-hoc test).
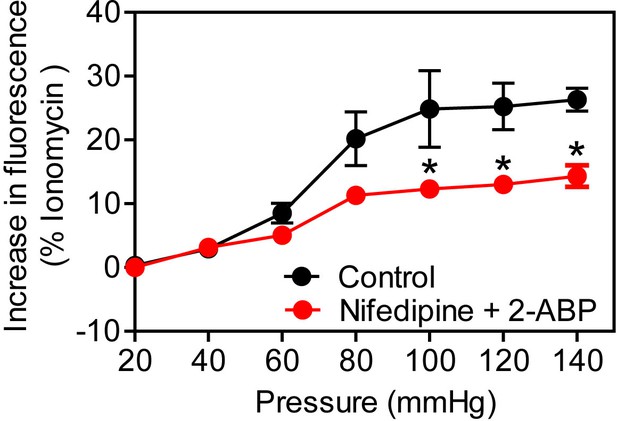
Effect of L- type and TRP channel inhibitors on myogenic tone.
Determination of intracellular [Ca2+] during myogenic tone induction in first order mesenteric arteries incubated for 30 min in the presence (red circles) or absence (black circles) of L-type channel inhibitor (nifedipine, 1 µM) and TRP channel inhibitor (2-Aminoethoxydiphenyl borate, 1 µM) (n = 3 mice for both groups). PDA, passive diameter. All values are mean values ± SEM. *, p≤0.05 (2-way ANOVA and Bonferroni’s post-hoc test).
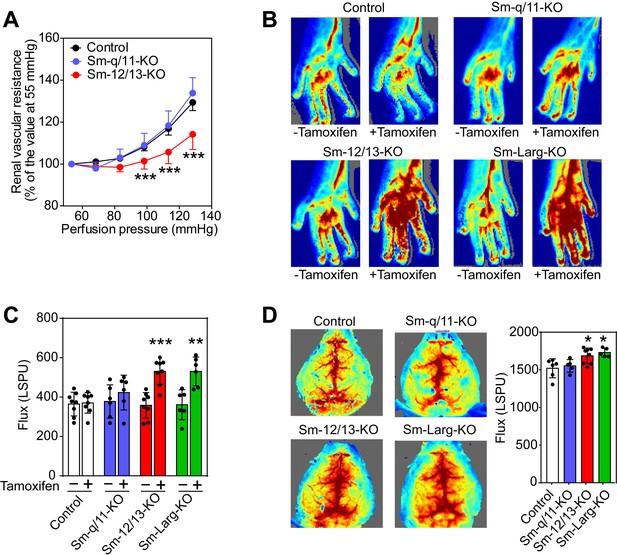
Effect of vascular smooth muscle-specific Gα12/Gα13 deficiency on vascular resistance and perfusion in peripheral organs.
(A) Flow-induced increase in vascular resistance in isolated perfused kidneys of control (black circles), Sm-12/13-KO (red circles) and Sm-q/11-KO mice (blues circles) (n = 12 mice (control) and n = 7 (Sm-q/11-KO and Sm-12/13-KO)). (B, C) Laser speckle perfusion imaging of the hind limb from wild-type (control), Sm-q/11-KO, Sm-12/13-KO and Sm-Larg-KO mice before and 2 weeks after tamoxifen treatment (n = 8 mice (control and Sm-12/13-KO) and n = 6 mice (Sm-q/11-KO and Sm-Larg-KO)). (D) Laser speckle perfusion imaging of the brain from wild-type (control), Sm-q/11-KO, Sm-12/13-KO and Sm-Larg-KO mice (n = 6 mice (control), n = 5 mice (Sm-q/11-KO and Sm-Larg-KO) and n = 8 mice (Sm-12/13-KO)). Shown are representative images as well as the statistical evaluation (bar diagrams). LSPU, laser speckle perfusion units. All values are mean values ± SEM. *, p≤0.05; **, p≤0.01; ***, p≤0.001 (Bonferroni’s post-hoc test (in A); unpaired t-test (in C and D; compared to control in D)).
-
Figure 2—source data 1
Analysis of kidney, hind limb and brain perfusion in smooth muscle-specific Gαq/Gα11, Gα12/Gα13, and ARHGEF12 (LARG) deficient mice.
- https://doi.org/10.7554/eLife.49374.009
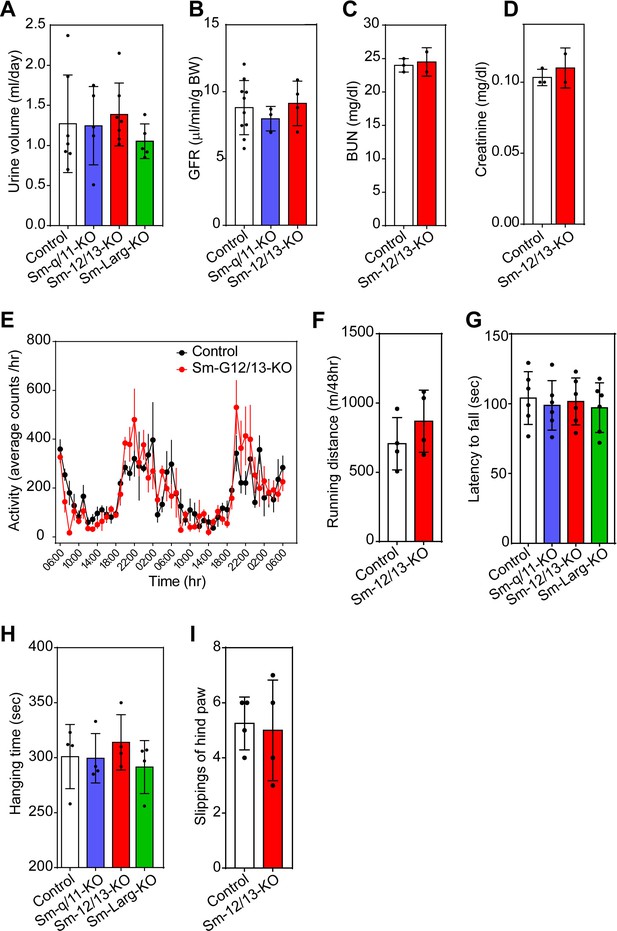
Effect of vascular smooth muscle-specific Gα12/Gα13 deficiency on Kidney and brain function.
(A–D) Urine volume. (A), glomerular filtration rate (GFR) (B), blood urea nitrogen (BUN) (C) and plasma creatinine levels (D) in wild-type and the indicated mutant mice (n = 7 mice (control and Sm-12/13-KO in A), n = 5 mice (Sm-q/11-KO and Sm-Larg-KO in A), n = 10 mice (control in B), n = 3 mice (Sm-q/11-KO in B and control in C and D), n = 4 mice (Sm-12/13-KO in B), n = 2 mice (Sm-12/13-KO in C and D)). (E–I) day and night activity (E), running distance (F), rotarod analysis (G), mesh grip analysis (H) and beam cross test (I) in wild-type and the indicated mutant mice (n = 4 mice (control and mutant mice in E,F,H and I), n = 4 mice (control and mutant mice in G)). All values are mean values ± SEM. n.s. = non significant (student’s t-test (A–D, F–I) and 2-way ANOVA and Bonferroni’s post-hoc test (E).
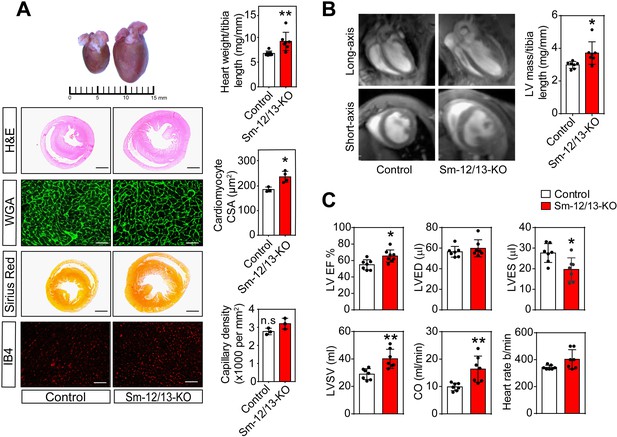
Cardiac structure and function of smooth muscle specific Gα12/Gα13-deficient mice.
(A) Hearts of wild-type (control, left) and Sm-12/13-KO mice (right) were stained 4 weeks after induction with hematoxylin and eosin (H and E), wheat germ agglutinin-AF488 (WGA-AF488), picrosirius red or IB4. Quantification of heart weights (normalized to tibia length), cardiomyocyte cross-sectional area (CSA) and capillary density is represented by bar graphs. Scale bar: 2 mm (H and E- as well as Sirius red-stained sections) and 50 µm (WGA- and IB4-stained sections) (n = 7 mice per group (heart weight per tibia length), n = 4 mice per group (Cardiomyocyte area) and n = 3 mice (capillary density)). (B) MRI images in long-axis four chamber view and short-axis view from control and Sm-12/13-KO mice. Left ventricular mass, as calculated from the MRI data, normalized to tibia length of wild-type and Sm-12/13-KO mice is represented as bar graph (n = 7 mice per group). (C) MRI assessment of LV function in control (black bar) and Sm-12/13-KO mice (red bar). LVEF, left ventricular ejection fraction; LVED, left ventricular end diastolic volume; LVES, left ventricular end systolic volume; LVSV, left ventricular stroke volume; CO, cardiac output (n = 7 mice per group). All values are mean values ± SEM. *, p≤0.05; **, p≤0.01 (unpaired t-test; compared to control).
-
Figure 3—source data 1
Histological and MRI analysis of hypertrophy in smooth muscle-specific Gα12/Gα13deficient mice.
- https://doi.org/10.7554/eLife.49374.012
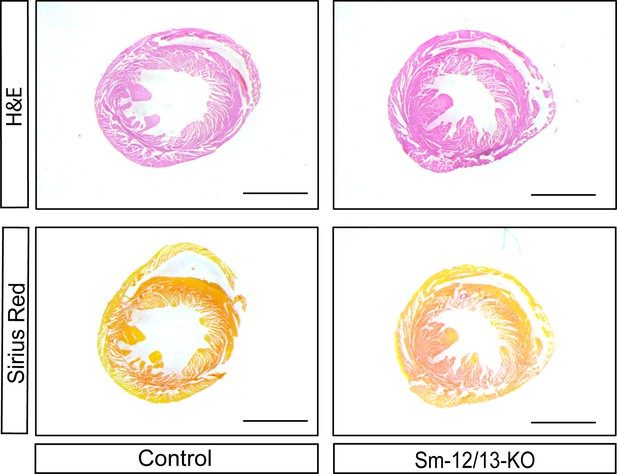
Hearts of wild-type (control) and Sm-12/13-KO mice were stained 34 weeks after tamoxifen induction with hematoxylin and eosin (H and E), as well as picrosirius red.
Scale bar: 5 mm.
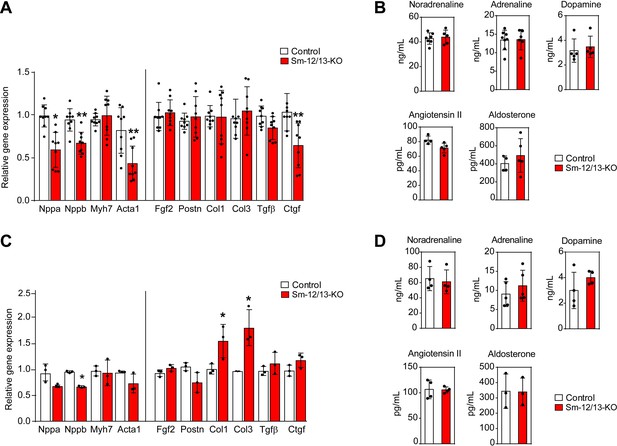
Cardiac gene expression and systemic levels of mediators in Sm-12/13-KO mice.
(A and C) relative expression of myocardial genes and fibrosis marker genes in the hearts of control (white bars) and Sm-12/13-KO mice (red bars) 4 weeks (A) and 35 weeks (C) after tamoxifen treatment (n = 9 mice (A) and n = 3 mice (C)). (B and D) plasma levels of catecholamines, angiotensin-II and aldosterone in control (white bars) and Sm-12/13-KO mice (red bars) 4 weeks (B) and 35 weeks (D) after induction (n = 3–7 mice). All values are mean values ± SEM. *, p≤0.05 (compared to control; unpaired t-test).
-
Figure 4—source data 1
Analysis of hypertrophy associated genes, catecholamines, aldosterone and angiotensin in smooth muscle-specific Gα12/Gα13deficient mice.
- https://doi.org/10.7554/eLife.49374.014

Effect of vascular smooth muscle-specific Gα12/Gα13 and LARG deficiency on LPS-induced hypotension.
(A and B), telemetric blood pressure measurements (systolic in A, diastolic in B) were performed in wild-type (control), Sm-12/13-KO and Sm-Larg-KO mice. At the indicated time point animals were injected with 10 mg/kg of lipopolysaccharide (LPS), and the blood pressure was monitored for 24 hr (n = 8 mice (control and Sm-Larg-KO) and n = 7 mice (Sm-12/13-KO)). The bar diagrams show the statistical evaluation of the average systolic (A) and diastolic blood pressure (B) during the period between 10 and 24 hr after LPS injection. Shown are mean values ± SEM. **, p≤0.01; ***, p≤0.001 (compared to control; unpaired t-test).
-
Figure 5—source data 1
Effects of LPS on blood pressure in Gα12/Gα13and ARHGEF12 (LARG) deficient mice.
- https://doi.org/10.7554/eLife.49374.016
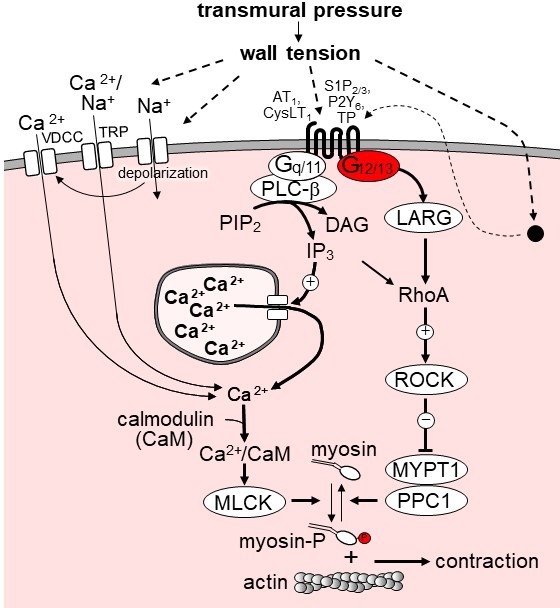
Model of the role of G12/G13 in vascular myogenic tone.
VDCC, voltage-dependent Ca2+ channels; TRP, transient receptor potential channels; PLC-β, phospholipase C-β; PIP2, phosphatidyl inositol bisphosphate; DAG, diacyl glycerol, IP3, inositol-1,4,5-triphosphate; LARG, RhoGEF protein ARHGEF12; MYPT1 and PPC1, regulatory and catalytic subunit of myosin phosphatase, respectively; ROCK, Rho-kinase. ROCK phosphorylates MYPT1 and thereby inhibits myosin phosphatase activity. AT1, angiotensin AT1 receptor; CysLT1, cysteinyl leukotriene receptor 1; S1P2/3, sphingosine-1-phosphate receptors 2 and 3; P2Y6, purinergic receptor Y6; TP, thromboxane A2 receptor. AT1 and CysLT1 have been shown to be activated by increased vascular pressure in a ligand independent manner, whereas activation of S1P2/3, TP and P2Y6 is believed to require formation or release of the respective receptor ligand. For details see text.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | Gna12−/− | PMID: 12077299 | RRID:MGI:3819345 | |
| Genetic reagent (Mus musculus) | Gna13flox/flox | PMID: 14528298 | RRID:MGI:3819345 | |
| Genetic reagent (Mus musculus) | Gnaqflox/flox | PMID: 11689889 | RRID:MGI:3819271 | |
| Genetic reagent (Mus musculus) | Gna11−/− | PMID: 9687499 | RRID:MGI:3819271 | |
| Genetic reagent (Mus musculus) | Largflox/flox | PMID: 18084302 | RRID:MGI:3819344 | |
| Genetic reagent (Mus musculus) | Myh11-CreERT2 | PMID: 18084302 | RRID:IMSR_JAX:019079 | |
| Antibody | anti-phospho-MYPT1 (rabbit polyclonal) | Merck Millipore | Catalog: 36–003 RRID:AB_310812 | WB (1:1000) |
| Antibody | anti-MYPT1 (rabbit polyclonal) | Cell Signalling Technologies | Catalog: 2634 RRID:AB_915965 | WB (1:500) |
| Antibody | anti-GAPDH (rabbit monoclonal) | Cell Signalling Technologies | Catalog: 2118 RRID:AB_561053 | WB (1:1000) |
| Commercialassay or kit | Rho-GLISA | Cytoskeleton Inc | BK124 | for determination of Rho activity |
| Ccommercial assay or kit | 3-CAT ELISA-ImmuSmol | LDN | BA E-5600 | for determination of adrenaline, dopamine and noradrenaline levels |
| Commercial assay or kit | Angiotensin II ELISA kit | ENZO Life Sciences | ADI-900–204 | |
| Commercial assay or kit | Aldosterone ELISA kit | ENZO Life Sciences | ADI-900–173 | |
| Chemical compound, drug | Y-27632 | Sigma Aldrich | Y0503 | 10 µM |
| Chemical compound, drug | 2-Aminoethyl diphenylborinate (2-ABP) | Sigma Aldrich | D9754 | 1 µM |
| Chemical compound, drug | Nifedipine | Sigma Aldrich | N7634 | 1 µM |
| Chemical compound, drug | Fura-2AM | Thermo Fisher Scientific | F1225 | 12.5 µM |
| Chemical compound, drug | Ionomycin | Sigma Aldrich | I0634 | 1 µM |
| Chemical compound, drug | LPS (Lipopolysaccharide) | Sigma Aldrich | L2630 | 10 mg/kg b.w |
| Other | WGA-AF488 | Thermo Fisher Scientific | W11261 | Histology (20 µg/ml) |
| Other | IB4-AF488 | Thermo Fisher Scientific | I21411 | Histology (1:200) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.49374.018





