Neurotrophins induce fission of mitochondria along embryonic sensory axons
Figures
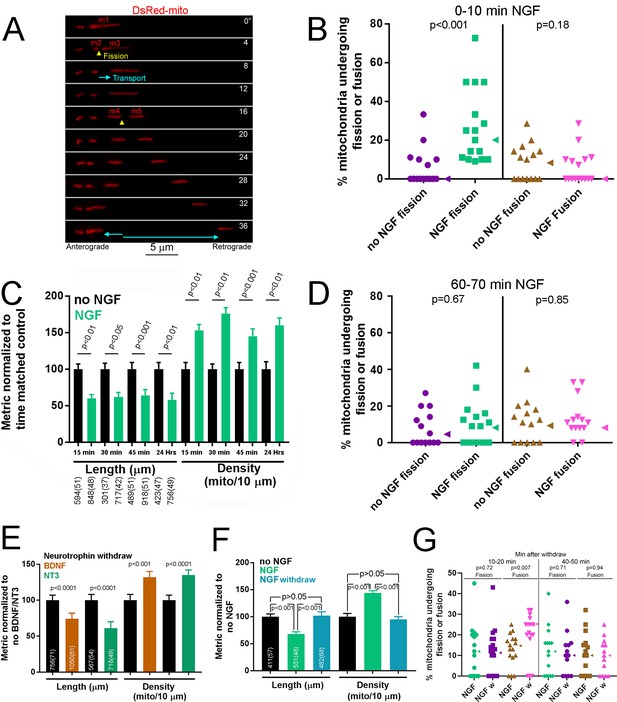
NGF induces a rapid bout of fission followed by maintenance of a new steady state of mitochondria length and density.
(A) Example of mitochondria labeled by expression of mitochondrially targeted DsRed (DsRed-mito) undergoing fission and subsequent transport during the first 5–10 min of NGF treatment (40 ng/mL throughout). By 4’ the mitochondrion labeled m1 undergoes fission giving rise to m2 and m3. The two emergent mitochondria move away from each other and at 16’ m3 fissions again to give rise to m4 and m5, and again both move away from each other. (B) Determination of the percentage of mitochondria that underwent fission or fusion during an initial 10 min treatment with NGF. Each data point reflects one axon (n = 95 and 100 mitochondria from 15 and 16 axons for no NGF and NGF groups respectively; Mann-Whitney test). Arrowheads to the right of data points denote the median. (C) Mitochondria length and densities in distal 50 μm of axons, excluding growth cones, after multiple durations of NGF treatment (sample sizes shown below bars using an x(y) format where x and y denote the number of mitochondria and axons respectively). Mean and SEM shown, Bonferroni posthoc tests. (D) Percentage of mitochondria that underwent fission or fusion during a 10 min period after a 1 hr treatment with NGF (n = 110 and 126 mitochondria from 14 axons/group for no NGF and NGF respectively; Mann-Whitney test). Arrowheads to the right of data points denote the median. (E) A 45 min treatment with either BDNF or NT3 (40 ng/mL for both) decreases mitochondria length and increases density (n shown in bars using the same format as panel C; Mean and SEM shown; Welch t-test for densities, Mann-Whitney test for length). (F) Removal of NGF with inclusion of a function blocking NGF antibody after a 30 min treatment (NGF withdraw) restores mitochondria length and density to no NGF treatment levels (no NGF) by 3.5 hr. In contrast, in the continued presence of NGF (NGF) mitochondria exhibit the same trends as expected based on the data in panel C. (n shown in bars using the same format as panel C; Bonferroni posthoc tests for density and Dunn’s posthoc tests for length). (G) Percentage of mitochondria that underwent fission or fusion during a 10 min period starting at 10 min or 40 min after NGF withdraw (NGF w) following an initial 30 min treatment with NGF. The time matched control groups (NGF) underwent similar medium exchanges as the NGF w group but the medium contained NGF. Mann-Whitney tests.
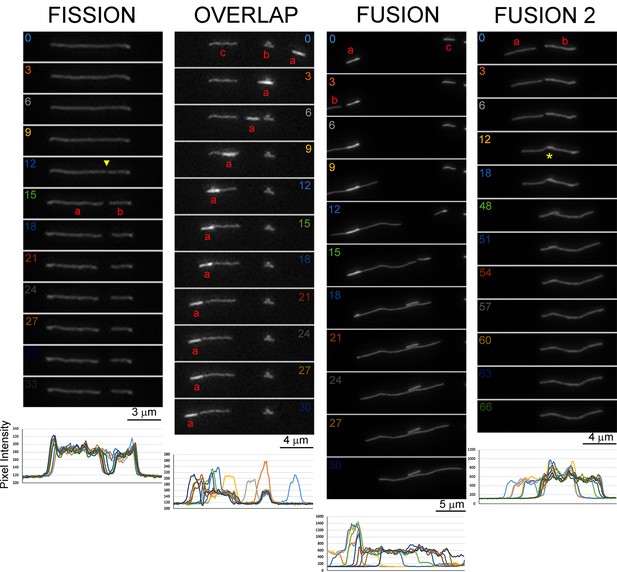
Examples of mitochondria fission, fusion and overlap without subsequent fusion.
In all cases lines scans color coded for time (seconds), as in the panels, are shown below the time lapse sequences. Fission: At 12 s a separation along the mitochondrion becomes evident (yellow arrowhead) and is reflected in the line scan as a drop in intensity to baseline levels at this and subsequent time points. During the fission the two ends of the emergent mitochondria (a and b) separate as the ends contract away from each other. In this example the fission did not coincide with subsequent transport of either of the emergent mitochondria, although there is a slight sub-micron movement of the left end of mitochondrion a between 12–15 s. Overlap: The mitochondrion labeled ‘a’ in red moves from right to left of the panel. At 3 s it overlaps with the mitochondrion labeled ‘b’ but moves past it by 6 s. Between 9–15 s a overlaps with c. At 21–30 s a stalls adjacent to c but does not fuse as indicated by (1) no equalization of fluorescence between the two mitochondria (a is brighter; compared to the fusion examples that follow) and (2) background level intensities between the two mitochondria as shown in the line scans. Fusion: Between 0–3 s mitochondrion b enters the field to the left of a. Mitochondrion b undergoes movement to the right of the panel between 3–15 s, during which there is overlap of a and b but no apparent change in the morphology or intensity of a. At 15 s the left end of b overlaps with a. Between 18–24 s a decrease in size and by 24 s a is no longer detectable as distinct from b. The emergent mitochondrion a+b is now of uniform intensity and there is no detectable overlap in fluorescence between a and b. This is interpreted as a and b having fused and the higher intensity initially apparent for a having redistributed over the longer length of a+b (in contrast to the preceding overlap sequence). During the sequence mitochondria c and b move toward each other but do not overlap and they remain adjacent to one another without any evidence of fusion or interaction. Furthermore, the length of mitochondria a and b at 12 s, during overlap, were 2.14 and 10.66 μm respectively. At 24 s and later the length of the fused mitochondrion (a+b) was 12.82 μm. Fusion 2: Between 0–12 s mitochondria a and b come into end-to-end contact. Between 12–18 s the ends overlap (denoted by yellow *). Between 18–54 s, when fusion is considered to have occurred and there is the loss of detectable overlap, the emergent mitochondrion undergoes rightward movement at both ends. Furthermore, while a and b initially exhibited differences in intensities, a having lower intensity that b at 0 s, by 54 s the intensity along the emergent mitochondrion (a+b) is uniform and there is no evidence of overlap as would be expected if a had migrated on top of b. Furthermore, at 0 s the summed length of a and b is 7.59 μm, and the length of the emergent mitochondrion a+b by 54 s and later is 7.52 μm.
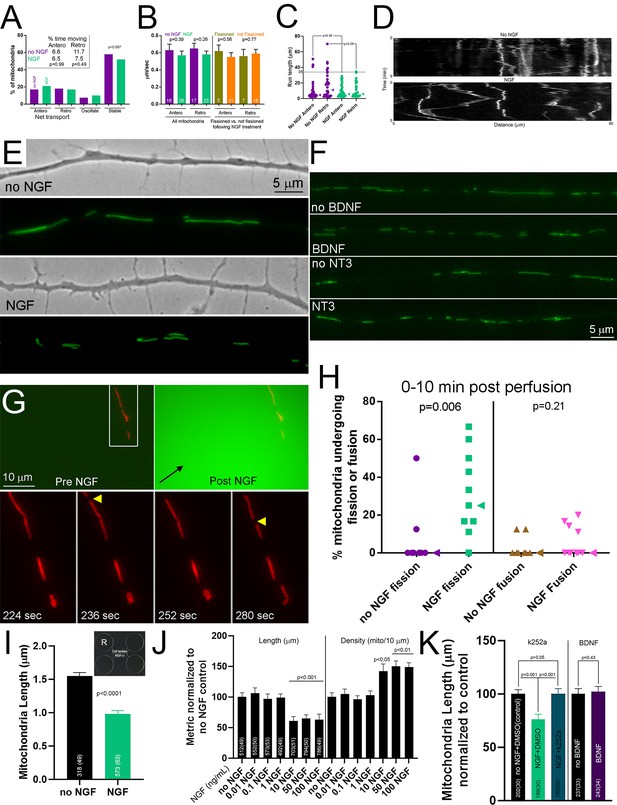
Net mitochondria transport and examples of neurotrophin treated axonal mitochondria.
(A) During the first 5–10 min post NGF treatment there was no detectable difference in individual behavioral categories of the entire population of axonal mitochondria as reflected by the proportions undergoing net anterograde (antero), retrograde (retro), oscillatory movements without net displacement (oscillatory) or exhibiting no detectable movement (stable) (n = 95 and 100 mitochondria from 15 and 16 axons for no NGF and NGF). However, analysis of the proportion undergoing any transport relative to those stalled approached significance (p=0.067; Fisher’s exact test) with a slight trend toward more transport in the NGF treatment group (38% and 34% for NGF and no NGF, respectively). The inset shows the values for the percent of time that mitochondria spent moving in the anterograde and retrograde directions (Fisher’s exact test, no NGF versus NGF within direction of movement). (B) Transport rates of mitochondria. No effects of NGF were noted on the net transport rate considering all mitochondria regardless of whether they underwent fission, and similarly there was no detectable difference in the rates of transport for mitochondria having undergone fission or not (n = mitochondria shown in bars, Welch t-tests). (C) Graph of the lengths of ‘runs’, periods of continuous transport, classed by NGF or no NGF treatment and whether the total length of the run was net anterograde or retrograde. Each datum is a mitochondrion. NGF did not affect the median (arrowhead) length of runs. The line demarcating the 35 μm length is intended to show that runs above this length were only detected in the no NGF group. (D) Kymographs showing the behavior of mitochondria undergoing runs. In the NGF group there are multiple instances of reversal of direction by mitochondria undergoing a run (55%), which are rare in the non NGF group wherein most mitochondria (85%) undergo continuous movement in only one direction during the run. (E) Examples of mitotracker green labeled mitochondria in axons treated with NGF for 30 min and no NGF treatment axons. (F) Examples of mitotracker green-labeled mitochondria in axons treated with either BDNF or NT3 for 45 min or no neurotrophin treatment. (G) Example of an axon being perfused by NGF and the ensuing fission of mitochondria. The top left panel shows the imaging field prior to commencing perfusion from the pipette located just outside of the field of view. The top right panel shows the perfusion with medium containing NGF and Cell Tracker Green (green), and the black arrow denotes the direction of perfusion. The bottom panels show two instances of mitochondria fission (yellow arrowheads) occurring at the shown seconds after initiation of NGF perfusion. (H) Graph showing the percentage of mitochondria undergoing fission or fusion in response to perfusion with NGF containing medium or control medium in all cases containing Cell Tracker Green to monitor perfusion. Median is denoted by arrowheads to the right of data points. (I) Graph showing the length of mitochondria in axons growing into the distal compartment of microfluidic chambers containing either no NGF or NGF (labeled NGF+/-). The cell body compartment contained no NGF. The inset shows an example of the microfluidic chambers used (R = reservoir). 49 and 63 axons were sampled from 13 and 6 chambers for the no NGF and NGF groups respectively. Mann-Whitney test. (J) Concentration response to NGF treatment (1 Hr). Dunn’s multiple comparison tests within metric relative to the no NGF group. (K) Graph showing the effects of k252a (100 nM 15 min pretreatment) on the NGF-induced decrease in mitochondria lengths (30 min NGF treatment) and the lack of an effect of treatment with 100 ng/mL BDNF (1 Hr) on the length of mitochondria in axons of neurons cultured in no NGF. Dunn’s multiple comparison tests for the k252a related data sets and Mann-Whitney test for the BDNF experiment.
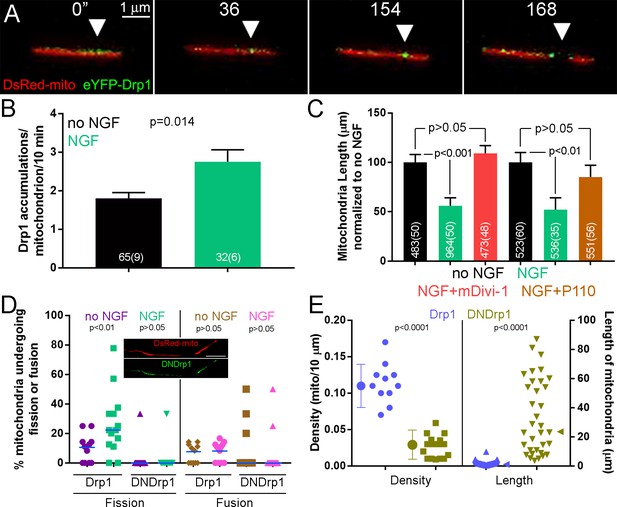
The Drp1 GTPase mediates NGF-induced fission.
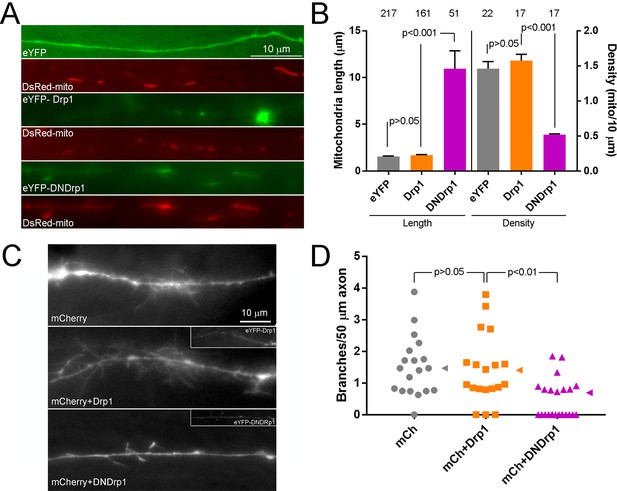
(A) Example of the formation of eYFP-Drp1 puncta (example from NGF treated axons). eYFP-Drp1 is initially heterogeneously distributed along the mitochondrion (0’). By 36 s Drp1 has begun to accumulate at the future site of fission, denoted by the arrowhead in all panels. During the ensuing period the accumulation of Drp1 becomes more focal and pronounced culminating with fission occurring at 154 s and the mitochondria moving apart by 168 s. (B) Quantification of the number of eYFP-Drp1 accumulations per mitochondrion during the first 10 min after treatment with NGF. n = mitochondria(axons) shown in bars, Mann-Whitney test. (C) Determination of mitochondria lengths with a 15 min pretreatment with 20 μM mDivi-1 followed by a 45 min treatment with NGF. Throughout this work mDivi-1 was used at 20 μM as this concentration inhibits fission without impacting complex I of the respiratory chain (Bordt et al., 2017; Smith and Gallo, 2017). Determination of mitochondria lengths after a 30 min pretreatment with 5 μM P110 followed by a 30 min treatment with NGF. For all pharmacological experiments in this report treatment with vehicle (DMSO) was performed as control. n = mitochondria(axons) shown in bars; Dunn’s posthoc tests. (D) Live imaging analysis of the rates of fission (% mitochondria/10 min) before and after NGF treatment (0–10 min) in the axons of eYFP-Drp1 (Drp1) or eYFP-DNDrp1 (dominant negative Drp1; DNDrp1) expressing neurons. Mitochondria were labeled through co-expression of mitochondrially targeted DsRed. Each data point reflects one axon. Mann-Whitney tests. Blue lines denote median. The inset shows and example of DNDrp1 expression. As with wild type Drp1 DNDrp1 targeted to mitochondria. Bar = 5 μm. (E) Mitochondria length and densities in the axons of neurons expressing Drp1 or DNDrp1 in the no NGF condition prior to NGF treatment. Data points for density represent axons and for lengths individual mitochondria within those axons. Mann-Whitney test and Welch t-test for length and density comparisons respectively. Mean and SD are shown to the left of data points for density, and median is denoted by arrowheads to the right of data points for length.
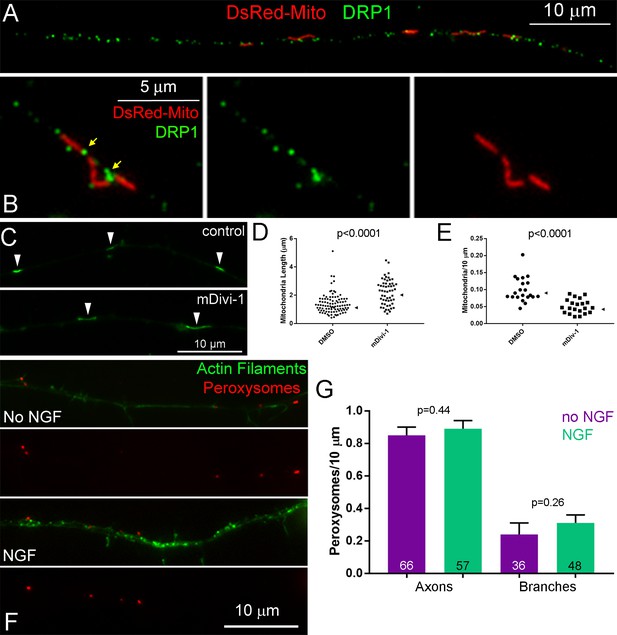
Involvement of Drp1 in mitochondria fission but not the regulation of peroxysomes.
(A) Immunostaining of endogenous Drp1 in axons also showing mitochondria labeled through expression of mitochondrially targeted DsRed. (B) Close up of axonal mitochondria that may have undergone fission at the time of fixation, as indicated by their proximity, showing association of endogenous Drp1 with the ends of apposed mitochondria (yellow arrows). (C) Examples of mitotracker green labeled mitochondria (white arrowheads) in axons treated for 3 hr with DMSO or mDivi-1. (D) and (E) quantification of mitochondria length and density, respectively, in axons treated with DMSO (control) or mDivi-1 for 3 hr. Data points reflect mitochondria and axons in (D) and (E), Mann-Whitney tests for both panels. Median is denoted by arrowheads to the right of data points. (F) Examples of peroxisome distribution in axons with no NGF or a 30 min NGF treatment. Peroxisomes were detected through immunostaining with antibodies to the peroxysomal marker PMP70. (G) Quantification of peroxisome densities in axons and branches of no NGF or NGF (30 min) treated neurons as in (F). n = axons shown in the bars labeled Axons; n for branches from this set of axons is denoted in the bars labeled Branches, Mann-Whitney tests.
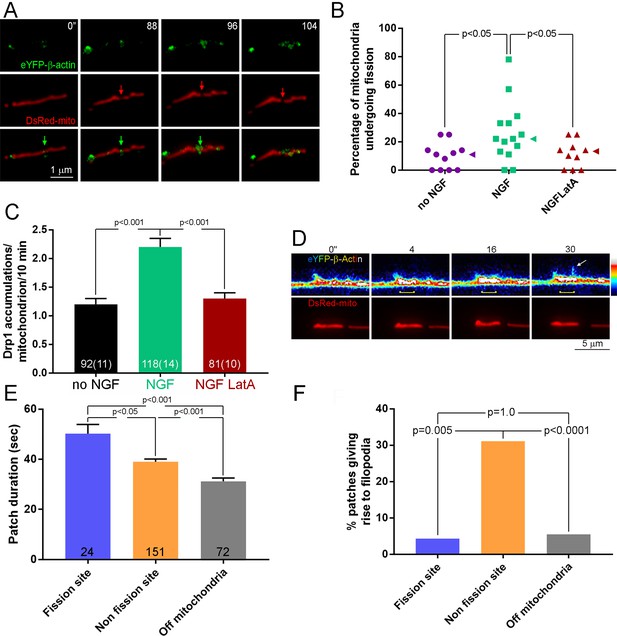
Patches of actin filaments mediate NGF-induced fission.
(A) Timelapse sequence showing the formation of an actin filament patch at the site of fission during the first 10 min of NGF treatment. The initiation of the actin patch is shown at time 0 s (green arrow) and tracked throughout the process of fission. The patch elaborates at the future site of fission (red arrow) that becomes evident at 96 s. Other actin patches formed along the mitochondrion are not associated with sites of fission. (B) Pretreatment (15 min) with the actin depolymerizing drug Latrunculin A (LatA; 4 μM) prior to NGF treatment (0–10 min) blocks the NGF-induced increase in the rate of mitochondria undergoing fission (% mitochondria/10 min). Each data point reflects one axon. Dunn’s posthoc multiple comparison tests. NGF and no NGF treatments included treatment with the DMSO vehicle. Median is denoted by arrowheads to the right of data points. (C) LatA pretreatment blocks the NGF-induced increase in the formation of Drp1 accumulations along mitochondria. Same experimental design as in (B), n = mitochondria(axons) shown in bars. Dunn’s posthoc multiple comparison tests. (D) Example of actin patch formation associated with a mitochondrion that gives rise to the emergence of a filopodium but not fission. By 4 s an actin patch has begun forming at the site populated by the mitochondrion (yellow bracket at 4–30 s). At 30 s a filopodium emerges from the patch (arrow). The heat map ranges 0–4095 in pixel intensities. (E) Analysis of the duration of actin patches during the first 10 min of NGF treatment as a function of their relationship to mitochondria and sites of fission. n = number of patches from 19 axons. Dunn’s posthoc multiple comparison tests. (F) Analysis of the probability that an actin patch will give rise to a filopodium as a function of their relationship to mitochondria and sites of fission. Same data set as in (E). Fisher’s exact tests.
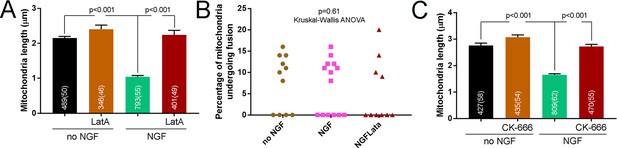
Requirement of Arp2/3 nucleated actin filaments in NGF-induced fission.
Examples of fission, fusion and mitochondrial overlap. Related to Materials and methods. (A) Pretreatment (15 min) with latrunculin A (LatA) prevents the NGF-induced (15 min treatment) decrease in mitochondria length. n = mitochondria(axons) shown in bars, Dunn’s multiple comparison tests. (B) Analysis of the percentage of mitochondria undergoing fusion (same data set as in Figure 3B). Kruskal-Wallis ANOVA, p=0.61. (C) Pretreatment (15 in) with the Arp2/3 inhibitor CK-666 prevents the NGF-induced (30 min treatment) decrease in mitochondria length. n = number of mitochondria (axons) shown in the bars, Dunn’s multiple comparison tests.
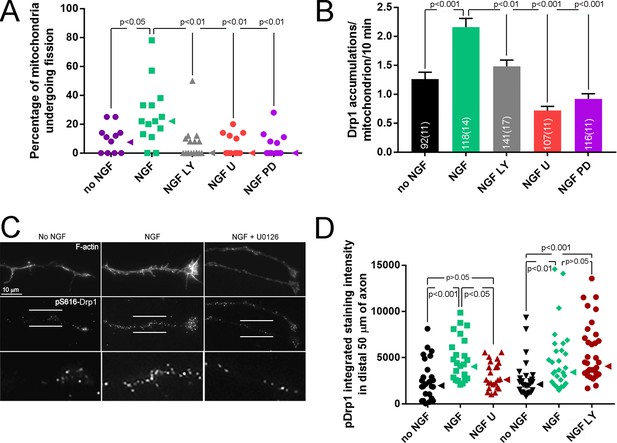
PI3K and Mek-Erk signaling are required for NGF-induced mitochondria fission.
(A) Inhibition of PI3K (LY294002, LY, 25 μM) or Mek-Erk (U0216, U, 50 μM; PD325901, PD, 1 μM) both prevented the NGF-induced increase in the rate of mitochondria undergoing fission during the first 10 min of NGF treatment (% mitochondria/10 min). Each data point reflects an axon. The no NGF and NGF groups received the DMSO vehicle and are the same as shown in Figure 3B as pharmacological experiments were performed in parallel. Dunn’s posthoc multiple comparison tests performed using no NGF, NGF and NGF+drug group within drug treatment. Median is denoted by arrowheads to the right of data points. (B) LY and U/PD pretreatment blocks the NGF induced increase in the formation of Drp1 accumulations along mitochondria. Same experimental design as in (A), n = mitochondria(axons) shown in bars. Dunn’s posthoc multiple comparison tests performed using no NGF, NGF and NGF+drug group within drug treatment. (C) Examples of axons stained with anti-pS616-Drp1 antibodies and phalloidin to reveal actin filaments (F-actin). The pS616-Drp1 staining pattern was punctate. NGF elevated the staining levels and pretreatment with U prevented the NGF-induced increase. The bottom panels show empty magnification examples of the axonal domains denoted by the parallel lines in the pS616-Drp1 panels above. For presentation purposes, all images in panel were equally digitally brightened to enhance visual appreciation of the signal. (D) Quantification of the total intensity of pS616-Drp1 staining in distal axons. Each datum reflects one axon, Dunn’s posthoc multiple comparison tests within drug treatment experiment. Median is denoted by arrowheads to the right of data points.
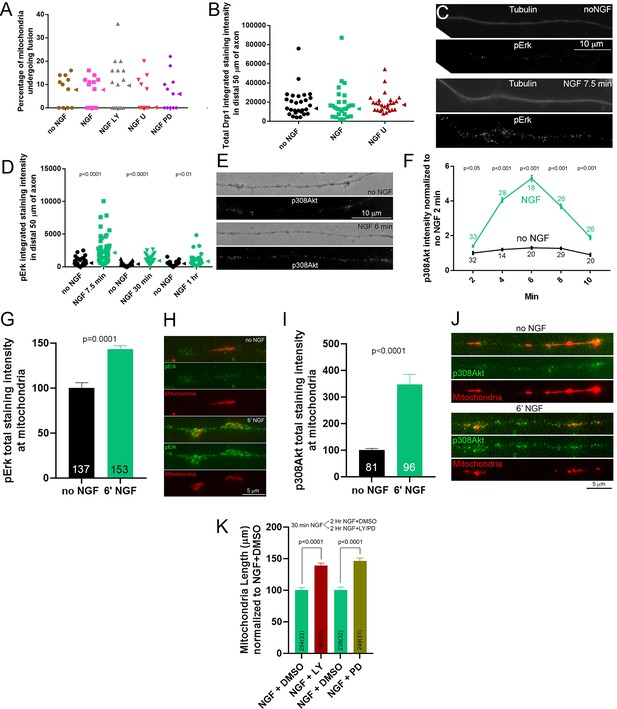
Signaling mechanism of NGF-induced fission.
(A) Inhibition of PI3K (LY294002, LY) or Mek-Erk (U0216, U; PD325901, PD) did not affect the rate of fusion (% mitochondria showing fusion/10 min). Kruskal-Wallis ANOVA p=0.79 and p=0.86 for LY and U/PD experiments, respectively. Median is denoted by arrowheads to the right of data points. (B) NGF treatment did not impact the levels of total Drp1 protein in axons and treatment with NGF and U did not alter levels relative to NGF alone. Kruskal-Wallis ANOVA p=0.63. Median is denoted by arrowheads to the right of data points. (C) Examples of pErk staining along axons counter-stained to show tubulin. For presentation purposes, all images in panel were equally digitally brightened to enhance visual appreciation of the signal. (D) Quantification of the total intensity of pErk staining along axons at 7.5 min post NGF treatment, when fission is occurring, and at 1 hr after NGF when the new steady state is established. Each datum reflects one axon, Mann-Whitney tests. Median is denoted by arrowheads to the right of data points. (E) Examples of axons stained with antibodies to Akt phosphorylated at T308. Phase contract image of axon is also shown. For presentation purposes, all images showing fluorescence in panel were equally digitally brightened to enhance visual appreciation of the signal. (F) Graph showing the staining intensity along the distal 50 μm of axons stained with antibodies to Akt phosphorylated at T308 normalized to the intensity for the 2 min no NGF treatment. n = number of axons shown above each time point. Results of analysis performed on raw data using Dunn’s posthoc time-matched multiple comparisons between NGF and no NGF groups are shown. (G) Graph of the total intensity of pErk in segments of axons defined by the presence of mitochondria in no NGF treatment and 6 min NGF treatment groups. n in bars represents mitochondria from 37 and 38 axons, respectively. Welch t-test. (H) Examples of pErk staining along axons expressing DsRed-mito in no NGF and at 6 min of NGF treatment groups. (I) Graph of the total intensity of p308Akt in segments of axons defined by the presence of mitochondria in no NGF treatment and 6 min NGF treatment groups. n in bars represents mitochondria from 32 and 34 axons, respectively. Welch t-test. (J) Examples of p308Akt staining along axons expressing DsRed-mito in no NGF and at 6 min of NGF treatment groups. (K) Graph showing the effects of inhibiting PI3K (LY) or Mek-Erk (PD) on mitochondria length during the NGF-induced steady state. Following a 30 min NGF treatment cultures were treated with either LY or PD for an additional 2 hr in the continued presence of NGF. Mann-Whitney tests.
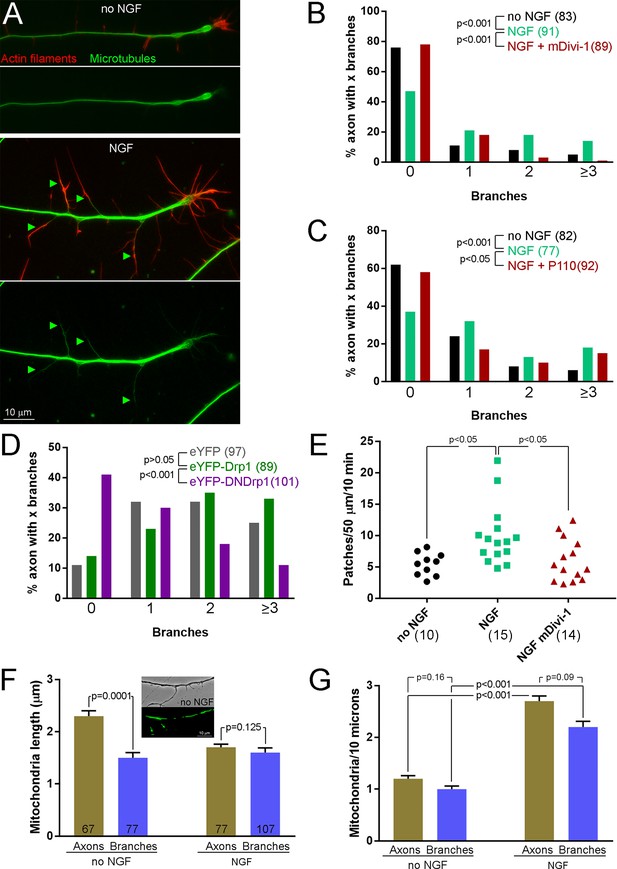
Inhibition of fission impairs NGF-induced axon branching.
(A) Example of NGF-induced axon branching. Top panels show axonal morphology in the no NGF treatment group. Some axonal filopodia are present but not branches. Bottom panels show an NGF-treated axons with four branches (green arrowheads). Mature branches contain microtubules and distally actin filaments. (B) Quantification of axon branches ± mDivi-1 or DMSO pretreatment. For panels (B)-(D) n = axon numbers shown in (). Mann-Whitney test. (C) Quantification of axon branches ± P110 or vehicle pretreatment. Mann-Whitney test. (D) Quantification of axon branches in dissociated neurons cultured overnight in NGF expressing eYFP (baseline control), eYFP-Drp1 or eYFP-DNDrp1. The differences in baseline branch number reflect the use of dissociated neurons relative to explant cultures as in panels (B) and (C) for the acute NGF treatment experiments. Mann-Whitney test. (E) Quantification of the rates of actin patch formation ±mDivi-1 or DMSO treatment. Each data point reflects one axon and the number of axons is shown in () below the data. Dunn’s posthoc multiple comparison tests. (F) and (G) Quantification of mitochondria length and density, respectively, in the axons and branches of axons emanating from explants raised in either no NGF or NGF overnight. n = axons shown in the bars labeled Axons; n for branches from this set of axons is denoted in the bars labeled Branches. Mean and SEM. Mann-Whitney tests.
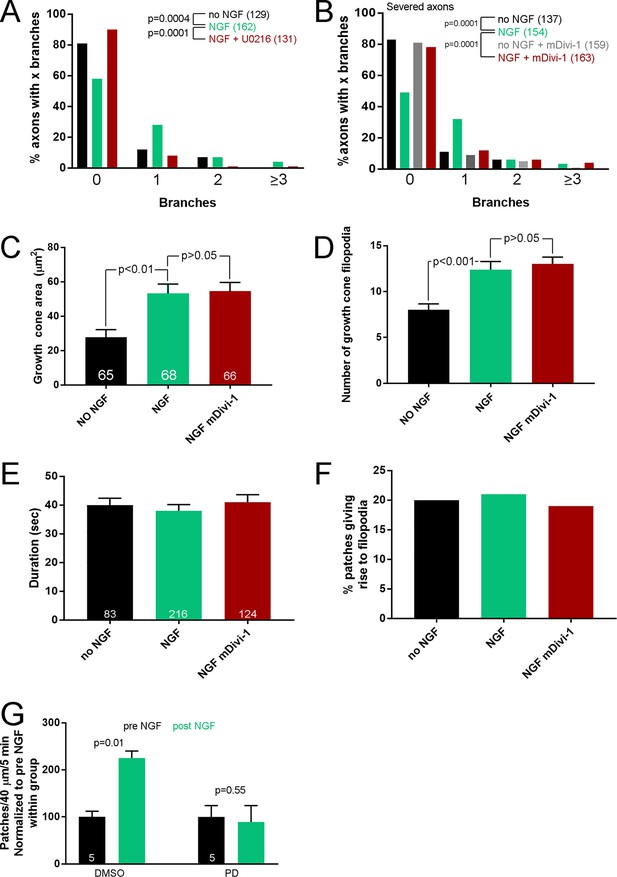
Role of fission in branching.
(A) Pretreatment (15 min) with the Mek-Erk inhibitor PD325901 (PD) blocks NGF-induced axons branching (40 min treatment). Mann-Whitney tests. (B) Severing of axons to isolate them from the cell bodies does not prevent the inhibition of NGF-induced axon branching (40 min treatment) by a 15 min pretreatment with mDivi-1 started immediately after axon severing. (C) A 15 min pretreatment with mDivi-1 does not impact the increase in growth cones area induced by a 10 min treatment with NGF. n = growth cones, one growth cone per axon, shown in the bars. Bonferroni multiple comparison tests. (D) Pretreatment with mDivi-1 similarly does not affect the NGF-induced increase in the number of filopodia at growth cones. Same sets of growth cones as in (B). Dunn’s multiple comparison tests. (E) mDivi-1 did not impact the duration of actin patches formed 30 min after NGF treatment. n = number of patches shown in bars from the axons shown in Figure 5E. Bonferroni multiple comparison tests. (F) mDivi-1 did not alter the percentage of patches that gave rise to filopodia. Same data set as in panel (D), Fischer’s exact tests. Fisher’s exact tests. (G) Analysis of actin patch formation rates in a pre-post NGF treatment design within axons pretreated with DMSO or PD for 15 min prior to sampling the pre-NGF rates. n = axons shown in bars, paired t-tests. Comparison of the pre NGF rates did not show an effect of PD relative to DMSO (p=0.87, Welch t-test).
In vivo/ovo expression of DNDrp1 impair the developmental branching of sensory axons.
(A) Examples of DsRed-mito labeled mitochondria in eYFP, eYFP-Drp1 or eYFP-DNDrp1 co-expressing axon in the acutely explanted living spinal cord of an E7 embryo. (B) Quantification of the length and density of mitochondria in axons in the living spinal cord as shown in panel (A). Number of mitochondria and axons are shown above the bars for length and density measurements respectively. 3–4 spinal cords/group. The sample sizes shown over the bars for density reflect the number of axons sampled. Dunn’s posthoc multiple comparison tests. (C) Examples of the morphology in sensory axons extending into the E7 embryonic spinal cord. The images are maximum intensity projections from Z-stacks. Control axons expressing only mCherry, to visualize morphology, were similar to those expressing mCherry and wild type Drp1. In contrast, the axons expressing mCherry and DNDrp1 exhibited simplified morphologies. For the groups expressing Drp1 constructs the insets show the signal from the eYFP label. (D) Quantification of the number of branches (axonal projections longer than 10 μm) per unit length of axon in the living spinal cord. Each data point reflects one axon sampled from 3 to 4 spinal cords/group. Dunn’s multiple comparison tests. Median is denoted by arrowheads to the right of data points.
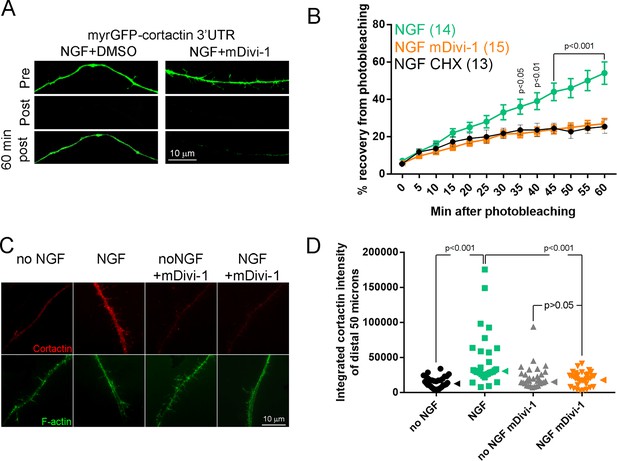
Inhibition of fission impairs the NGF-induced translation of axonally targeted cortactin mRNA.
(A) Example of FRAP experiments showing pre-photobleaching, just post-photobleaching and 60 min post-photobleaching images. The NGF treated axon recovers fluorescence more extensively than the mDivi-1 pretreated axon. (B) Graph showing the quantification of the relative FRAP (% recovery from time 0 value to pre-photobleaching value) in NGF+DMSO and NGF+mDivi-1 treatment groups (n = axons). Differences between NGF+DMSO and NGF+mDivi-1 groups are detected starting at 35 min using Bonferroni posthoc time-matched multiple comparison tests. The NGF+CHX (35 μM cycloheximide 30 min pretreatment before NGF treatment as in Spillane et al., 2012) shows the extent of recovery due to translation independent diffusion or transport of the myrGFP reporter from the axon proximal to the region of bleaching. The recovery in the NGF+CHX and NGF+mDivi-1 groups was not different at any time point using Bonferroni posthoc time-matched multiple comparison tests. (C) Examples of the staining levels of cortactin in axons. A 30 min treatment with NGF increases cortactin levels and the increase is impaired by pretreatment with mDivi-1. Pretreatment with CHX blocks the increase in axonal cortactin levels induced by NGF (Spillane et al., 2012). (D) Quantification of the levels of cortactin in distal axons (total integrated staining intensity). Each datum represents one axon. Dunn’s posthoc multiple comparison tests. Median is denoted by arrowheads to the right of data points.
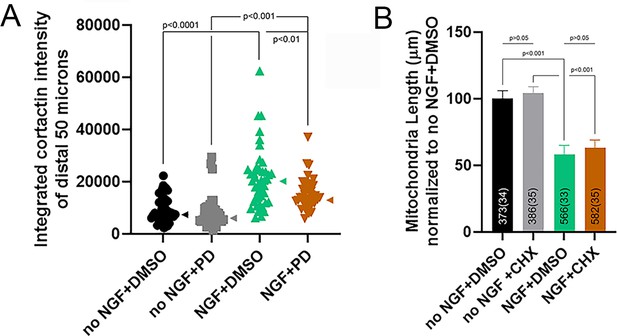
Effects of Mek-Erk inhibition on cortactin translation and inhibition of translation of NGF-induced fission.
(A) Pretreatment with PD attenuates the NGF-induced increase in the axonal protein levels of cortactin, determined as in Figure 7C,D. Dunn’s multiple comparison tests. (B) Pretreatment with cycloheximide (CHX; as in Figure 7B) does not alter the shortening of mitochondria in response to NGF treatment (30 min). Dunn’s multiple comparison tests.
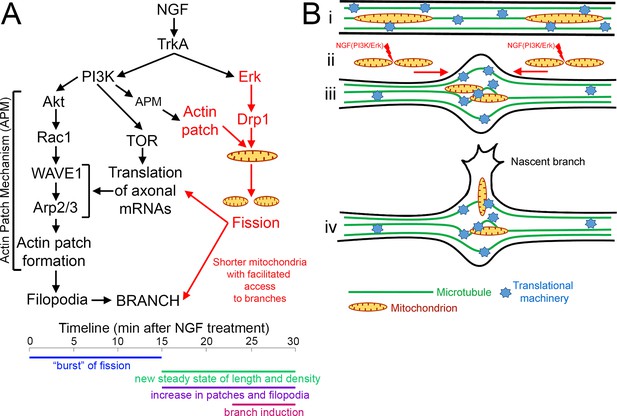
Summary and hypothetical mechanism for the role of fission in allowing mitochondria to target to sites of NGF-induced sensory axon collateral branching.
(A) Schematic summary of previously established components of the mechanism of NGF-induced axon branching (black text; modified from Spillane et al., 2012) and the role of NGF-induced mitochondria fission elucidated herein (red text). The formation of actin patches is dependent on a PI3K-Rac1-Arp2/3 based signaling and cytoskeletal mechanism (actin patch mechanism, APM). This mechanism driving formation of actin patches is dependent on PI3K-Akt signaling both in the context of NGF-signaling and in the absence of NGF (Ketschek and Gallo, 2010). NGF increases the rate of actin patch formation, in part, by driving the intra-axonal translation of relevant actin regulatory molecules (cortactin, Arp2, WAVE1; Spillane et al., 2012) required for patch formation. Herein, we show that NGF also induces the fission of axonal mitochondria during the first 10 min of signaling, prior to branching and the axonal translation-dependent increase in actin patch formation. The fission requires NGF-induced activation of Drp1 through Erk signaling. The fission also requires actin filaments that present as patches at the site of fission. Based on the requirement for actin filaments, PI3K-Akt signaling and the Arp2/3 complex for the formation of actin patches and fission, we suggest the role of PI3K in fission is mediated through the previously described APM also regulating the actin patch component of the fission machinery. The ensuing fission results in mitochondria with lengths that facilitate their targeting into nascent branches. The fission is also required for the NGF-induced and mitochondria-dependent intra-axonal translation of cortactin (Spillane et al., 2013). The hypothetical mechanism by which fission may contribute to axonal translation is presented in the panel B. The approximate time line of the events described above is presented below. The NGF-induced burst of fission occurs during the first 10–15 min of signaling, after which the new steady state of length and density is established along axons. The fission precedes the subsequent axonal translation dependent increase in the formation of actin patches and filopodia (Ketschek and Gallo, 2010; Spillane et al., 2012; Spillane et al., 2013). The emerge of new branches from the axon follows becoming evident by after 20 min of NGF treatment (Spillane et al., 2012). (B) Graphical representation of the hypothetical contribution of fission to the establishment of localized sites of intra-axonal protein synthesis during NGF-induced axon collateral branching. This hypothetical model is derived from the synthesis of from multiple prior studies showing the accumulation of organelles and translational machinery at sites of branching (Yu et al., 1994; Spillane et al., 2013; Courchet et al., 2013; Wong et al., 2017), the correlation of sites of branching with localized microtubule splaying (Dent and Kalil, 2001; Ketschek et al., 2015) and the observation that NGF induces microtubule splaying by 5 min post treatment (Ketschek et al., 2015), the time when mitochondria are undergoing fission and redistribution within the axon. (i) before NGF treatment mitochondria are longer, and translational machinery is undergoing transport and stalling throughout the axon in an unregulated manner. (ii) Signaling through PI3K and Erk is required for the fission of axonal mitochondria in response to NGF, and the fission correlates with transport mediated redistribution of mitochondria within the axon. (iii) as mitochondria undergo redistribution they encountered segments of the axon within which microtubules have undergone splaying apart and correlate with sites of potential branching. Through an unspecified mechanism transport cargoes undergo stalling in the axon segments where microtubules have splayed and accumulate resulting in the mitochondria and translational machinery. This converge then assists in the establishment of axon segments with high translation potential that correlate with sites of potential axon branching. (iv) Axon branches can then arise from these specialized domains through a multifaceted process involving the regulation of the actin cytoskeleton, microtubules, intra-axonal protein synthesis and directed transport into branches. The shorter length of mitochondria following fission is permissive for the entry of mitochondria into nascent branches.
Tables
Data set used for estimation of the probability of random overlap between actin patches and sites of fission along mitochondria.
| Mitochondria length (µm) | Summed length of actin patches present on mitochondrion at the time of fission (µm) | Proportion of mitochondria length occupied by actin patches (B/A) |
|---|---|---|
| 7.17 | 0.47 | 0.07 |
| 6.90 | 0.69 | 0.10 |
| 12.00 | 1.1 | 0.09 |
| 8.15 | 0.69 | 0.08 |
| 16.78 | 3.69 | 0.22 |
| 7.35 | 0.53 | 0.07 |
| 3.94 | 0.65 | 0.16 |
| 10.24 | 0.75 | 0.07 |
| 9.65 | 0.75 | 0.08 |
| 4.10 | 0.69 | 0.17 |
| 7.48 | 0.75 | 0.10 |
| 6.65 | 0.69 | 0.10 |
| 16.78 | 0.23 | 0.01 |
| 5.87 | 0.69 | 0.12 |
| 4.70 | 0.50 | 0.11 |
| 8.30 | 0.75 | 0.09 |
| 6.90 | 0.80 | 0.12 |
| 7.70 | 0.78 | 0.10 |
| 11.30 | 0.86 | 0.08 |
| 4.40 | 0.56 | 0.13 |





