CXCL12-induced rescue of cortical dendritic spines and cognitive flexibility
Figures
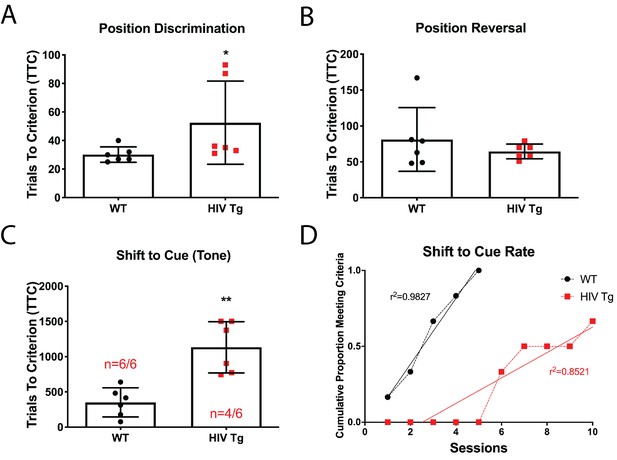
Male adult HIV-Tg rats are deficient in strategy shifting and initial rule discrimination, but not reversal learning.
(A) HIV-Tg rats performed worse on the position discrimination task compared to age- and sex-matched WT animals. N = 6 animals/group, *p<0.05. (B) No significant differences were observed between WT and HIV-Tg rats on position reversal. N = 6 animals/group (C) Fewer HIV-Tg rats reached criterion on the shift to cue phase and took significantly greater number of trials to reach criterion. N = 6 animals/group, **p<0.01. (D) HIV-Tg rats took significantly longer to reach criterion as assessed via linear regression. N = 6 animals/group, (F(1,11)=15.94, p=0.0021).
-
Figure 1—source data 1
HIV-Tg behavior raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig1-data1-v2.xlsx
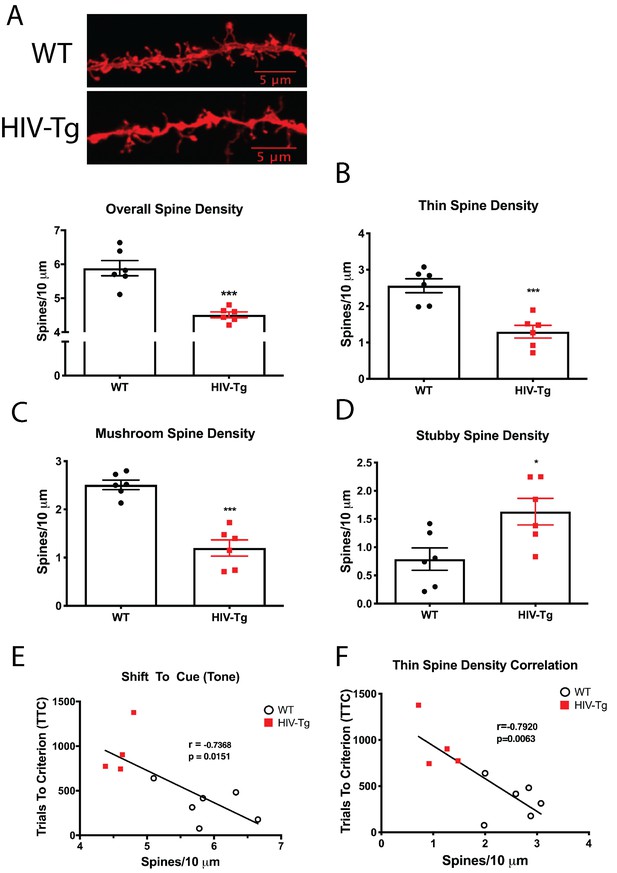
Layer II/III pyramidal neurons in the PrL region of the mPFC of HIV-Tg rats have alterations in spine density and morphology.
(A) Dendritic spine density and morphology were assessed using Neurolucida 360 software. Male HIV-Tg rats had a reduction in overall dendritic spine density in layer II/III pyramidal neurons in the mPFC. N = 6 animals/group, 8 dendrites measured for each animal and averaged into single data point. ***p<0.001. (B) On the same set of dendrites, thin spine density was significantly decreased in male HIV-Tg rats compared to sex-matched WT controls. N = 6 animals/group. ***p<0.001. (C) As seen with overall and thin spine density, HIV-Tg rats had reduced mushroom spine density compared to WT controls. N = 6 animals/group. ***p<0.001. (D) In contrast, stubby spine density was significantly elevated in male HIV-Tg rats. N = 6 animals/group. *p<0.05. (E) Across both groups, overall dendritic spine density in layer II/III pyramidal neurons in the mPFC were negatively associated with trials to criterion in the shift to cue phase. N = 12 animals; Pearson’s r = −0.7368, p=0.0151. (F) When only thin spine density was considered, the relationship with shift to cue trials to criterion became even stronger. N = 12 animals; Pearson’s r = −0.7920, p=0.0063.
-
Figure 2—source data 1
HIV-Tg dendritic spines raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig2-data1-v2.xlsx
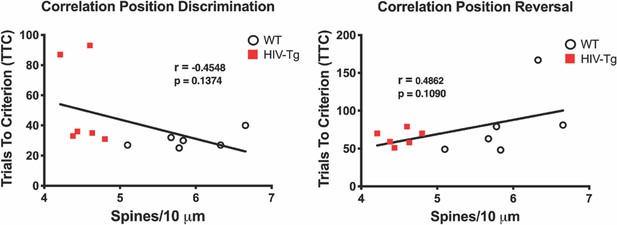
There was no relationship between overall dendritic spine density and the first two phases of the behavioral task.
As expected, there was no observed correlation between dendritic spine density and either position discrimination or position reversal.
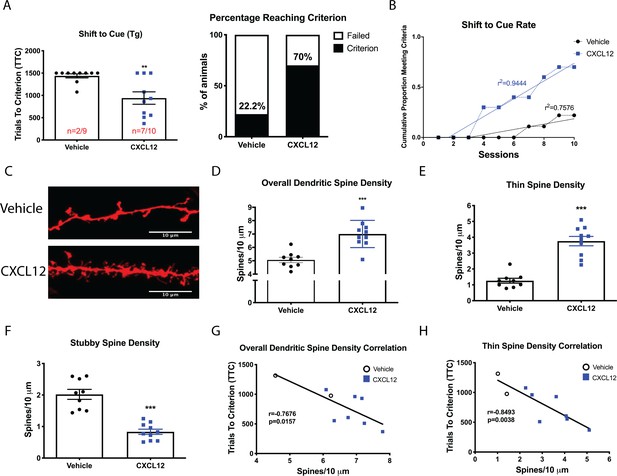
Once daily CXCL12 treatment significantly enhanced set-shifting abilities of HIV-Tg rats and completely restored dendritic spine density and morphology in the mPFC.
(A) A greater percentage of CXCL12-treated rats reached criterion on the shift to cue phase and also took significantly fewer trials to complete the task. N = 9 animals/vehicle and 10 animals/CXCL12, p=0.03. (B) The rates at which the groups reached criterion were significantly different, with CXCL12-treated animals learning at a faster rate. N = 9 animals/vehicle and 10 animals/CXCL12, F(1,16)=44.3781, p<0.001. (C) Representative image of DiOlistically labeled mPFC slices from HIV-Tg rats treated with either vehicle or CXCL12. (D) Adult male HIV-Tg rats treated with CXCL12 had significantly higher overall dendritic spine density in layer II/III pyramidal neurons of the mPFC. N = 9 animals/vehicle and 10 animals/CXCL12, 8 dendrites measured for each animal and averaged into single data point, ***p<0.001 (E) Further analysis on spine morphology revealed a specific increase in the number of thin spines. N = 9 animals/vehicle and 10 animals/CXCL12; ***p<0.001. (F) CXCL12 treatment resulted in a significant reduction in stubby spine density in HIV-Tg rats. N = 9 animals/vehicle and 10 animals/CXCL12; ***p<0.001 (G) As previously observed, overall dendritic spine density was significantly negatively associated with trials to criterion on the set-shifting phase. N = 19 animals, Pearson’s r = −0.7676, p=0.0157. (H) This relationship became even stronger when only thin spines were correlated with the number of trials required to complete the task. N = 19 animals, Pearson’s r = −0.8493, p=0.0038.
-
Figure 3—source data 1
WT and HIV-Tg ±CXCL12 raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig3-data1-v2.xlsx
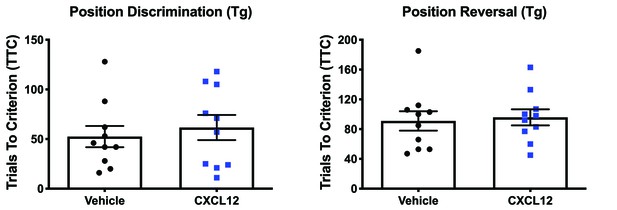
Vehicle and CXCL12-treated HIV-Tg rats performed similarly during position discrimination and position reversal.
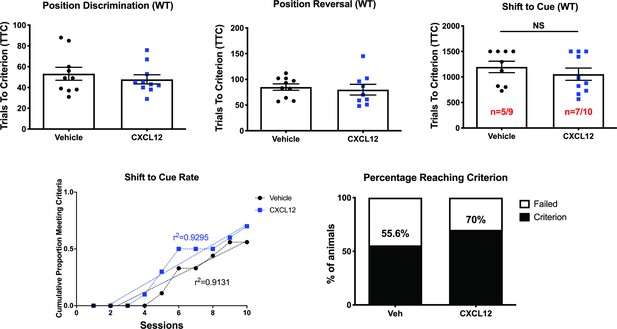
Vehicle and CXCL12-treated male WT rats performed equally in the attentional set-shifting task.
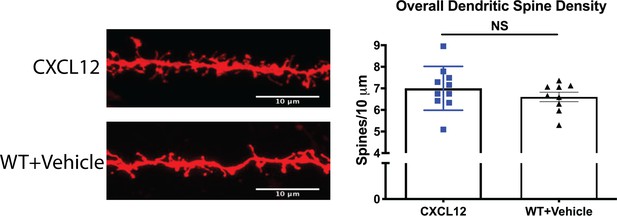
CXCL12-treated HIV-Tg and vehicle-treated WT rats had no difference in overall dendritic spine density.
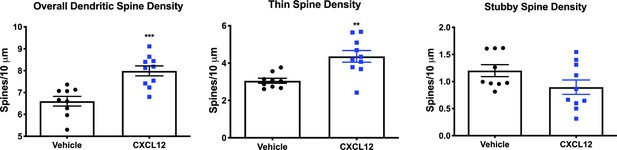
CXCL12-treated WT rats display higher overall and thin dendritic spine density in the mPFC.
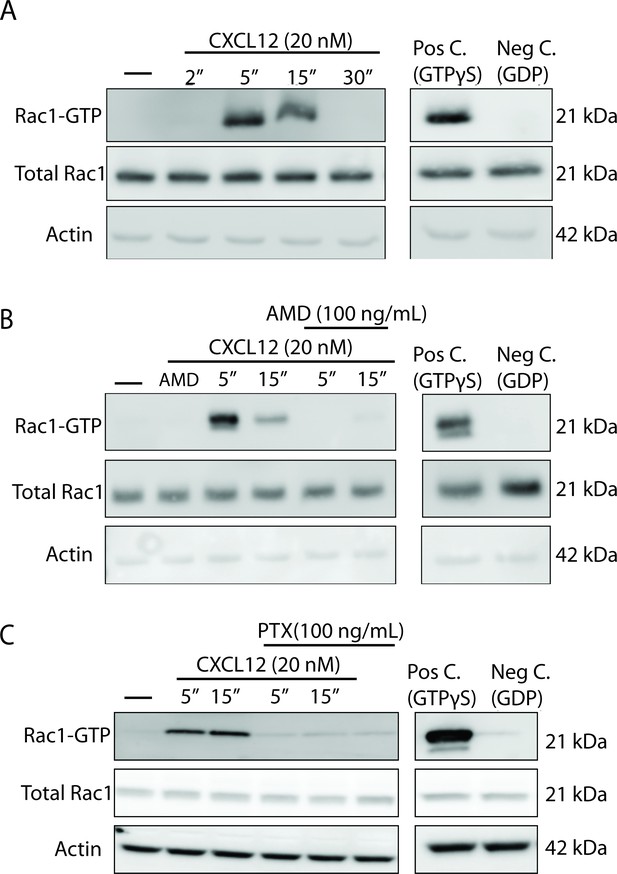
CXCL12 activates the small GTPase Rac1 in cultured cortical neurons in a CXCR4- and Gαi-dependent manner.
(A) Cultured cortical neurons (21 DIV) were treated with CXCL12 (20 nM) for the indicated time points and subjected to pulldown using PAK agarose beads. Immunoblotting revealed a significant activation of Rac1 at 5 and 15 min and a return to baseline at 30 min. Positive and negative controls were performed by incubating lysates with GTPγS and GDP, respectively. N = 3 (B) Pretreatment with the CXCR4 antagonist, AMD3100 (100 ng/mL; 20 min) attenuated CXCL12-induced activation of Rac1. Positive and negative controls were performed by incubating lysates with GTPγS and GDP, respectively. N = 3 (C) Pretreatment with the Gαi inhibitor pertussis toxin (PTX, 100 ng/mL; 18 hr) completely prevented activation of Rac1 by CXCL12. Positive and negative controls were performed by incubating lysates with GTPγS and GDP, respectively. N = 3.
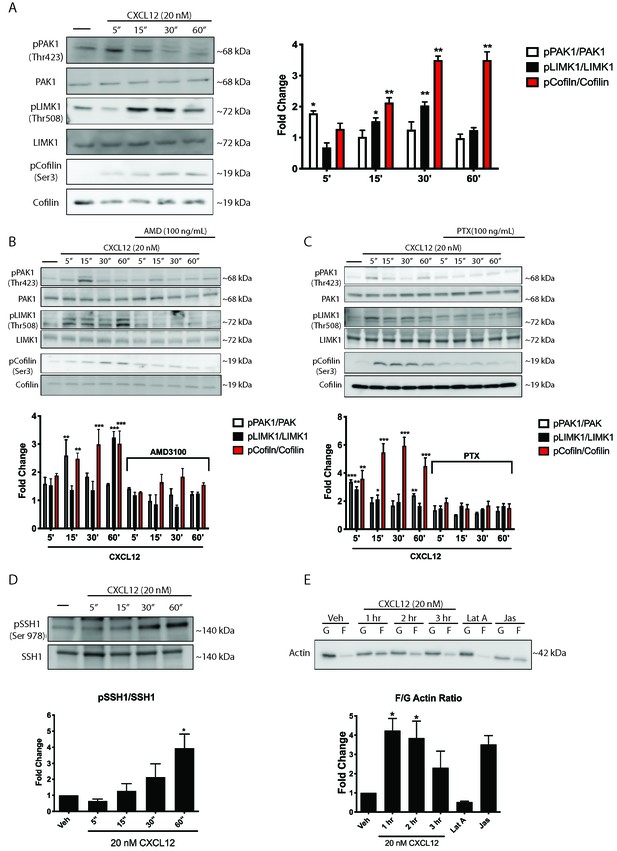
CXCL12 phosphorylates downstream mediators of the Rac1 pathway and results in changes to actin polymerization in cortical neurons.
(A) Cultured cortical neurons (21 DIV) were exposed to CXCL12 (20 nM) for the indicated time points and the phosphorylation status of PAK1, LIMK1, and cofilin was examined. CXCL12 treatment resulted in a time-dependent increase in the phosphorylation of all three proteins. N = 3, *p<0.05, **p<0.01. (B) Pretreatment with AMD3100 (100 ng/mL; 20 min) blocked CXCL12-induced phosphorylation of Rac1 downstream mediators. N = 3, **p<0.01, ***p<0.001. (C) Inhibition of Gαi signaling (via PTX) blocked the ability of CXCL12 to phosphorylate Rac1 downstream mediators. N = 3, *p<0.05, **p<0.01, ***p<0.001. (D) Following treatment with CXCL12, the protein phosphatase SSH1 is phosphorylated (and inactivated) in a time-dependent manner. N = 3, *p<0.05. (E) Separation of F and G-actin in cortical neurons revealed a shift in favor of F-actin following CXCL12 treatment. Latrunculin A (5 μM, 2 hr), a potent actin polymerization inhibitor, and jaspakinolide (5 μM, 2 hr), an inducer of actin polymerization, were used as internal controls for the assay. N = 3; *p<0.05.
-
Figure 5—source data 1
Densitometry statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig5-data1-v2.xlsx
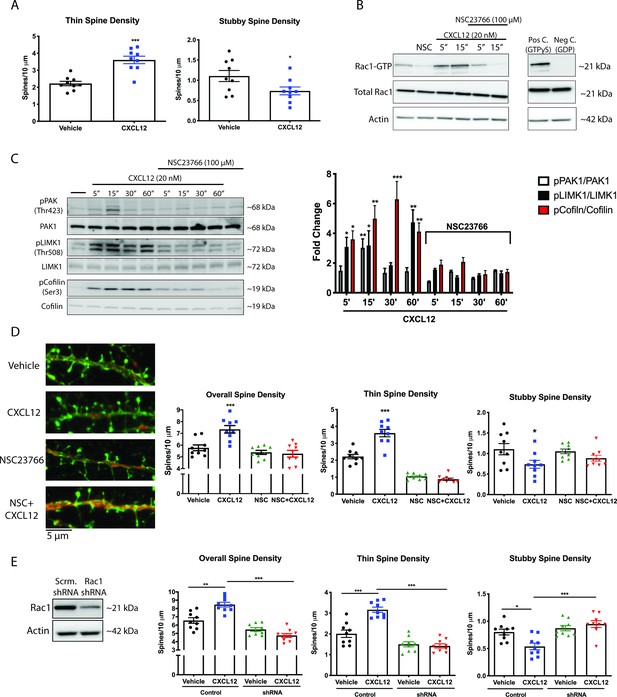
CXCL12 specifically modulates thin spine density via activation of Rac1.
(A) Cultured cortical neurons (21 DIV) were treated with CXCL12 (20 nM, 3 hr), resulting in a specific increase in thin spine density and a decrease in stubby spine numbers. N = 9 coverslips/group, 4 dendrites measured/coverslip and averaged into single data point, 3 separate experiments, *p<0.05, ***p<0.001. (B) Pretreatment with the specific Rac1 inhibitor NSC23766 (100 μM, 15 min) completely blocked CXCL12-induced activation of Rac1 in cortical neurons. N = 3 (C) Subsequently, inhibition of Rac1 activation by NSC23766 prevented phosphorylation of downstream mediators by CXCL12. N = 3, *p<0.05, **p<0.01, ***p<0.001. (D) Inhibition of Rac1 activation by NSC23766 blocked the ability of CXCL12 to modulate overall dendritic spine density, as well as thin and stubby spine density. N = 9 coverslips/group, 4 dendrites measured/coverslip and averaged into single data point, 3 separate experiments, *p<0.05, ***p<0.001. (E) Cortical neurons (18 DIV) were infected with control or Rac1-shRNA viral particles and GFP-positive neurons were analyzed (21 DIV) following treatment with either vehicle or CXCL12. Knockdown of Rac1 inhibited CXCL12-mediated alterations in spine density and morphology. N = 9 coverslips/group, 4 dendrites measured/coverslip and averaged into single data point, 3 separate experiments, *p<0.05, **p<0.01, ***p<0.001.
-
Figure 6—source data 1
In vitro raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig6-data1-v2.xlsx
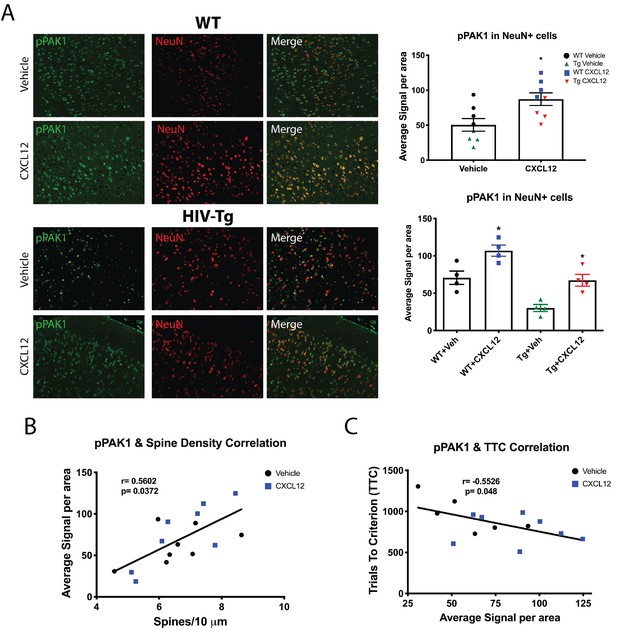
CXCL12 administration increases phosphorylation of PAK1 in layer II/III pyramidal neurons in the mPFC of male WT and HIV-Tg rats.
(A) Sections from the contralateral hemisphere used for dendritic spine analysis were subjected to immunohistochemical and multispectral analysis. Both adult male WT and HIV-Tg rats treated with CXCL12 had a significant increase in pPAK1 levels in NeuN+ cells in the mPFC. N = 4/group, *p<0.05 for WT+Veh vs WT+CXCL12, *p<0.05 for WT+CXCL12 vs Tg+CXCL12, and *p<0.05 for Tg+Veh vs Tg+CXCL12. (B) Phosphorylation of PAK1 in the mPFC was positively associated with overall dendritic spine density in the same set of animals. N = 14 animals, Pearson’s r = 0.5602, p=0.0372. (C) Levels of phosphorylated PAK1 are negatively correlated with the number of trials to criterion on the set shift phase. N = 14 animals, Pearson’s r = −0.5526, p=0.048.
-
Figure 7—source data 1
Tyramide IHC raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig7-data1-v2.xlsx
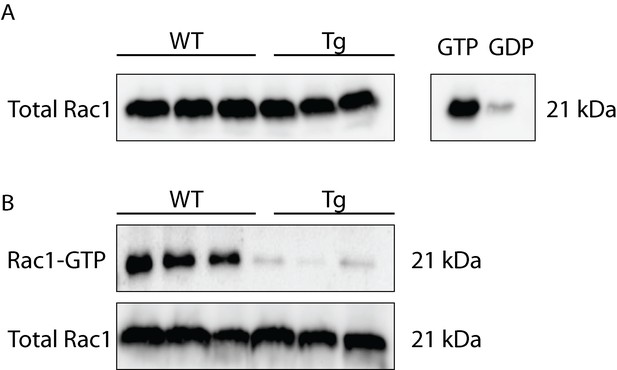
HIV-Tg rats show reduced levels of activated Rac1 and no changes in total Rac1 protein expression in frontal cortex lysates.
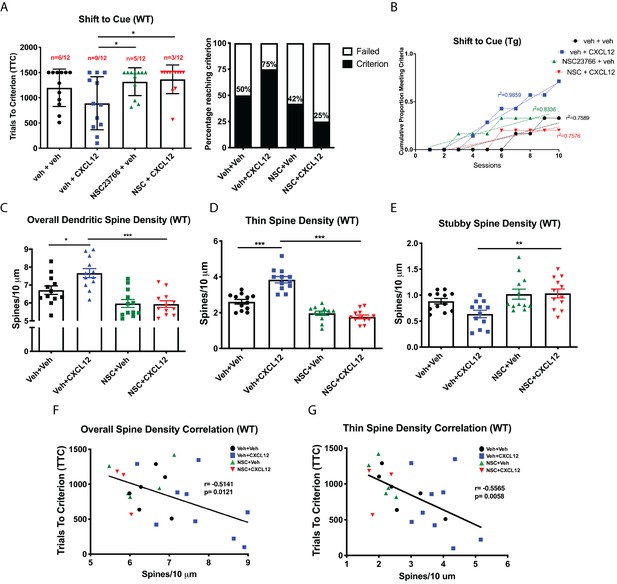
Blockade of Rac1 activation prevents alterations in cognitive flexibility and dendritic spines by CXCL12.
(A) Treatment with NSC23766 mitigated the ability of CXCL12 to increase the number of animals reaching criterion on the shift to cue phase. N = 12 animals/group, p=0.0498. (B) The rate at which animals reached criterion was significantly delayed with NSC23766 pretreatment as assessed via linear regression. N = 12 animals/group, F(3,32)=12.6622, p<0.001. (C) Blockade of Rac1 activation by NSC23766 prevents CXCL12-mediated upregulation of overall dendritic spine density in the mPFC. N = 12 animals/group, 8 dendrites measured for each animal and averaged into single data point, **p<0.01. (D) Additionally, NSC23766 prevented changes in thin spine density induced by CXCL12. N = 12 animals/group, 8 dendrites measured for each animal and averaged into single data point, ***p<0.001. (E) The ability of CXCL12 to decrease stubby spine density was blocked by NSC23766. N = 12 animals/group, 8 dendrites measured for each animal and averaged into single data point, **p<0.01. (F) As previously observed, overall dendritic spine density was negatively associated with trials to criterion on the set-shifting phase of the behavioral task. N = 23 animals, Pearson’s r = −0.7862, p=0.0127. (G) This relationship became even stronger when only thin spine density was considered. N = 23 animals, Pearson’s r = −0.8350, p=0.0051.
-
Figure 8—source data 1
WT ±CXCL12/NSC23766 raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig8-data1-v2.xlsx

NSC23766 inhibits activation of Rac1 in vivo.
In a pilot study, animals were treated with either 1ug/uL or 2ug/uL of NSC23766 had significant reductions in Rac1-GTP compared to vehicle treated animals. Sections from the contralateral hemisphere used for the Rac1-GTP assay were subjected to immunohistochemical and multispectral analysis. NSC23766 reduced phosphorylation of PAK1 in a dose-dependent manner. n=2 animals/group; for Western blot, each lane represents one animal; for IHC, 6 images were taken per animal and averaged into a single data point.
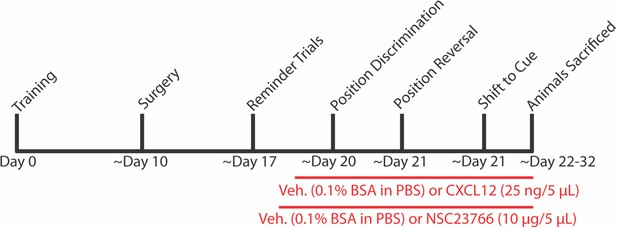
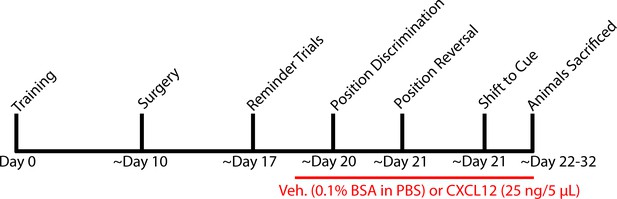
Experimental timeline for behavior and ICV infusions in Figures 8 and 9.
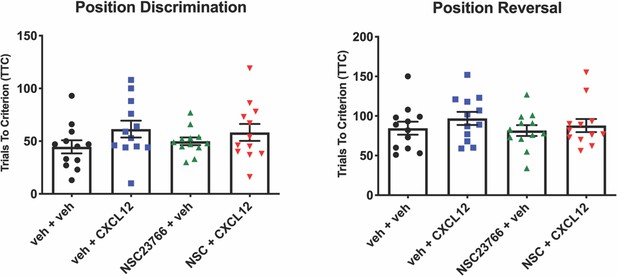
NSC23766 treatment did not alter performance on position discrimination or position reversal in WT rats.
There were no significant differences among all groups during the first two phases of the behavioral task. N=12 animals/group.
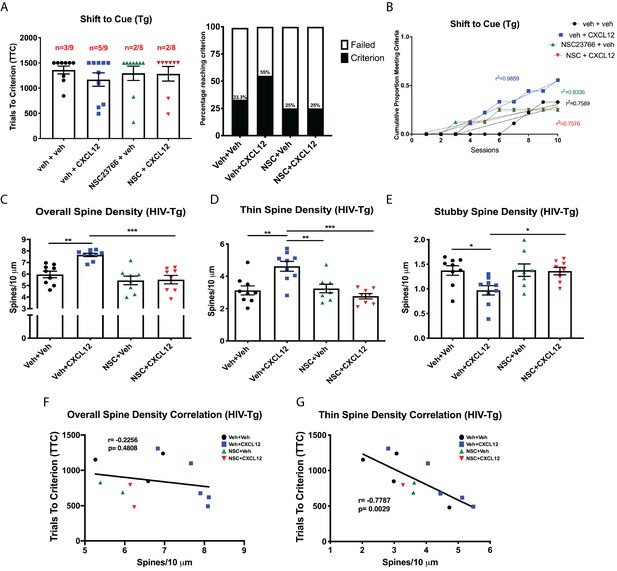
CXCL12 depends on Rac1 activation to rescue cognitive flexibility and dendritic spine density in the HIV-Tg rat.
(A) Inhibition of Rac1 activity blocked the ability of CXCL12 to positively enhance cognitive flexibility in an attentional set-shifting task. N = 9 for veh+veh, N = 9 for veh+CXCL12, N = 8 for NSC+veh, and N = 8 for NSC+CXCL12. (B) NSC23766 co-treatment significantly attenuated the rate at which animals reached criterion on the shift to cue phase as assessed by linear regression. N = 9 for veh+veh, N = 9 for veh+CXCL12, N = 8 for NSC+veh, and N = 8 for NSC+CXCL12, F(3,32)=7.503, p=0.0006. (C) Blockade of Rac1 activation abrogated the effect of CXCL12 on overall dendritic spine density in HIV-Tg rats. N = 9 for veh+veh, N = 9 for veh+CXCL12, N = 8 for NSC+veh, and N = 8 for NSC+CXCL12, eight dendrites measured for each animal and averaged into single data point, **p<0.01, ***p<0.001. (D) CXCL12’s impact on thin spines was mitigated by co-treatment with NSC23766. N = 9 for veh+veh, N = 9 for veh+CXCL12, N = 8 for NSC+veh, and N = 8 for NSC+CXCL12, eight dendrites measured for each animal and averaged into single data point, **p<0.01, ***p<0.001. (E) NSC23766 prevented decreases in stubby spine density mediated by CXCL12 treatment. N = 9 for veh+veh, N = 9 for veh+CXCL12, N = 8 for NSC+veh, and N = 8 for NSC+CXCL12, eight dendrites measured for each animal and averaged into single data point, *p<0.05. (F) There was no significant relationship observed between overall dendritic spine density and trials to criterion on the set-shifting phase of the behavioral task. N = 12 animals, Pearson’s r = −0.2256, p=0.4808. (G) Thin spine density was negatively associated with the number of trials to criterion in the shift to cue phase of the behavioral task. N = 12 animals, Pearson’s r = −0.7787, p=0.0029.
-
Figure 9—source data 1
HIV-Tg ±CXCL12/NSC23766 raw data and statistical analysis.
- https://cdn.elifesciences.org/articles/49717/elife-49717-fig9-data1-v2.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (R. Norvegicus) | F344/NHsd (Male) | Envigo and University of Maryland | RRID:RGD_61109 | |
| Strain, strain background (R. Norvegicus) | HSD:HIV-1 (F344) (Male) | Envigo and University of Maryland | ||
| Cell line (H. sapiens) | HEK293T | ATCC | RRID:CVCL_0063 | |
| Transfected construct (R. Norvegicus) | Rac1 shRNA | Origene Technologies | Cat # TL712781 | |
| Recombinant DNA reagent | pCMVR8.74 | AddGene | RRID:Addgene_22036 | |
| Recombinant DNA reagent | pMD2.G | AddGene | RRID:Addgene_12259 | |
| Antibody | Anti-pPAK1 rabbit polyclonal (Thr423) | Cell Signaling Technology | RRID:AB_330220 | 1:1000 |
| Antibody | Anti-PAK1 rabbit polyclonal | Cell Signaling Technology | RRID:AB_330222 | 1:1000 |
| Antibody | Anti-pLIMK1 rabbit polyclonal (Thr507/508) | EMD Millipore | RRID:AB_568901 | 1:1000 |
| Antibody | Anti-LIMK1 rabbit polyclonal | Cell Signaling Technology | RRID:AB_2281332 | 1:1000 |
| Antibody | Anti-pCofilin rabbit polyclonal (Ser3) | Cell Signaling Technology | RRID:AB_330238 | 1:1000 |
| Antibody | Anti-Cofilin rabbit monoclonal | Cell Signaling Technology | RRID:AB_10622000 | 1:1000 |
| Antibody | Anti-pSSH1L rabbit polyclonal (Ser978) | ECM Biosciences | RRID:AB_10553849 | 1:500 |
| Antibody | Anti-SSH1 rabbit monoclonal | Cell Signaling Technology | RRID:AB_2798263 | 1:1000 |
| Antibody | Anti-Rac1 | Cell Biolabs | Included in #STA-401–1 | 1:6000 |
| Antibody | Anti-β-actin rabbit polyclonal | Sigma-Aldrich | RRID:AB_476693 | 1:6000 |
| Antibody | Anti-MAP2 rabbit polyclonal | EMD Millipore | RRID:AB_91939 | 1:1000 |
| Antibody | Anti-pPAK1 rabbit polyclonal (Thr423) | Invitrogen | RRID:AB_2554427 | 1:25 |
| Antibody | Anti-NeuN rabbit monoclonal | Cell Signaling Technology | RRID:AB_2651140 | 1:400 |
| Antibody | Goat polyclonal anti-rabbit Alexa Fluor 568 | Invitrogen | RRID:AB_143157 | 1:250 |
| Peptide, recombinant protein | Rat CXCL12 | Peprotech | Cat# 400-32A | |
| Commercial assay or kit | Rac1 Activity Assay Kit | Cell Biolabs | Cat# STA-401–1 | |
| Chemical compound, drug | NSC23766 | Tocris | Cat# 2161 | |
| Software, algorithm | GraphPad Prism | GraphPad Software | RRID:SCR_015382 | Version 8.0 |
| Software, algorithm | Neurolucida 360 | MBF Biosciences | RRID:SCR_016788 | Version 2017.01.4 |
| Software, algorithm | Nuance Multispectral Imaging Systems | Nuance | RRID:SCR_015382 | Version 2.10 |
| Software, algorithm | Med-PC | Med Associates | RRID:SCR_012156 | Version 4.1 |
| Other | Helios Gene Gun | Bio-Rad | Cat# 1652411 | |
| Other | Phalloidin Alexa Fluor 488 | Invitrogen | Cat # A12379 | 1:400 |
| Other | Alexa Fluor conjugated tyramide 488 | Invitrogen | Cat # B40953 | |
| Other | Alexa Fluor conjugated tyramide 555 | Invitrogen | Cat# B40955 | |
| Other | DiI Stain | Invitrogen | Cat# D282 | |
| Other | Hoechst | Invitrogen | H3570 | 1 μg/mL |