Exploring structural dynamics of a membrane protein by combining bioorthogonal chemistry and cysteine mutagenesis
Figures
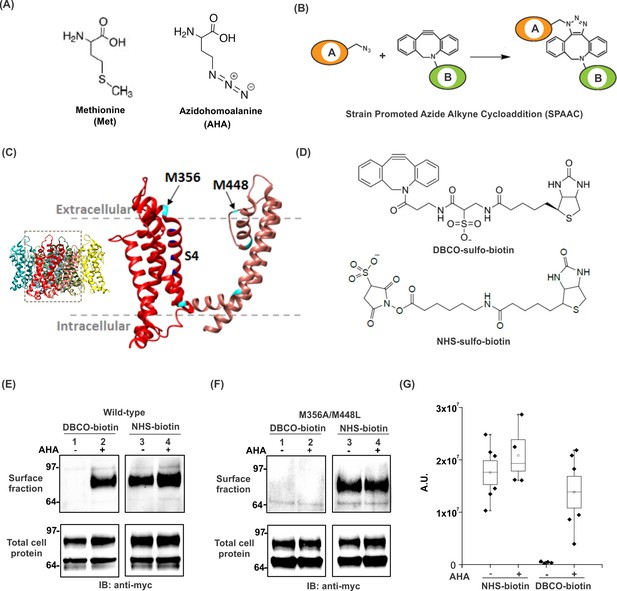
Incorporation and detection of azidohomoalanine into the Shaker Kv channel.
(A) Structures of Methionine and Azidohomoalanine. (B) A schematic for the strain promoted azide alkyne cycloaddition (SPAAC) reaction. (C) Transmembrane region of a single subunit of a Kv channel containing voltage sensing domain (red) and pore domain (pink). Methionine residues are colored in cyan. Inset shows tetrameric structure of the Kv1.2–2.1 paddle chimera crystal structure, 2R9R (Long et al., 2007). (D) Structures of biotin probes; DBCO-sulfo-biotin (top) and NHS-sulfo-biotin (bottom). (E–F) Anti-myc western blots for the surface fraction (top) and total cell protein (bottom) isolated from Xenopus laevis oocytes injected with ShakerΔ5-V478W-myc (Wild-type, E) or the mutant lacking the methionine residues facing the extracellular side (M356A/M448L, F). (G) Densitometry plots of anti-myc western blots for the surface fraction of the wild-type Shaker Kv channel in the absence or presence of AHA. A.U. refers to arbitrary units for absolute chemiluminescence intensity. Boxes represent SEM for n = 4–6. The small open squares and black horizontal lines represent the mean and weighted mean values, respectively, for the chemiluminescence intensity. Vertical black lines represent the full range of data.
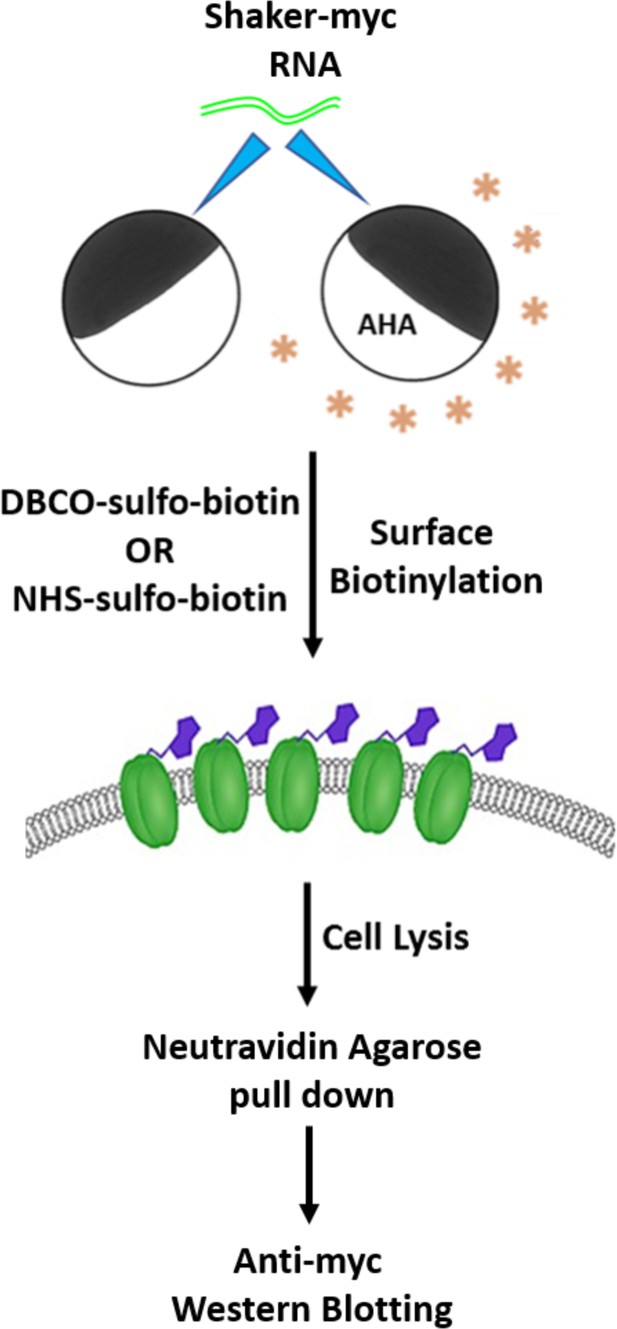
Scheme for detecting AHA incorporation into the Shaker Kv channel.
Xenopus laevis oocytes expressing the Shaker Kv channel are incubated in the absence or presence of 4 mM AHA and probed with azide or amine-reactive biotin probes (1 mM each) to label the cell surface proteins. Subsequently, oocytes are lysed, and biotinylated proteins are pulled down using NeutrAvidin agarose beads followed by anti-myc western blotting to detect the myc-tagged Shaker Kv channel containing AHA in place of methionine residues.
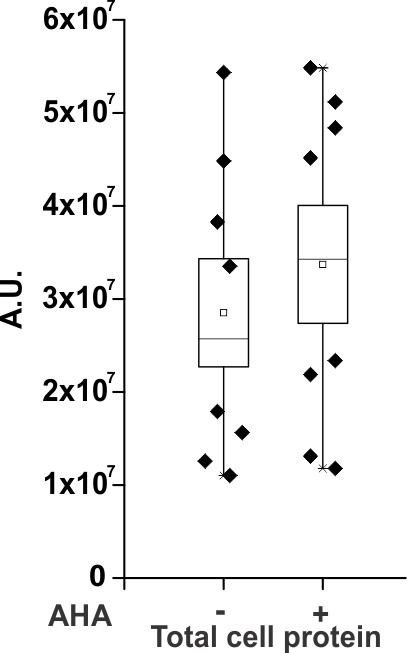
Densitometry plots of anti-myc western blots for the total cell protein obtained after cell lysis from oocytes injected with the ShakerΔ5-V478W-myc (wild-type, Figure 1E) in the absence and presence of AHA.
A.U. refers to arbitrary units for absolute chemiluminescence intensity. Box represents SEM for n = 8.
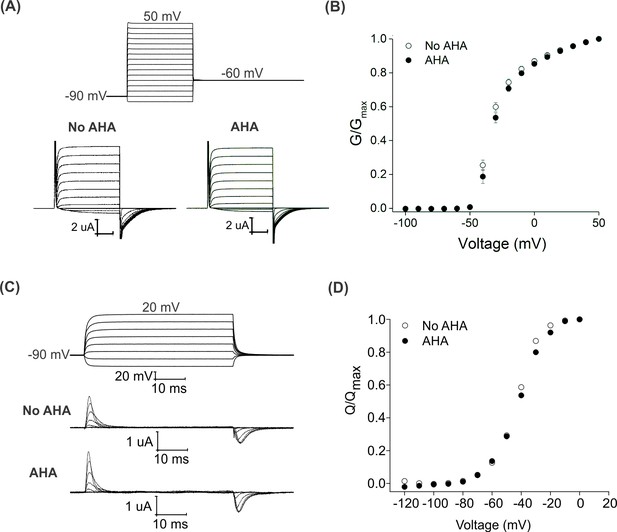
Effect of AHA on the gating behavior of the Shaker Kv channel.
(A) Ionic currents elicited by voltage steps for oocytes injected with Shaker-IR in the absence (left) or presence of AHA (right). Holding voltage = −90 mV, tail voltage = −60 mV. (B) G-V relationships obtained from tail currents at −60 mV in the absence (open circles) and presence of AHA (closed circles). All data points represent mean ± SEM (n = 3) (C) Gating currents obtained from Shaker-V478W, a non-conducting mutant of Shaker, in the absence (top) or presence of AHA (bottom). (D) Q-V relationships obtained from the gating currents elicited after stepping to different voltages from a holding voltage of −90 mV in the absence (open circles) and presence of AHA (closed circles).

Effect of AHA on the gating behavior of the Shaker Kv channel.
(A–F) Representative traces for the ionic currents elicited in the absence (black traces) or presence of 2.5 μM GxTx1E (red traces) at −10 mV (A–B), 50 mV (C–D) and 100 mV (E–F) from oocytes injected with ShakerΔ5 (No AHA, left and AHA, right), holding voltage = −90 mV, tail voltage = −60 mV. (G–H) Conductance-voltage (relationships obtained in the absence (black, control) and presence of GxTx1E (red, toxin) for oocytes injected with ShakerΔ5 in the absence (G) or presence (H) of AHA. G-V relations in control solution were obtained from tail currents elicited at −60 mV after stepping to test voltages and normalized to the maximal value following voltage steps to +100 mV. In the presence of toxin (red), steady state currents were used to calculate the G-V relationships due to rapid kinetics of the tail currents and normalized to the maximal conductance at +100 mV in control solution. All data points represent mean ± SEM (n = 3).
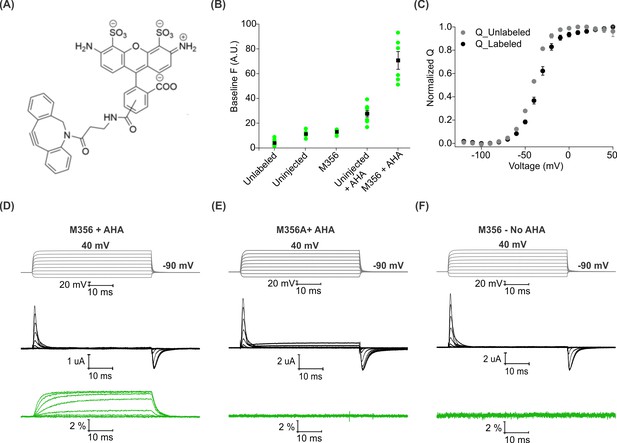
Voltage clamp fluorometry with AHA-modified Shaker Kv channels.
(A) Structure of azide reactive cyclooctyne-conjugated Alexa Fluor 488, AF488-DBCO. (B) Baseline fluorescence intensity (at −90 mV) obtained from unlabeled and labeled oocytes, either uninjected or injected with Shaker-M356 (M356/M448L) in the presence or absence of AHA (n = 5–6) (C) Q-V relationship obtained from AHA-modified Shaker-M356 before (gray) and after (black) labeling with AF488-DBCO. All data points represent mean ± SEM (n = 3). (D–F) Representative signals for gating currents (black) and fluorescence responses (green) obtained from AF488-DBCO labeled oocytes, injected with Shaker-M356 (D) or M356A (Shaker-M356A/M448L) (E) in the presence of AHA or Shaker-M356 in the absence of AHA (F).
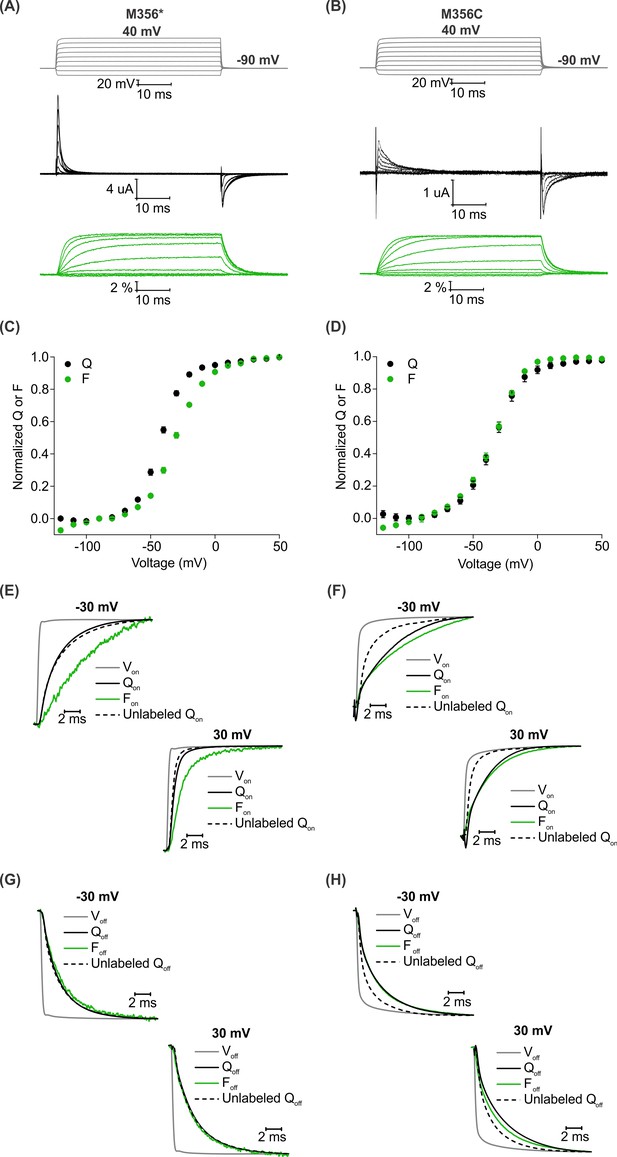
A comparison of AHA and cysteine-mediated voltage clamp fluorometry with the Shaker Kv channel.
(A–B) Representative traces for gating currents (black) and fluorescence responses (green) obtained from oocytes injected with Shaker-M356* (M356/M448L/C245V/C462A) (A) or Shaker-M356C (M356C/M448L/C245V/C462A) (B) after labeling with AF488-DBCO or AF488-C5-Maleimide, respectively. (C–D) Relationship between total gating charge displaced (Q, black) and change in fluorescence intensity (F, green) at steady state as a function of voltage for oocytes injected with Shaker-M356*, n = 18 (C) or Shaker-M356C, n = 5 (D). All data points represent mean ± SEM. (E–H) Kinetics of displacement of gating charge (black) and change in fluorescence intensity (green) during activation (E–F) and deactivation (G–H) of voltage sensors at weak (−30 mV, top) and strong depolarization (30 mV, bottom). Dashed lines represent the displacement of gating charge in unlabeled oocytes. Gray traces represent the integrated capacitive transient as a measure of the speed of the voltage clamp.
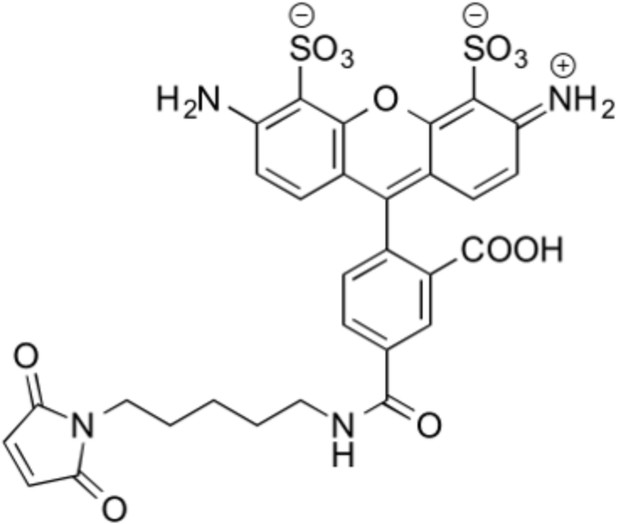
Structure of thiol reactive Alexa Fluor 488, AF488-C5-Maleimide.
https://doi.org/10.7554/eLife.50776.010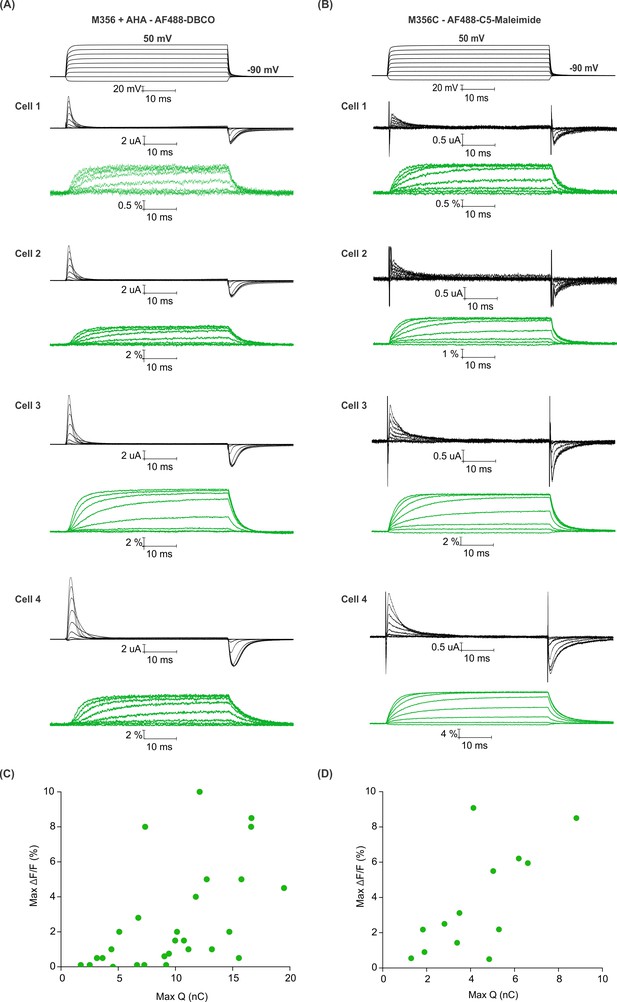
Efficiency of AHA and cysteine-mediated voltage clamp fluorometry with Shaker.
(A–B) Gating currents (black) and fluorescence responses (green) from AF488-DBCO labeled Shaker-M356 (M356/M448L) in the presence of AHA (A) or AF488-C5-maleimide labeled Shaker-M356C (M356C/M448L/C245V/C462A) (B). (C–D) Scatter plot for maximum fluorescence signal (Max ΔF/F, %) obtained as a function of total gating charge displaced (Max Q, nC) for oocytes labeled at M356 through AHA (C) or cysteine (D).
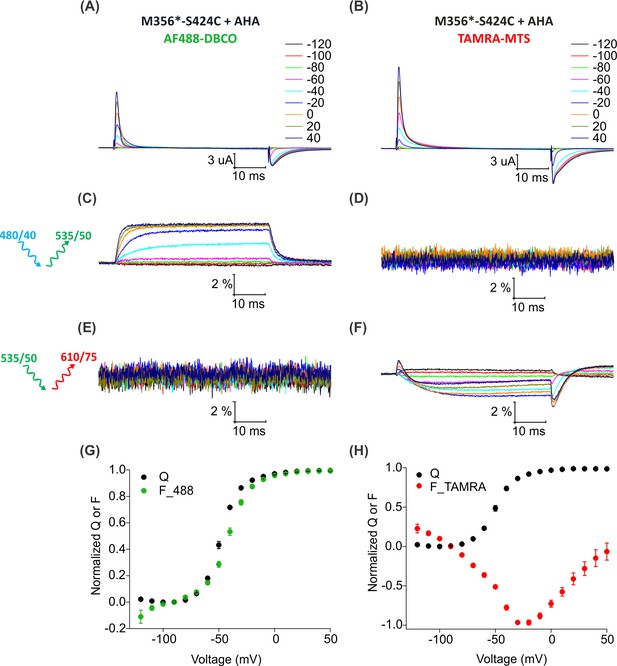
Fluorescence responses from the Shaker Kv channel labeled with azide or thiol-reactive fluorophores.
(A–B) Gating currents from oocytes expressing Shaker-M356*-S424C (M356/M448L/C245V/C462A/S424C) in the presence of AHA and labeled with AF488-DBCO (A) or TAMRA-MTS (B). (C–D) Fluorescence responses from oocytes labeled with AF488-DBCO (C) or TAMRA-MTS (D) through 488 filter cube (ex. 480/40 nm; em. 535/50 nm). (E–F) Fluorescence response from oocytes labeled with AF488-DBCO (E) or TAMRA-MTS (F) through TAMRA filter cube (ex. 535/50 nm; em. 610/75 nm). (G–H) Q-V (Q, black) and steady state F-V relationships (F_488; green, F_TAMRA; red) obtained from oocytes labeled with AF488-DBCO (G) or TAMRA-MTS (H) fluorophores. All data points are the mean ± SEM, n = 5–7.
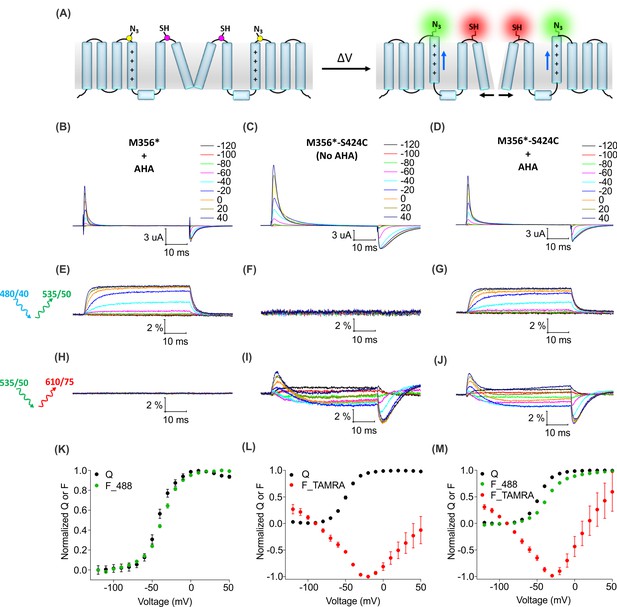
Two color labeling of the Shaker Kv channel through AHA and cysteine.
(A) A schematic for two-color labeling of the Shaker Kv channel. Each subunit contains an azide group from AHA (yellow) on the top of S4 within the voltage-sensing domain and a thiol group from cysteine (magenta) in the pore domain. Voltage-dependent conformational changes in the channel (blue and black arrows) result into a change in the fluorescence intensity of AF488-DBCO (green) and TAMRA-MTS (red) fluorophores. (B–D) Gating currents obtained from two-color labeled oocytes expressing Shaker-M356* (M356/M448L/C245V/C462A) in the presence of AHA (B) or Shaker-M356*-S424C (M356/M448L/C245V/C462A/S424C) in the absence (C) or presence of AHA (D). (E–J) Fluorescence responses from the two-color labeled oocytes through 488 filter cube (ex. 480/40 nm; em. 535/50 nm) (E–G) and TAMRA filter cube (ex. 535/50 nm; em. 610/75 nm) (H–J). (K–M) Q-V (Q, black) and steady-state F-V relationships (F_488; green, F_TAMRA; red) obtained from oocytes labeled with both AF488-DBCO and TAMRA-MTS. In all cases, data points are the mean ± SEM (n = 4–6). For Shaker-M356*, maximal ΔF/F (%) for TAMRA filter cube was 0.013 ± 0.003 (H). For Shaker-M356*-S424C without AHA, maximal ΔF/F (%) for 488 filter cube was 0.046 ± 0.011 (F).

Voltage-dependent fluorescence responses from Shaker-M356*-S424C after labeling with AF488-C5-Maleimide.
(A) Representative signals for gating currents (black) and fluorescence responses (green) obtained from oocytes expressing Shaker-M356*-S424C (M356/M448L/C245V/C462A/S424C) and labeled with AF488-C5-Maleimide. The gray traces represent the voltage steps from a holding voltage of −90 mV. (B) Relationship between total gating charge displaced (Q, black) and change in fluorescence intensity (F, green) at steady state as a function of voltage (n = 5). All data points represent mean ± SEM.
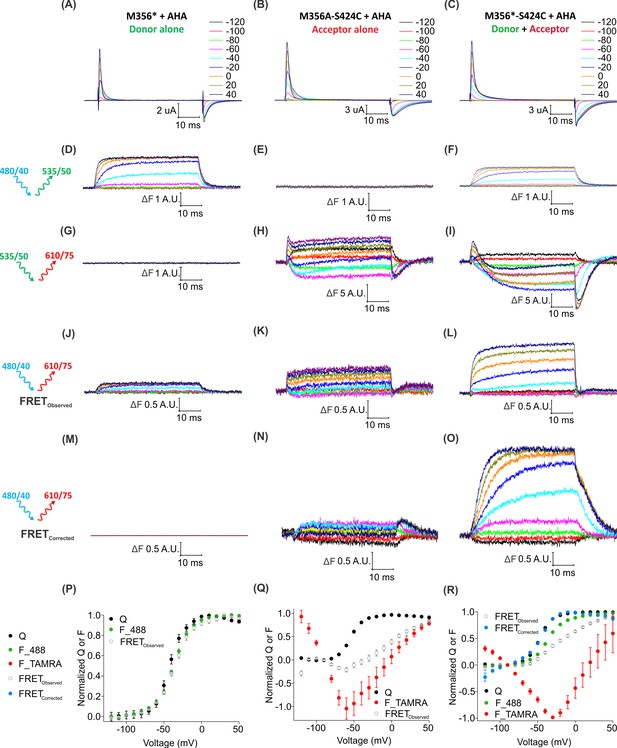
Two-color labeling through AHA and cysteine installs different fluorophores within the Shaker Kv channel.
(A–C) Gating currents obtained from two-color labeled oocytes expressing Shaker-M356* (M356/M448L/C245V/C462A) (A), Shaker-M356A-S424C (M356A/M448L/C245V/C462A/S424C) (B) or Shaker-M356*-S424C (M356/M448L/C245V/C462A/S424C) (C) in the presence of AHA. (D–O) Fluorescence responses from two-color labeled oocytes through 488 filter cube (ex. 480/40 nm; em. 535/50 nm) (D–F), TAMRA filter cube (ex. 535/50 nm; em. 610/75 nm) (G–I) or FRET filter cube (ex. 480/40 nm; em. 610/75 nm) before (J–L) and after (M–O) bleed through correction. (P–R) Q-V (Q, black) and steady-state F-V relationships (F_488; green, F_TAMRA; red, FRETObserved; gray and FRETCorrected; cyan) obtained from oocytes labeled with both AF488-DBCO and TAMRA-MTS. In all cases, data points are the mean ± SEM (n = 5–7). For Shaker-M356*, maximal ΔF for TAMRA filter cube was 0.131 ± 0.003 (G) and FRETCorrected was −0.00585 ± 0.004 (M). For Shaker-M356A-S424C, maximal ΔF for 488 filter cube was 0.010 ± 0.008 (E) and FRETCorrected cube was 0.032 ± 0.018 (N).

Structure of Kv1.2–2.1 paddle chimera (2R9R) indicating the Cα distances between residues corresponding to M356 and S424 in the Shaker Kv channel (Long et al., 2007).
https://doi.org/10.7554/eLife.50776.016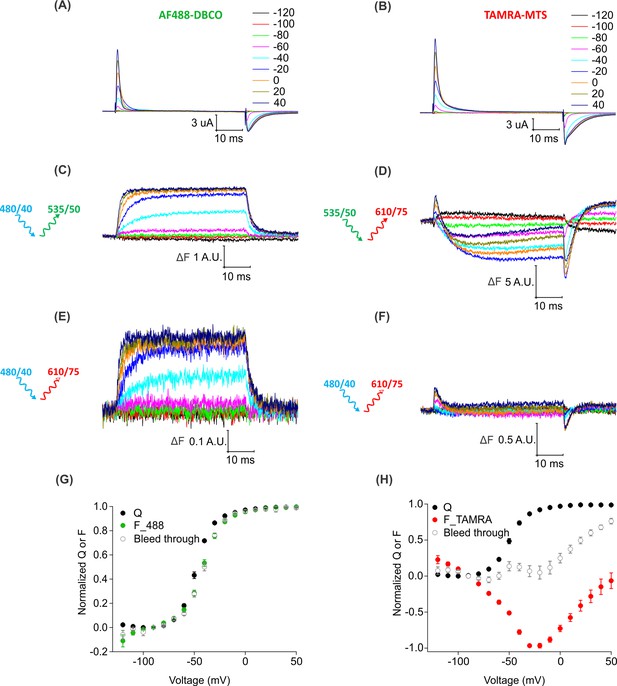
Bleed through of fluorescence from AF488 and TAMRA through FRET cube.
(A–B) Gating currents obtained from oocytes expressing Shaker-M356*-S424C (M356/M448L/C245V/C462A/S424C) in the presence of AHA and labeled with AF488-DBCO (A) or TAMRA-MTS (B). (C) Fluorescence responses from AF488-DBCO labeled oocytes through 488 filter cube (ex. 480/40 nm; em. 535/50 nm). (D) Fluorescence responses from TAMRA-MTS labeled oocytes through TAMRA filter cube (ex. 535/50 nm; em. 610/75 nm). (E–F) Bleed through fluorescence responses from oocytes labeled with AF488-DBCO (E) or TAMRA-MTS (F) through FRET filter cube (ex. 480/40 nm; em. 610/75 nm). (G–H) Q-V (Q, black) and steady-state F-V relationships (F_488; green, F_TAMRA; red, Bleed through; gray) from AF488-DBCO (G) or TAMRA-MTS (H) labeled oocytes.

Bleed through ratio of fluorescence from AF488 and TAMRA through FRET cube.
Ratio of maximal ΔF obtained through FRET cube (ex. 480/40 nm; em. 610/75 nm) and 488 filter cube (ex. 480/40 nm; em. 535/50 nm) or TAMRA filter cube (ex. 535/50 nm; em. 610/75 nm) for AF488-DBCO (green, n = 7) or TAMRA-MTS (red, n = 9) labeled oocytes expressing Shaker-M356*-S424C (M356/M448L/C245V/C462A/S424C).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Drosophila melanogaster) | Shaker | Gene ID: 32780 | ||
| Oocytes (Xenopus laevis, female) | Xenopus Oocytes or oocytes | Xenopus Express | IMP XL FM | |
| Antibody | Anti-myc antibody | ThermoFisher | Cat. No. 46–0603 | 1:1000 (2 µl in 2 ml) |
| Antibody | HRP-conjugated anti-mouse secondary antibody | Amersham ECL | Cat. No. NA931VS | 1:3750 (4 µl in 15 ml) |
| Recombinant DNA reagent | pGEMHE vector | Liman et al., 1992 | ||
| Peptide, recombinant protein | GxTx1E | This paper | Toxin | |
| Chemical compound, drug | NHS-sulfo-biotin | ThermoFisher | Cat. No. 21335 | 1 mM from 10X stock in ddH2O |
| Chemical compound, drug | DBCO-sulfo-biotin | Sigma | Cat. No. 760706 | 1 mM from 10X stock in ddH2O |
| Chemical compound, drug | AF488-DBCO | Click Chemistry Tools | Cat. No. 1278 | 100 µM from 100X stock in anhydrous DMSO |
| Chemical compound, drug | AF488-C5-Maleimide | Click Chemistry Tools | Cat. No. 1014 | 100 µM from 100X stock in anhydrous DMSO |
| Chemical compound, drug | TAMRA-MTS | Toronto Research Chemicals | Cat. No. T305175 | 10 µM from 1000X stock in ddH2O |
| Chemical compound, drug | Azidohomoalanine (AHA) | Bachem | Cat. No. F-4265 | 4 mM from100 mM stock in ddH2O |
Additional files
-
Supplementary file 1
Commercially available dibenzocyclooctyne (DBCO)-conjugated fluorophores https://clickchemistrytools.com/product-category/fluorescent-dyes/cu-free-click-chemistry/ - All these fluorophores are charged and membrane impermeable.
- https://doi.org/10.7554/eLife.50776.019
-
Supplementary file 2
Commercially available bicyclononyne (BCN)–conjugated fluorphores https://biotium.com/product/cf-dye-bcn/ - All these fluorophores are membrane impermeable.
- https://doi.org/10.7554/eLife.50776.020
-
Supplementary file 3
Prevalence and location of methionine and cysteine residues in a selection of membrane proteins previously studied with voltage-clamp fluorometry.
- https://doi.org/10.7554/eLife.50776.021
-
Transparent reporting form
- https://doi.org/10.7554/eLife.50776.022





