Interhemispherically dynamic representation of an eye movement-related activity in mouse frontal cortex
Figures
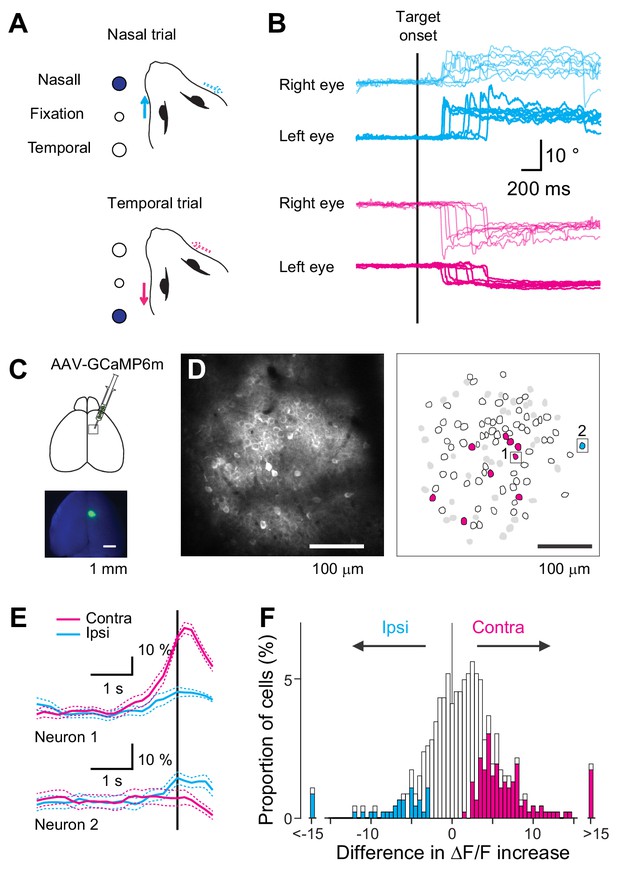
Neural representation in the MOs during a visually-guided eye movement task.
(A) Experimental design of a visually-guided eye movement task. After the mice fixated the central LED, nasal or temporal target LED was turned on, instructing the mice to shift their left eye toward the target. (B) Example traces of eye position recorded during one behavioral session. Traces are aligned to the target onset. Magenta traces: trials with temporal target (n = 8 trials). Cyan traces: trials with nasal target (n = 8 trials). (C) GCaMP6m was virally expressed in the MOs in the right hemisphere for two-photon calcium imaging. (D) Representative image and corresponding ROIs for neurons labeled with GCaMP6m. Magenta polygons: neurons exhibiting higher activity in the contraversive than ipsiversive condition just before eye movement onset. Cyan polygons: neurons exhibiting higher activity in the ipsiversive condition. White polygons: neurons showing significant movement-related activity. Gray polygons: neurons showing no significant movement-related activity. Black squares: example neurons shown in E. (E) Fluorescence changes for the two neurons shown in D. Average fluorescence changes for contraversive trials (magenta) and ipsiversive (cyan). Vertical line indicates eye movement onset. (F) Distribution of contraversive/ipsiversive difference in ΔF/F increase for neurons showing significant fluorescence change (n = 463). Magenta bars: neurons significantly selective for the contraversive condition (n = 114). Cyan bars: neurons significantly selective for the ipsiversive condition (n = 35).
-
Figure 1—source data 1
Contains numerical data plotted in Figure 1F.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig1-data1-v2.mat
-
Figure 1—source code 1
Displays the distribution of difference in DF/F increase.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig1-code1-v2.m
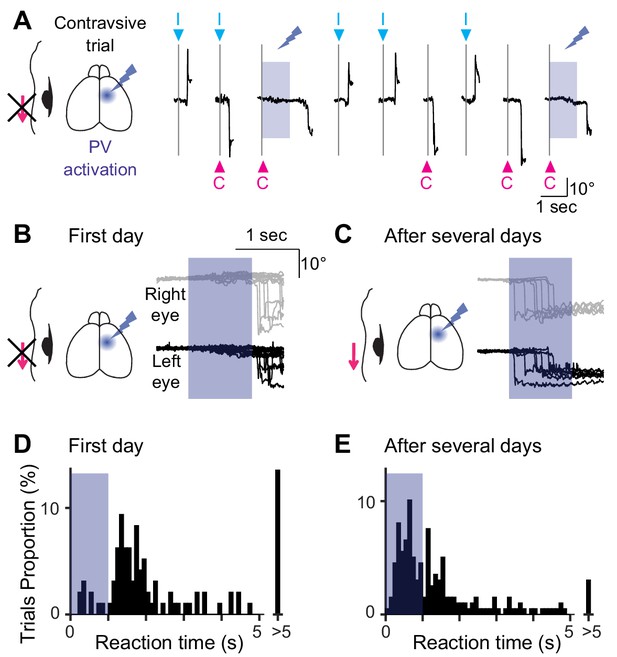
Optogenetic suppression of unilateral MOs during the visually-guided eye movement task for multiple days.
(A) The experimental design and example traces of eye position from one animal. Neuronal activity of the unilateral MOs was optogenetically suppressed during the eye movement task by activating PV+ interneurons. Optogenetic suppression was induced just after visual cues (blue shaded period) only for 1 s. When the blue light was illuminated, the mice could not produce temporal eye movements for the contraversive direction. (B, C) Effect of the unilateral optogenetic suppression during the task. Example traces from one animal. The suppression severely impaired the contraversive eye movements on the first day (B) but not after 4 days (C). Traces of the bilateral eyes are shown. The blue shades indicate the optogenetic suppression period. (D, E) Distribution of reaction time for the first day (1.76 ± 1.47 s, 96 out of127 trials, eight mice) and after 5.9 ± 1.0 days (1.06 ± 1.00 s, 198 out of 220 trials, eight mice). The reaction times were significantly different (p<10−9, Mann-Whitney U test).
-
Figure 2—source data 1
Contains numerical data plotted in Figure 2D,E.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig2-data1-v2.mat
-
Figure 2—source code 1
Displays distributions of reaction time.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig2-code1-v2.m
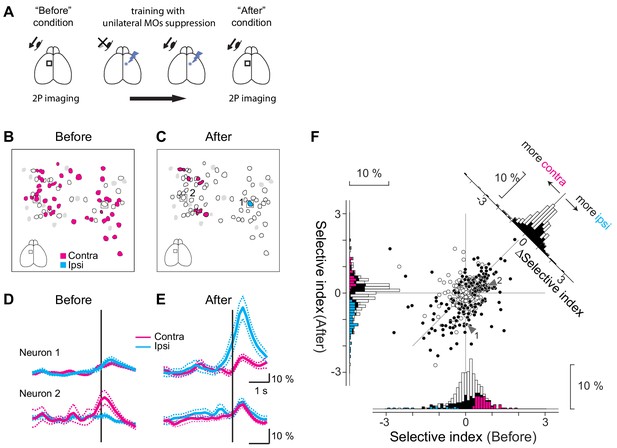
Repetitive suppression of unilateral MOs induces compensatory changes in neural encoding in the contralateral MOs.
(A) Experimental design. When the mice learned the visually-guided eye movement task, we performed two photon calcium imaging to investigate directional preference of the MOs neurons in one hemisphere (‘Before’ condition). Then optogenetic suppression was applied to the MOs in the contralateral hemisphere. While the mice were trained for several days, they regained the ability to make contraversive eye movements. We again performed two-photon calcium imaging (‘After’ condition) to obtain the neuronal response for the same neurons imaged in the ‘Before’ condition. (B, C) Training-induced changes in direction selectivity of the MOs neurons in a representative imaging session. In the ‘Before’ condition (B), many neurons preferred the contraversive direction (magenta polygons), but in the ‘After’ condition (C), these neurons got sparser, and one neuron selective for ipsiversive direction (cyan polygon) showed up. White polygons indicate movement-related cells, and gray polygons non-significant cells. Black squares: example neurons shown in D, E. (D, E) Examples of two neurons (indicated in B, C) where direction selectivity changed between the ‘Before’ and ‘After’ conditions. Average fluorescence changes for trials with ipsiversive movements (cyan traces: Before, n = 30; After, n = 31 trials) and contraversive movements (magenta traces: Before, n = 35; After, n = 32 trials). (F) Training for several days induced difference in selectivity index for MOs neurons (six mice). White dots in the scatter plot (n = 225) are neurons showing significant movement-related activity, and black dots (n = 190) are neurons showing significant selectivity on top of the movement-related activity (total of 415 neurons are shown). The x-axis indicates selectivity index for the Before condition and the y-axis for the After condition. The histograms for the selectivity index are shown along the x-axis (Before) and the y-axis (After). Black bars are neurons showing significant direction preference either ‘before’ or ‘after’ conditions (n = 190 neurons). On top of black bars, magenta and cyan bars are overlaid for neurons showing preference for contraversive and ipsiversive directions (‘before’ condition: contraversive, magenta, n = 78 neurons, ipsiversive, cyan, n = 18 neurons, ‘after’ condition: contraversive, magenta, n = 40 neurons, ipsiversive, cyan, n = 69 neurons). Change in the selectivity index is shown in a histogram in the top right corner. White bars indicate the movement-related neurons (n = 225) and black bars the direction-selective neurons (n = 190).
-
Figure 3—source data 1
Contains numerical data plotted in Figure 3F.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig3-data1-v2.mat
-
Figure 3—source code 1
Displays the scatterplot of Selective Indices.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig3-code1-v2.m
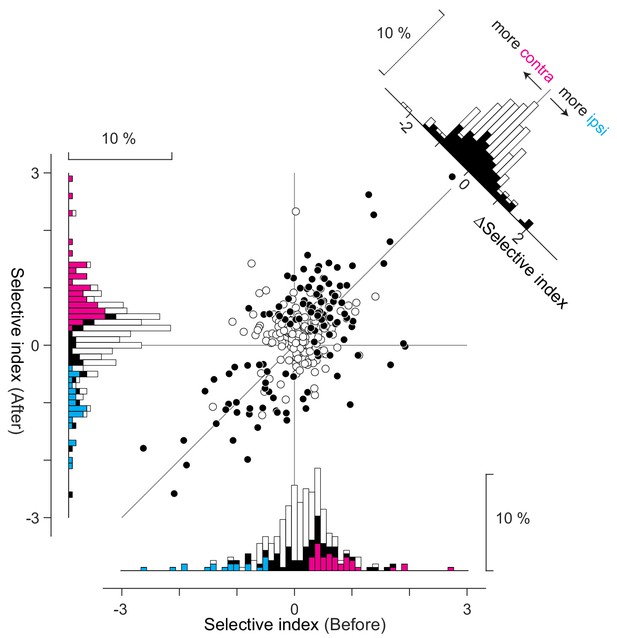
Several days of training induces slight increase in contraversive preference in neural encoding.
Training for several days caused slight changes in selectivity index difference in control mice that virally expressed GFP (n = 4 mice). The tendency of the change was opposite to Figure 3F. White dots in the scatter plot (n = 159) are neurons showing significant movement-related activity, and black dots (n = 123) are neurons showing significant selectivity on top of the movement-related activity (total of 282 neurons are shown). The x-axis indicates the selectivity index for the Before condition and the y-axis for the After condition. The histograms for the selectivity index are shown along the x-axis (Before) and the y-axis (After). Black bars are neurons showing significant direction preference either ‘before’ or ‘after’ conditions (n = 123 neurons). On top of black bars, cyan and magenta bars are overlaid for neurons showing preference for contraversive and ipsiversive directions (‘before’ condition: contraversive, magenta, n = 39 neurons, ipsiversive, cyan, n = 23 neurons, ‘after’ condition: contraversive, magenta, n = 69 neurons, ipsiversive, cyan, n = 27 neurons). Change in the selectivity index is shown in a histogram in the top right corner. White bars indicate the movement-related neurons (n = 159) and black bars the direction-selective neurons (n = 123). Color convention is the same with Figure 3F.
-
Figure 3—figure supplement 1—source data 1
Contains numerical data plotted in Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig3-figsupp1-data1-v2.mat
-
Figure 3—figure supplement 1—source code 1
Displays the scatterplot of Selective Indices for control data.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig3-figsupp1-code1-v2.m
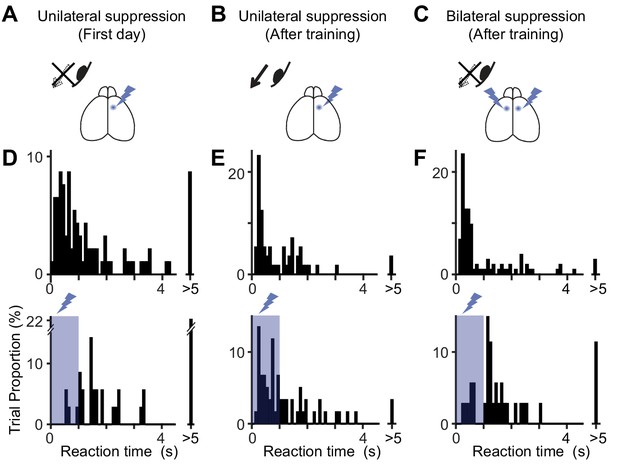
Distribution of reaction times after unilateral and bilateral MOs suppression.
(A–C) Optogenetic suppression design. On the first day of optogenetic suppression (A), mice showed difficulty in performing the contraversive eye movements within 1 s of optogenetic suppression. After several days of training (B), the mice were able to make contraversive eye movements (‘After training’). However, bilateral suppression of the MOs (C) induced the difficulty again. (D–F) The distribution of reaction times for A-C. Top and bottom histograms are from trials without and with optogenetic suppression. On the first day (D), the reaction time is rarely within the 1 s period of optogenetic suppression (10.3% of 39 trials with suppression vs. 52.1% of 96 trials without suppression, p<10−5, Pearson’s chi-square test, n = 4 mice). After several days of training (E), the reaction time could be shorter than 1 s (55.7% of 61 trials with suppression vs. 55.7% of 61 trials without suppression, p=1, Pearson’s chi-square test). However, bilateral suppression of the MOs prolonged the reaction time again (18.9% of 37 trials, with suppression vs. 66.7% of 108 trials without suppression, p<10−6, Pearson’s chi-square test).
-
Figure 4—source data 1
Contains numerical data plotted in Figure 4D.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig4-data1-v2.mat
-
Figure 4—source data 2
Contains numerical data plotted in Figure 4E.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig4-data2-v2.mat
-
Figure 4—source data 3
Contains numerical data plotted in Figure 4F.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig4-data3-v2.mat
-
Figure 4—source code 1
Displays the distributions of reaction time.
- https://cdn.elifesciences.org/articles/50855/elife-50855-fig4-code1-v2.m
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | PV-Cre, C57Bl/6 | PMID: 15836427 | RRID:IMSR_JAX:008069 | The Jackson Laboratory (#008069) |
| Strain, strain background (Mus musculus) | Ai32, C57Bl/6 | PMID: 22446880 | RRID:IMSR_JAX:012569 | The Jackson Laboratory (#012569) |
| Recombinant DNA reagent | AAV2/1-syn-GCaMP6m | PMID: 23868258 | RRID:Addgene_100841 | Upenn Vector Core |
| Recombinant DNA reagent | AAV2/1-EF1α-DIO-hChR2(H134R)-EYFP | http://www.optogenetics.org | RRID:Addgene_ 20298 | Upenn Vector Core |
| Software, algorithm | Matlab | https://www.mathworks.com/products/matlab.html | RRID:SCR_001622 |





