Retinal oxygen supply shaped the functional evolution of the vertebrate eye
Figures
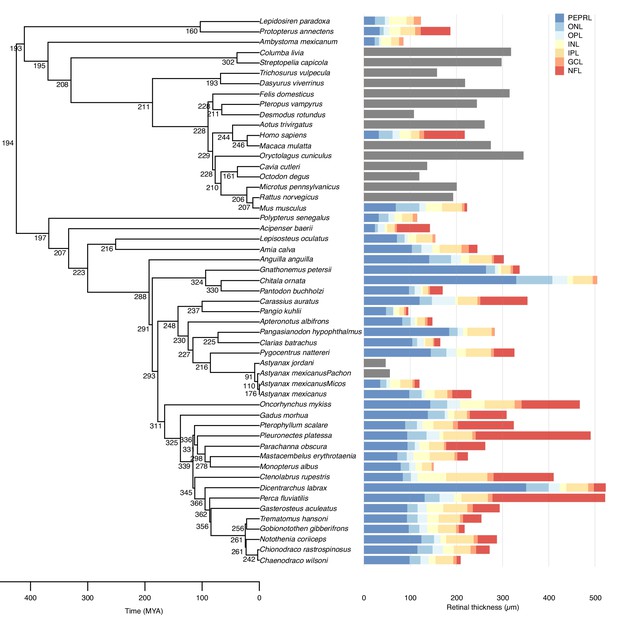
Evolution of maximal retinal thickness in 53 bony fishes.
Measured (bars) and reconstructed (internal nodes) values for maximal retinal thickness plotted on a bony fish phylogeny. Stacked colours represent thicknesses of individual retinal layers (retinal thickness in species without data on retinal layers thickness are shown in grey bars). Retinal thickness was measured using in vivo ultrasound, histology, or acquired from the literature, and retinal layer thickness was measured on histological sections. Ancestral states were inferred using maximum likelihood. Retinal layer abbreviations: PEPRL, pigment epithelium and photo receptor layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; NFL, nerve fiber layer.
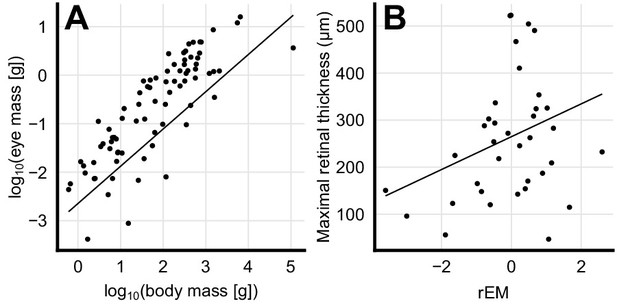
Scaling of eye mass and retinal thickness.
(A) Species-mean values of eye mass and body mass (dots) showing an allometric scaling relationship. Solid line depicts a phylogenetic general least squares (PGLS) fit to the data (log10(eye mass [g])=0.77 log10(body mass [g]) – 2.64, t = 12.7, p<0.001, n = 79). (B) Positive correlation between retinal thickness and residual eye mass (rEM) that are the residuals of the PGLS fit, which are body mass-independent measures of eye mass. Solid line depicts a PGLS fit to the data (retinal thickness = 34.9 rEM + 265, t = 21.6, p<0.001, n = 36).
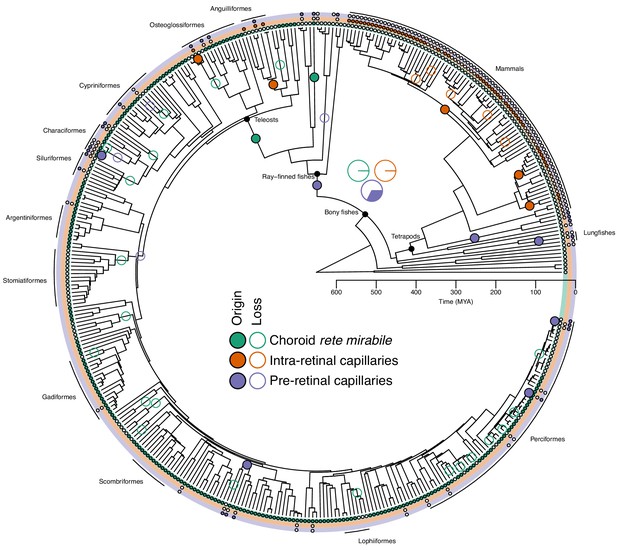
Evolution of retinal oxygen supply mechanisms in vertebrates.
The time-calibrated phylogeny of jawed vertebrates plotted with the inferred evolution of additional retinal oxygen supply mechanisms using observations from this study and the literature. Labels on terminal branches indicate the retinal oxygen supply phenotype of each species, where empty and filled circles represent the absence and presence of specific mechanisms (no circle represents no information available). Empty and filled circles in internal nodes represent inferred origins and losses of these mechanisms based on stochastic character mapping. Pie charts in the centre shows the Bayesian posterior probability for the presence of the three types of capillaries in the most recent common ancestor of bony fishes. Arches in the periphery denote mammals, lobe-finned fishes, and larger orders of ray-finned fishes. See Figure 3—figure supplement 3 for a phylogeny with species names.
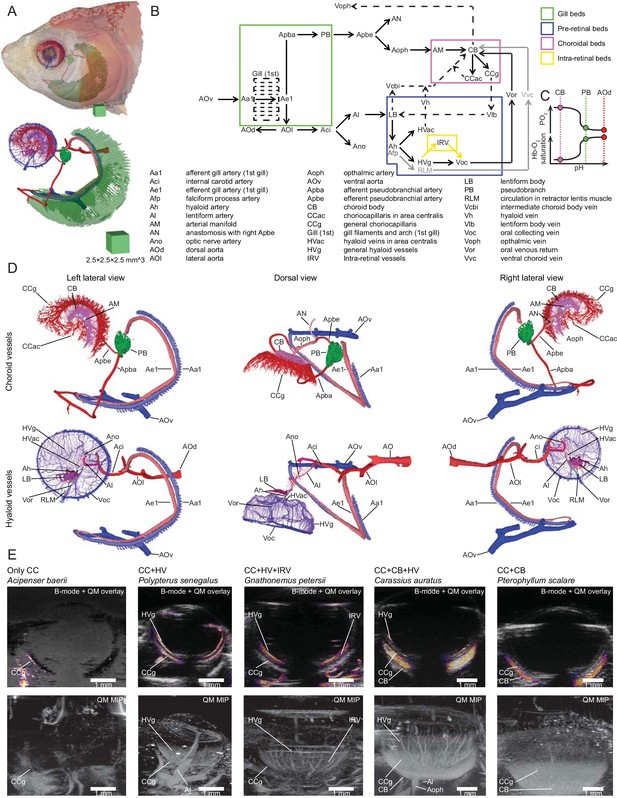
Circulation in the bony fish eye.
(A) Retinal circulation in the goldfish Carassius auratus, which displays all of the vertebrate supply systems with the exception of intra-retinal circulation. (B) Flow chart of circulation in the ray-finned fish eye connecting the branchial and retinal capillary beds (coloured boxes). Dashed lines are vessels not visualised in goldfish, grey lines are vessels in species with a falciform process (Copeland, 1980), and yellow lines are vessels in species with intra-retinal circulation. (C) Activation of the Root effect represented by a reduction of blood pH in the pseudobranch and choroid body, leading to a reduction in haemoglobin oxygen saturation and an increase in oxygen partial pressure in the choroid body. (D) Left lateral, dorsal, and right lateral views of the choroid and pre-retinal structures in goldfish. (E) 2D brightness-mode ultrasound images with coloured overlays from quadratic averaging (QM) showing blood movement (top), and maximum intensity projections of QM images of whole eyes (bottom) in species with five different circulation types in eyes of ray-finned fish (E).
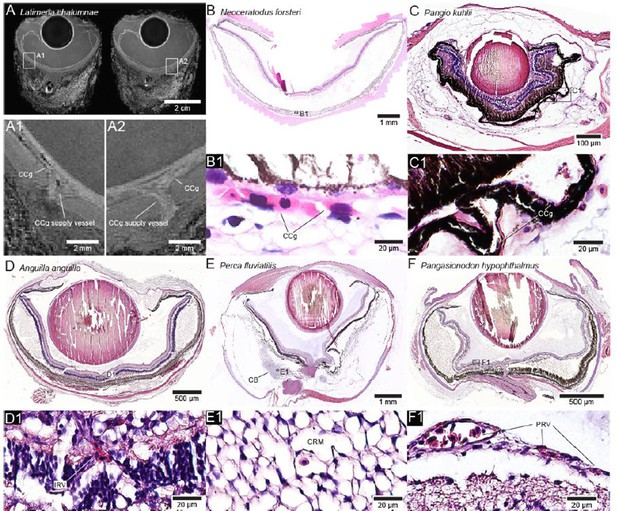
Examples of retinal blood supply types in bony fishes.
Basal sarcopterygian fishes such as the coelacanth (A) and the Australian lungfish (B) rely solely on the general choriocapillaris (CCg) with no additional retinal oxygen supply mechanisms, which was most likely the case in the most recent common ancestor of all bony fishes. Likewise, some phyletically derived teleosts such as loaches (C) have secondarily lost specialised mechanisms for oxygen delivery to the retina and also rely on the CCg to supply and possess relatively thin retinae. Inter-retinal vessels (IRV) are found in the European eel (D). The specialised choroid body (CB) containing the choroid rete mirabile (CRM) is found in Amia and in several teleost fishes such as the perch (E). Some bony fishes that have secondarily lost oxygen secretion such as the striped catfish (F) have evolved an extensive network of pre-retinal capillaries to supply the inner side of the retina. All eyes are presented as H and E stained histological sections except the coelacanth eye (A) that underwent non-destructive high field MRI.
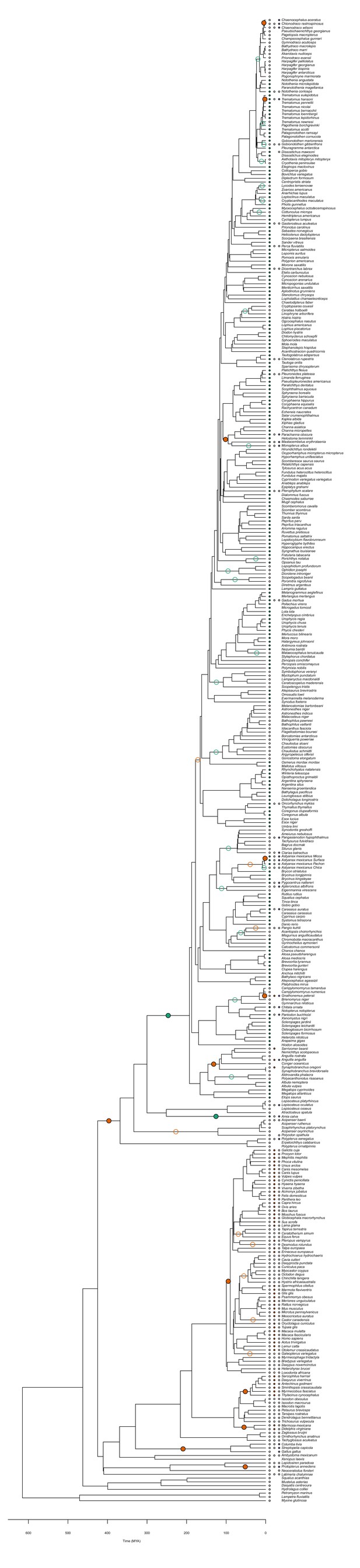
Evolution of retinal oxygen supply mechanisms in vertebrates.
Figure 3 plotted with species names.
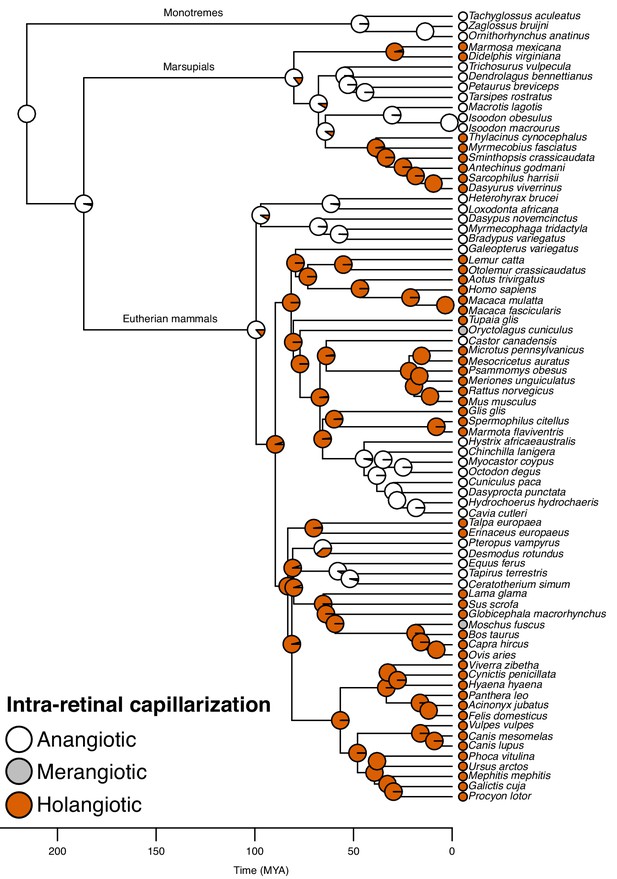
Evolution of retinal capillarization in mammals.
Mammalian phylogeny showing retinal capillarization examined in extant species (tips; literature data: Chase, 1982; Leber, 1903; Samorajski et al., 1966; Moritz et al., 2013; Kolmer, 1927; Bellhorn, 1997; McMenamin, 2007) and reconstructed retinal capillarization (internal branches), showing of holangiotic (capillarization of the whole retina; orange), merangiotic (capillarization confined to the retina around the optic nerve; grey), and anangiotic capillarization (little or no capillarization; white). Pie charts indicate ancestral states on internal nodes showing posterior probabilities summarised from stochastic character mapping.
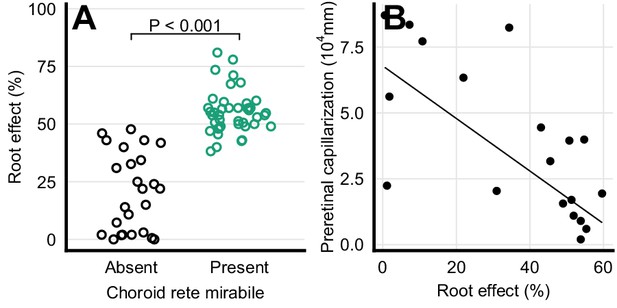
Haemoglobin function and retinal capillarization.
(A) Frequency distribution of Root effect magnitude in ray-finned fishes with and without a choroid rete mirabile. The effect of the presence of the choroid rete mirabile was tested by phylogenetic analysis of variance simulation (F = 122, p<0.001, n = 68). (B) Pre-retinal capillarization and Root effect magnitude in ray-finned fishes, showing a negative correlation as tested by phylogenetic general least squares (t = -4.62, p<0.001, n = 19). Each dot represents mean values for each species. Pre-retinal capillarization is the volume of capillaries on the inner side in mm3 of the retina per retinal surface area in mm2. Root effect is the per cent haemoglobin desaturation at pH 5.5 compared to pH 8.5 in air-equilibrated buffers.
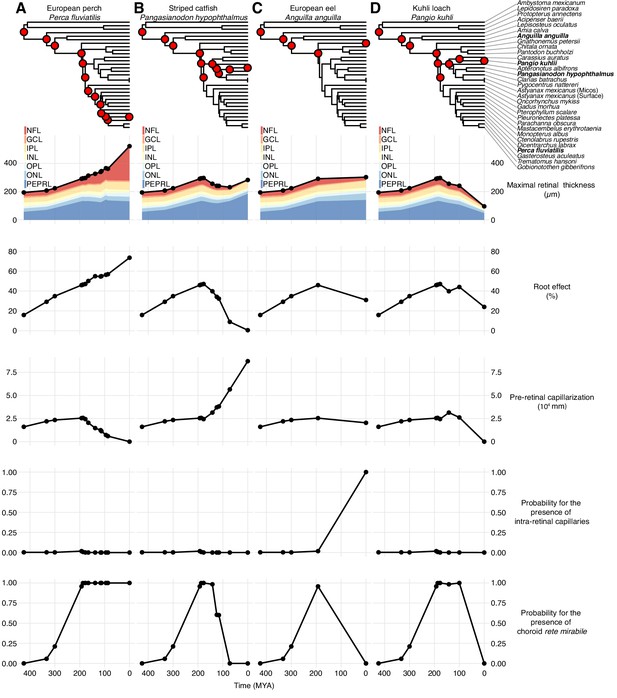
Evolutionary trajectories of retinal oxygen supply and morphology.
Each column displays the evolution of physiological and anatomical parameters in the lines of descent connecting the most recent common ancestor of bony fishes to either European perch, Perca fluviatilis (A), striped catfish, Pangasianodon hypophthalmus (B), European eel, Anguilla anguilla (C), or kuhli loach, Pangio kuhli (D). The right-most symbols in each column are measured values and points to the left are reconstructed values of in all internal nodes in the phylogeny connecting to the most recent common ancestor of bony fishes (see top phylogeny, where the four species are marked in bold). Maximal retinal thickness is plotted in black where stacked shaded areas below represent measured and reconstructed thickness of the individual retinal layers. Pre-retinal capillarization is the volume of capillaries on the inner side in mm3 of the retina per retinal surface area in mm2. Root effect is the per cent haemoglobin desaturation at pH 5.5 compared to pH 8.5 in air-equilibrated buffers. Maximum total retinal thickness, thickness of individual retinal layers, and pre-retinal capillarization were reconstructed using maximum likelihood, and the presence of intra-retinal capillaries or the choroid rete mirabile was reconstructed using stochastic character mapping. Retinal layer abbreviations: PEPRL, pigment epithelium and photo receptor layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; NFL, nerve fiber layer. Trajectories to all extant species in the data set are deposited on GitHub (https://github.com/christiandamsgaard/Retinaevolution).
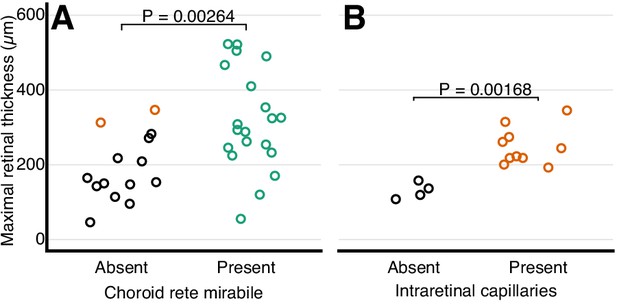
Relationship between retinal oxygen supply and morphology.
Frequency distribution of retinal thickness in ray-finned fishes with and without a choroid rete mirabile (A), and in mammals with and without intra-retinal capillaries (B). In (A), orange symbols indicate species with intra-retinal capillarization. Each dot represents mean values for each species. The effect of the choroid rete mirabile or intra-retinal capillaries on retinal thickness were assessed by phylogenetic analysis of variance simulation. Root effect is the per cent haemoglobin desaturation at pH 5.5 compared to pH 8.5 in air-equilibrated buffers.
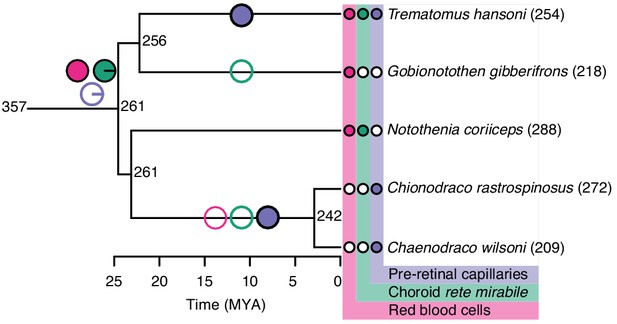
Evolution of retinal oxygen supply and morphology in Notothenioids, including the haemoglobin-less Antarctic icefishes (C. rastrospinosus and C. wilsoni).
Measured and reconstructed values of maximal retinal thickness plotted on the Notothenioid phylogeny. Ancestral state values for retinal thickness were estimated by maximum likelihood. The observed absence (open symbols) and presence (filled symbols) of red blood cells (pink), choroid rete mirabile (green), and pre-retinal capillaries (purple) are indicated at the tips, and inferred origins and losses of these traits are marked on internal branches by filled and open symbols, respectively. Pie charts at the most recent common ancestor of notothenioids indicate the Bayesian posterior probability of these traits being present.
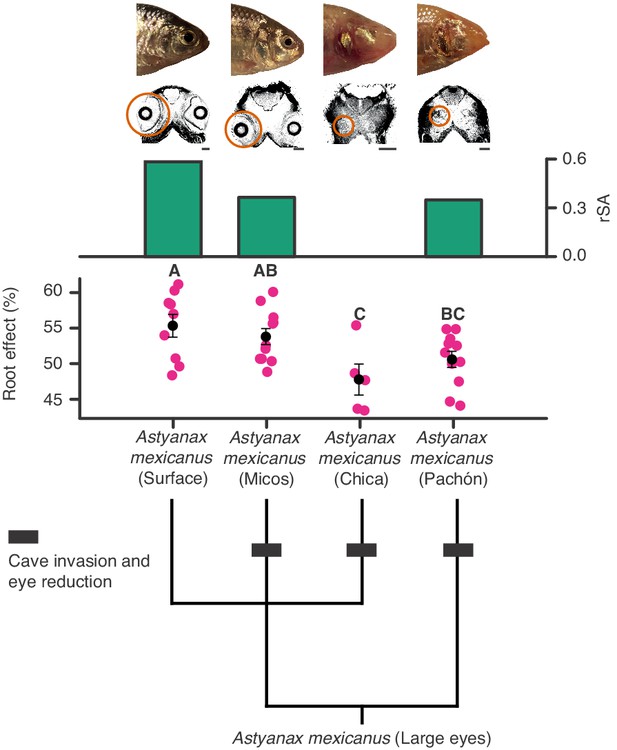
Regressive evolution of oxygen secretion in troglobitic Mexican tetras.
Representative photographs and computed tomography scans of a surface and three cave forms of Astyanax mexicanus: Surface (large eyes), Micos (invaded caves about 10–20,000 years ago, variably reduced eyes), Chica (invaded caves around 10–20,000 years ago, highly reduced eyes), and Pachón (invaded caves at earliest around 3 Ma but most likely during the last glaciation, highly reduced eyes). Orange circles on computed tomography scans mark the actual size and location of eyes. Green bars show residuals of log10(choroid rete mirabile endothelium surface area [mm]) (rSA) on log10(body mass [g]). There was no choroid rete mirabile in A. mexicanus (Chica). Root effects magnitudes are marked as pink dots, and black dots and bars are means and standard error of mean. There were significant differences in mean Root effect between ecotypes as determined by one-way ANOVA (F3, 32 = 4.617, p=0.009) with a Student-Newman-Keuls posthoc test and indicated by letters above bars (n = 9, 11, 11, and five for Surface, Micos, Pachón and Chica, respectively). Lower panel shows the phylogenetic relationships between different forms of A. mexicanus.
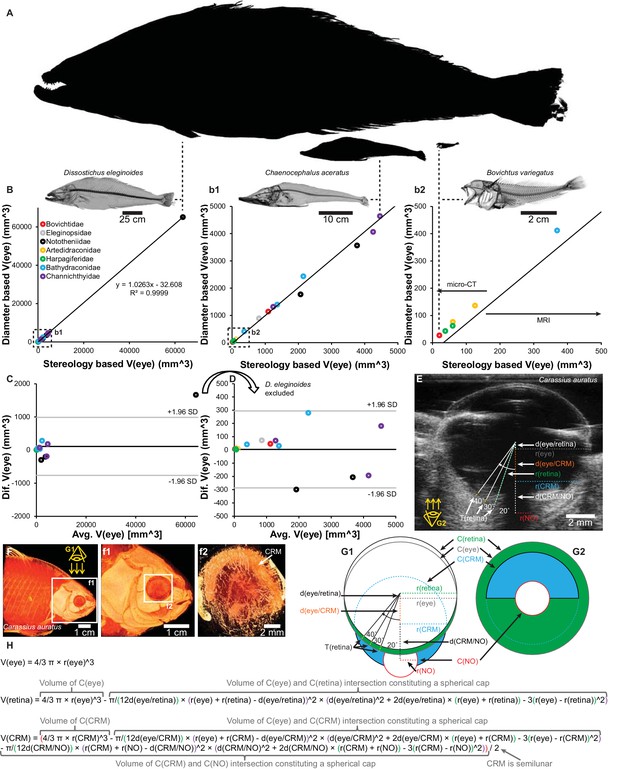
Validation of eye volume [V(eye)] measurement from eye radius and schematic of quantitative anatomical measurements.
Estimations of V(eye) in 16 species of notothenioids spanning 4 orders of magnitude in body size (A, relative sizes of largest, a medium sized and smallest species) showed a proportional relationship between V(eye) based on eye radius and stereological measurements from three-dimensional magnetic resonance imaging (MRI) and micro-CT imaging (B, correlation plot with magnifications (b1 and b2). C and D, Bland-Altman plot containing all specimens (C) and with the markedly larger Dissostichus eleginoides excluded (D)). Retinal thickness, T(retina), was measured at 20°, 30° and 40° to the optic nerve at both sides (only one side shown in figure) on ultrasound (E) and micro-CT (F, volume reconstruction to show shape of choroid rete mirabile) datasets and similarly on histological slides. Eye volume, retina volume, V(retina) and choroid rete mirabile volume, V(CRM), were calculated based on geometrical assumptions (G1, G2, and H. See Materials and methods for written description) and the measurement of the radii, r(x), of the circle describing the eye, C(eye), and the imaginary circles constituted by the inner side of the retina, C(retina), the choroid rete mirabile, C(CRM), the optic nerve, C(NO) and the displacement, d(x), of the center of these circles.
Videos
Two-dimensional ultrasound videos in B-mode, quadratic average mode, and colour Doppler mode in a mid-coronal plane through the eyes of fishes.
Ambystoma mexicanum, Lepidosiren paradoxa, Protopterus annectens, Polypterus senegalus, Acipenser baerii, Lepisosteus oculatus, Anguilla anguilla, Pantodon bucholzi, Gnathonemus petersii, Chitala ornata, Carassius auratus, Pangio kuhlii, Pangasianodon hypophthalmus, Clarias batrachus, Apteronotus albifrons, Pygocentrus nattereri, Astyanax mexicanus (Surface), Astyanax mexicanus (Micos), Astyanax mexicanus (Pachòn), Astyanax mexicanus (Chica), Oncorhynchus mykiss, Gadus morhua, Ctenolabrus rupestris, Dicentrarchus labrax, Gasterosteus aculeatus, Perca fluviatilis, Pterophyllum scalare, Pleuronectes platessa, Parachanna obscura, Mastacembelus erythrotaenia, and Monopterus albus.
Three-dimensional, quadratic-averaged ultrasound slice videos through the coronal plane of the eyes of five species with fundamentally different eye circulatory patterns.
Acipenser baerii, Only choriocapillaris; Polypterus senegalus, Choriocapillaris and pre-retinal capillaries; Gnathonemus petersii, Choriocapillaris, pre-retinal capillaries and intra-retinal vessels; Pterophyllum scalare, Choriocapillaris and choroid rete mirabile; and Carassius auratus, Choriocapillaris, choroid rete mirabile and pre-retinal capillaries.
Additional files
-
Supplementary file 1
Number of species included in the data analysis for each physiological or anatomical trait.
Most data points were generated within this study, but literature data on maximal retinal thickness and Root effect magnitude as well as literature observations on different capillary types was included in the analysis.
- https://cdn.elifesciences.org/articles/52153/elife-52153-supp1-v1.docx
-
Supplementary file 2
Concentration of benzocaine to achieve anaesthesia, ultrasound transducer frequency, and resolution of micro-CT.
CT, computed tomography; n, numbers of replicates for ultrasound; NA, not available; US, ultrasound. Values are means ± standard deviations.
- https://cdn.elifesciences.org/articles/52153/elife-52153-supp2-v1.docx
-
Supplementary file 3
Coefficient of variance for retinal thickness, volume of retina and volume of eyes in species used in micro-ultrasound.
The coefficient of variance (CV) was calculated as the ratio between standard deviation and sample mean. Values are percentages. CRM, choroid rete mirabile; n, number of replicates for ultrasound CV analysis; T, thickness; V, Volume.
- https://cdn.elifesciences.org/articles/52153/elife-52153-supp3-v1.docx
-
Supplementary file 4
Interactive 3D model.
Three-dimensionally rendered interactive model generated from micro-computed tomography imaging of a goldfish after an arterial injection of a BaSO4-based contrast agent. The interactive file should be viewed in Adobe Acrobat Readernineor higher. To activate the 3D feature, click the model. Using the cursor, it is possible to rotate, zoom, and pan the model. All segments of the model can be turned on/off or made transparent. The model tree is a hierarchy containing several sublayers that can be opened (+). Pre-defined views similar to Figure 3—figure supplement 1A and C can be selected below the model tree
- https://cdn.elifesciences.org/articles/52153/elife-52153-supp4-v1.pdf
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/52153/elife-52153-transrepform-v1.docx





