C1 neurons are part of the circuitry that recruits active expiration in response to the activation of peripheral chemoreceptors
Figures
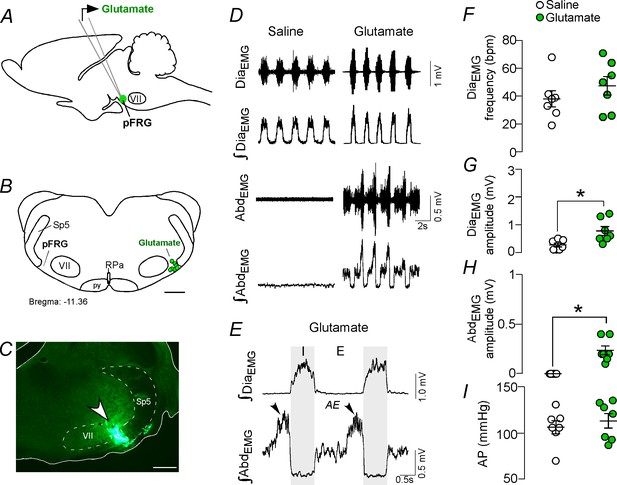
Activation of glutamatergic receptors in the pFRG region evoked active expiration.
(A) Experimental design. (B) Injection sites of glutamate (10 mM, 50 nL) into the pFRG region. (C) Photomicrography showing a typical injection site of glutamate into the pFRG region. (D) Traces showing breathing parameters (diaphragm electromyography [DiaEMG] and abdominal electromyography [AbdEMG]) from a representative experiment after the injection of saline or glutamate into the pFRG. (E) Expanded traces after glutamate injection into the pFRG. Black arrows show active expiration (AE) in the expanded traces. (F–I) Individual data for each parameter — (F) DiaEMG frequency (bpm), (G) DiaEMG amplitude (mV), (H) AbdEMG amplitude (mV) and (I) AP (mmHg) — after unilateral injections of saline (white circles; N = 7) or glutamate (green circles; N = 7) into pFRG. Lines show mean and SEM. *Different from saline, Paired Student’s t-test, p<0.05. Abbreviations: AbdEMG, abdominal electromyogram; AE, active expiration; AP, arterial pressure; bpm, breaths per minute; DiaEMG, diaphragm electromyogram; pFRG, parafacial respiratory group; py, pyrimidal tract; RPa, raphe pallidus; Sp5, spinal trigeminal tract; VII, facial motor nucleus. Scale bar in B = 1 mm and C = 0.5 mm.
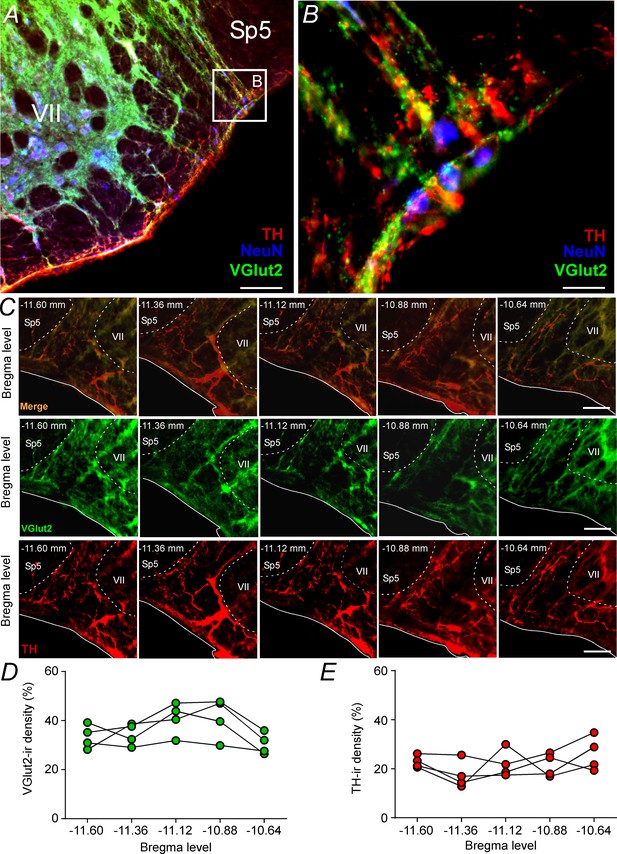
Glutamatergic and catecholaminergic innervations within the pFRG region.
(A, B) Single case showing the close apposition of glutamatergic (VGlut2; green) and/or catecholaminergic (TH; red) terminals in the pFRG neurons (NeuN; blue). (C) Rostro-caudal distribution of glutamatergic (VGlut2; green) and catecholaminergic (TH; red) terminal in the pFRG region (Bregma level: −11.60 at 10.64 mm). Note the overlap between the VGlut2 and TH terminals. (D) VGlut2-immunoreactivity density in a rostro-caudal distribution (Bregma level from −11.6 to −10.64 mm). (E) TH-immunoreactivity density in a rostro-caudal distribution (Bregma level from −11.6 to −10.64 mm). Abbreviations: NeuN, nuclear neuronal marker; Sp5, spinal trigeminal tract; TH, tyrosine hydroxylase; VGlut2, vesicular glutamate transporter 2; VII, facial motor nucleus;. Scale bar in panel (A) = 1 mm, in panel (B) = 10 μm, and in panel (C) = 100 μm.
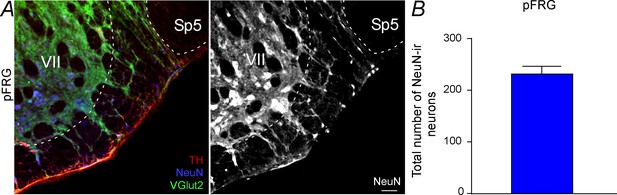
Total number of NeuN neurons located in the pFRG region.
(A) Photomicrography showing immunoreactive markers for tyrosine hydroxylase (TH, red) NeuN (blue) and VGlut2 (green) expression in the pFRG region and a split figure showing only NeuN expression in pFRG. (B) Rostro-caudal distribution of NeuN immunoreactive cells located in the pFRG region. Abbreviations: pFRG, parafacial respiratory group; Sp5, spinal trigeminal tract; VII, facial motor nucleus. Scale bar = 100 μm.
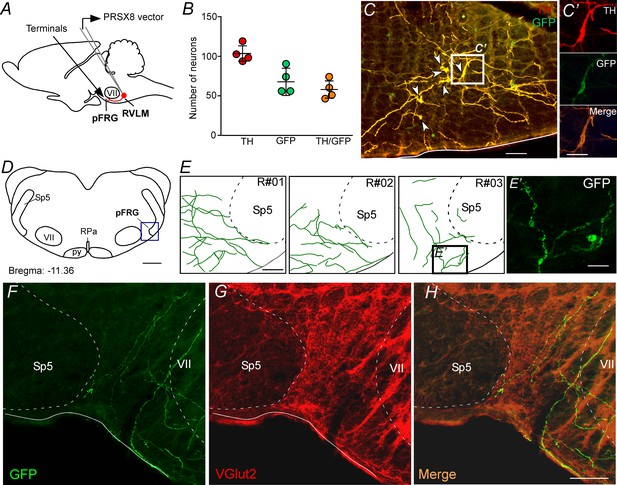
pFRG receives glutamatergic inputs from catecholaminergic C1 neurons.
(A) Experimental design. (B) The number of TH and GFP neurons in the RVLM (N = 4). Cell count was obtained in six coronary brain sections (40 μm with 240 μm intervals between slices) from each rat. (C, C’) Single case showing that the C1 neurons are transfected by the lentivirus PRSX8-ChR2-eYFP. (D, E) Schematic drawing of a coronal brain section showing the distribution of GFP terminals in the pFRG region in three rats (bregma level: −11.36 mm in accordance with Paxinos and Watson, 2007). (E’) Photomicrography showing terminals into the pFRG region of the R#03. (F–H) Photomicrography showing fibers and terminals expressing lentivirus (GFP, green) and glutamate (VGlut2, red) in the pFRG region. Abbreviations: Fn, facial nerve; GFP, green fluorescent protein; pFRG, parafacial respiratory group; NTS, nucleus of the solitary tract; py, pyramid tract; RPa, raphe pallidus; RVLM, rostral ventrolateral medulla; Sp5, spinal trigeminal tract; TH, tyrosine hydroxylase; VGlut2, vesicular glutamate transporter 2; VII, facial motor nucleus. Scale bars in panels (C) and (E–H) = 50 μm, (C’), (E’) and (H’) = 20 μm, (D) = 1 mm.
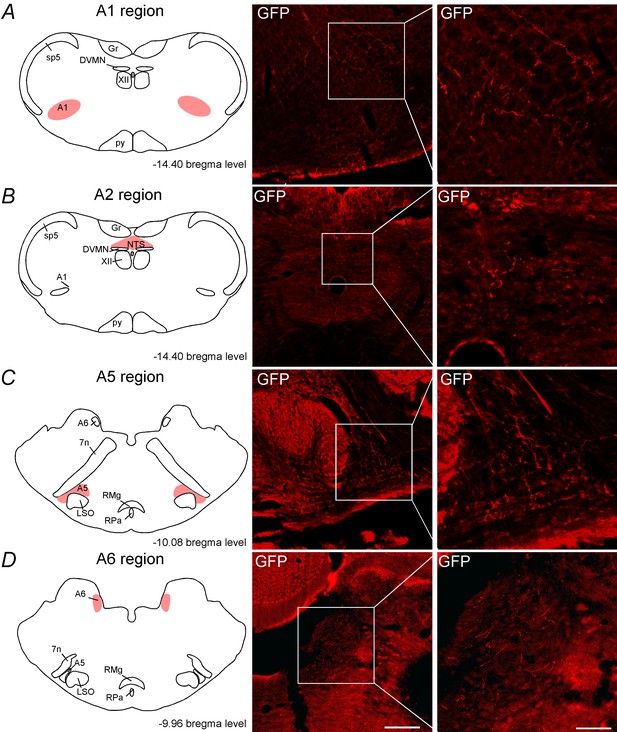
C1 neurons project to the noradrenergic neurons.
Schematic drawing showing the location of the catecholaminergic (A) A1 region, (B) A2 region, (C) A5 region and (D) A6 region. The photomicrographs show fibers and terminals arising from C1 neurons. Abbreviations: DVMN, dorsal vagus motor nucleus; Fn, facial nerve; Gr, gracile nucleus; LSO, lateral superior olive; NTS, nucleus of the solitary tract; py, pyramid tract; RMg, raphe magnus; RPa, raphe pallidus; Sp5, spinal trigeminal tract; XII, hypoglossal nucleus. Scale bar = 100 μm.
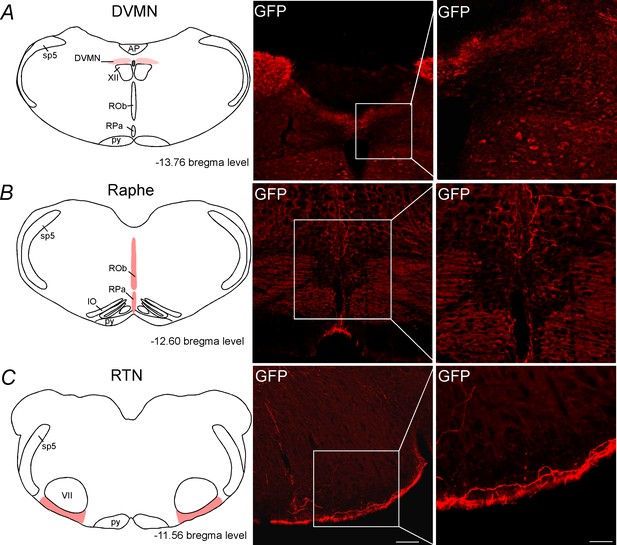
C1 neurons project to DVMN, medullary aphe and RTN regions.
Schematic drawing showing the location of the (A) dorsal vagus motor nucleus (DVMN), (B) the medullary raphe and (C) the retrotrapezoid nucleus (RTN). The photomicrographs show fibers and terminals arising from C1 neurons. Abbreviations: AP, area postrema; DVMN, dorsal vagus motor nucleus; IO, inferior olive; py, pyramid tract; ROb, raphe obscurus; RPa, raphe pallidus; Sp5, spinal trigeminal tract; XII, hypoglossal nucleus. Scale bar = 100 μm.
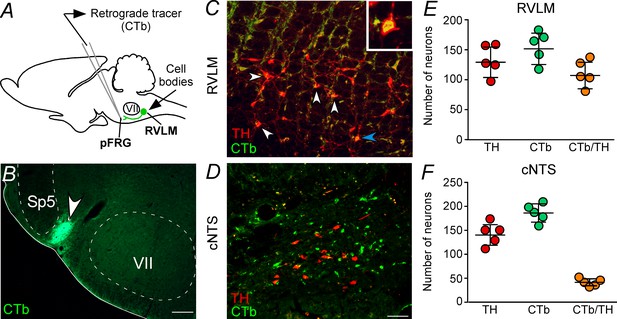
Catecholaminergic C1 neurons project to the pFRG region.
(A) Experimental design. (B) Single case showing a positive injection of the retrograde tracer CTb (1%, 30–50 nL) into the pFRG region. (C, D) Photomicrography of catecholaminergic (TH, red) and CTb (green)-labelled cells located in the RLVM and cNTS of one rat with CTb-positive injection into pFRG. White arrows indicate some examples of double labeled cells, whereas the blue arrow indicates the high magnification double label (CTb+/TH+) in the RVLM region. (E, F) Number of CTb and/or TH-labeled cells in the RVLM and cNTS (N = 5). Cell count was obtained in coronary brain sections (six sections from the RVLM and three sections from the cNTS, each of 40 μm thickness with 240 μm of intervals between slices) from each rat. Abbreviations: cNTS, commissural nucleus of the solitary tract; CTb, Cholera Toxin b; pFRG, parafacial respiratory group; RVLM, rostral ventrolateral medulla; Sp5, spinal trigeminal tract; TH, tyrosine hydroxylase; VII, facial motor nucleus. Scale bars in panel (B) = 0.5 mm and in panel (D) = 50 μm (this scale bar applies to panels [C] and [D]).
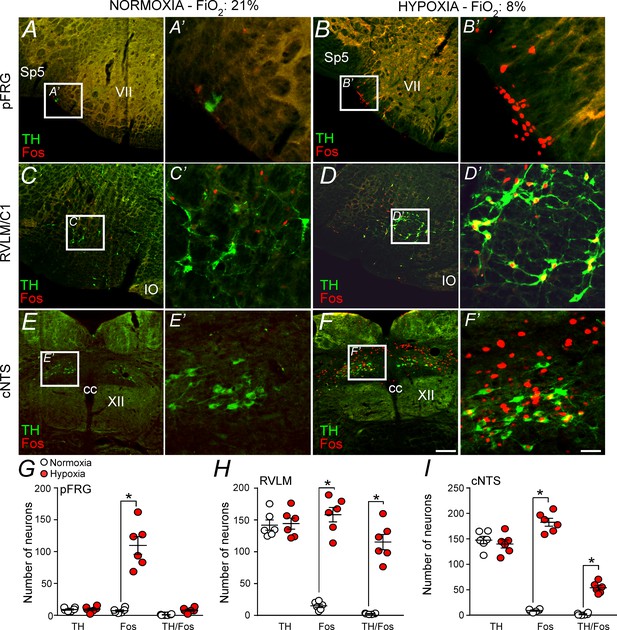
Hypoxia activates neurons in the pFRG, RVLM and cNTS.
(A–F) Photomicrography showing representative cases of TH- and Fos-labelled cells located in(A, B) the pFRG, (C, D) the RLVM and (E, F) the cNTS after normoxia (21% O2, balanced with N2; N = 6) and after hypoxia (3 hr in 8% O2, balanced with N2; N = 6). (A’–F’) Higher magnification of pFRG, RVLM and cNTS showing neurons labeled by Fos and/or TH. (G–I) Number of Fos and/or TH-labeled cells in the pFRG, RVLM and cNTS. Cell count was obtained in coronary brain sections (five sections from the pFRG, six sections from the RVLM and three sections from the cNTS of 40 μm in thickness with 240 μm intervals between slices) from each rat. Abbreviations: cc, central canal; cNTS, commissural nucleus of the solitary tract; IO, inferior olive; pFRG, parafacial respiratory group; RVLM, rostral ventrolateral medulla; Sp5, spinal trigeminal tract; TH, tyrosine hydroxylase; VII, facial motor nucleus; XII, hypoglossal nucleus. *Different from normoxia. Scale bar in panel (F) = 100 μm, this scale bar also applies topanels (A–F), whereas the scale bar in panel (F’) = 20 μm applies to panels (A’-F’).
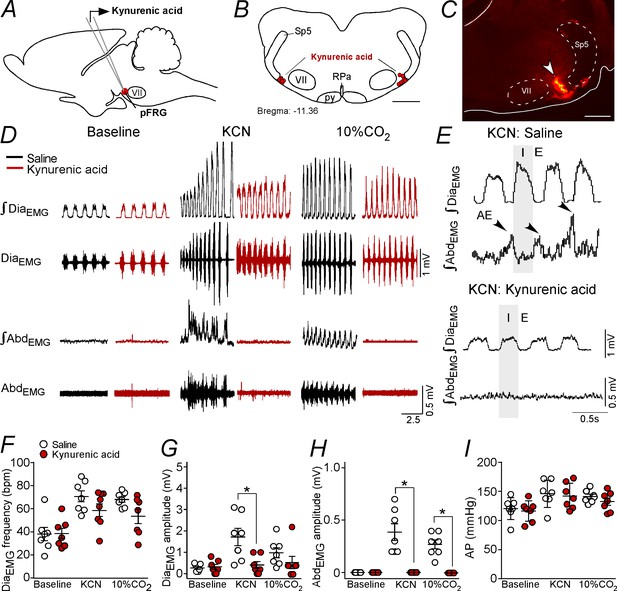
Kynurenic acid injection into the pFRG region blunted active expiration induced by hypoxia and hypercapnia.
(A) Experimental design. (B) Bilateral sites for the injection of kynurenic acid (100 mM, 50 nL) into pFRG. (C) Photomicrography showing a typical site of kynurenic acid injection into pFRG. (D) Traces showing breathing parameters (diaphragm electromyography [DiaEMG] and abdominal electromyography [AbdEMG]) from a representative experiment after the injection of saline or kynurenic acid into the pFRG in a condition of cytotoxic hypoxia (KCN) or hypercapnia (10% CO2). (E) Expanded traces showing the presence of active expiration (AE) (black arrow) after intravenous injection of KCN in a saline-treated rat and the absence of AE induction by KCN when ionotropic glutamatergic receptors are blockaded at the level of pFRG region. (F–I) Individual data for each parameter: (F) DiaEMG frequency (bpm), (G) DiaEMG amplitude (mV), (H) AbdEMG amplitude (mV) and (I) AP (mmHg) after bilateral injections of saline (white circles; N = 7) or kynurenic acid (red circles; N = 7) into pFRG under baseline, KCN or 10% CO2 conditions. Lines show mean and SEM. *Different from saline; two-way ANOVA, p<0.05. Abbreviations: AbdEMG, abdominal electromyogram; AP, arterial pressure; bpm, breaths per minute; DiaEMG, diaphragm electromyogram; KCN, potassium cyanide; pFRG, parafacial respiratory group; py, pyriamidal tract; RPa, raphe pallidus; Sp5, spinal trigeminal tract; VII, facial motor nucleus. Scale bar in panel (B) = 1 mm and in panel (C) = 0.5 mm.
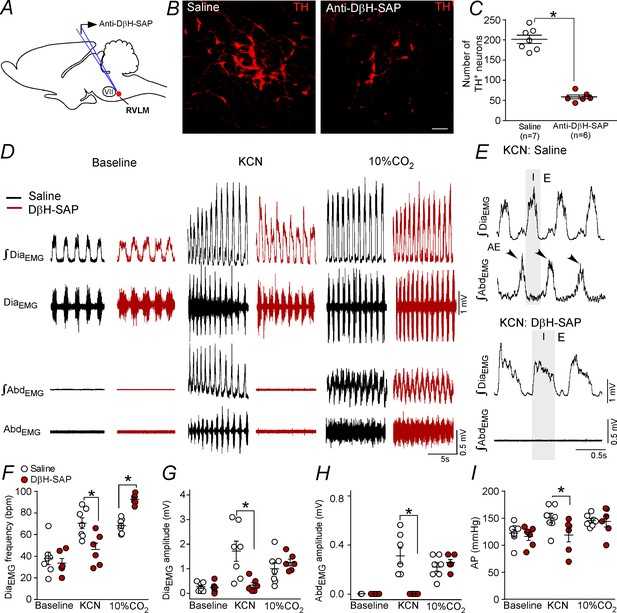
Ablation of the catecholaminergic C1 neurons blunted the active expiration induced by hypoxia, but not by hypercapnia.
(A) Experimental design. (B) Photomicrography showing the C1 regions after bilateral injection of saline or anti-DβH-SAP (2.4 ng/100 nL) into the RVLM. (C) Number of TH neurons into the RVLM of the saline and anti-DβH-SAP-treated groups. (D) Traces showing breathing parameters (diaphragm electromyography [DiaEMG] and abdominal electromyography [AbdEMG]) from a representative experiment in a rat treated with saline or anti-DβH-SAP into the C1 region in a condition of cytotoxic hypoxia (KCN) or hypercapnia (10% CO2). (E) Expanded traces showing the presence of active expiration (AE) (black arrow) after intravenous injection of KCN into a saline-treated rat and the absence of AE by KCN in an anti-DβH-SAP-treated rat. (F–I) Individual data for each parameter: (F) DiaEMG frequency (bpm), (G) DiaEMG amplitude (mV), (H) AbdEMG amplitude (mV) and (I) AP (mmHg) after bilateral injection of saline (white circles; N = 7) or anti-DβH-SAP (red circles; N = 6) into the RVLM under baseline KCN or 10% CO2. Lines show mean and SEM. *Different from saline; two-way ANOVA, p<0.05. Abbreviations: AbdEMG, abdominal electromyogram; Anti-DβH-SAP, immunotoxin anti-dopamine β-hydroxylase-saporin; AP, arterial pressure; bpm, breaths per minute; DiaEMG, diaphragm electromyogram; KCN, potassium cyanide; RVLM, rostral ventrolateral medulla; VII, facial motor nucleus. Scale bar in panel (B) = 25 μm.
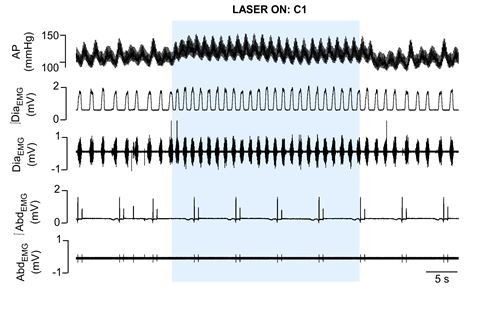
Cardiorespiratory effects elicited by optogenetic activation of C1 cells in urethane anaesthetized, vagotomized and artificially ventilated adult rats.
The arterial pressure (AP); diaphragm electromyography activity (DiaEMG); and abdominal electromyography (AbdEMG) were measure before, during and after the 30 seconds of C1 photostimulation (474 nm blue laser).





