Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway
Figures
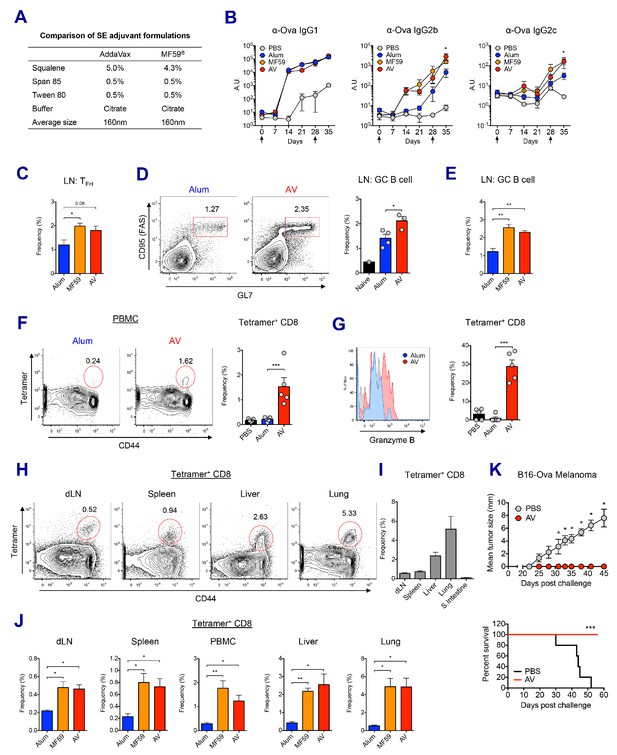
MF59 and AddaVax induce robust antibody and CD8 T cell responses.
(A) Formulation comparison of two SE adjuvants; AddaVax and MF59 (B) WT B6 mice were primed and boosted with Ova mixed with MF59, AV or alum at indicated time points (arrows). Levels of Ova-specific IgG1, IgG2b and IgG2c in serum were determined by ELISA. *p<0.05 (t-test). (C–J) After the prime-boost vaccination with Ova plus MF59, AV or alum, immune responses were measured at day 7 post-boost. (C) Frequency of follicular helper T cells (CXCR5+ PD1+ CD4+) *p<0.05 (t-test) (D and E) Frequency of germinal center B cells *p<0.05 (t-test). **p<0.01 (t-test) (F) Ova-specific CD8 T cell response was assessed by class I MHC-peptide tetramer staining. ***p<0.001 (t-test). (G) Granzyme B expression was measured among Ova-specific CD8 T cells. ***p<0.001 (t-test). (H, I and J) FACS plots and bar graphs displaying Ova-specific CD8 T cell responses in different organs. (K) 10 days after the prime-boost vaccination with Ova alone or Ova plus AV, WT B6 mice were challenged with Ova-expressing B16 melanoma. Graphs show size of tumor (upper panel) and survival of mice (lower panel). *p<0.05 (ANOVA). For the survival curve, p value was determined by Log-rank (Mantel-Cox) test. Data are representative of two to three independent experiments (mean and s.e.m.).
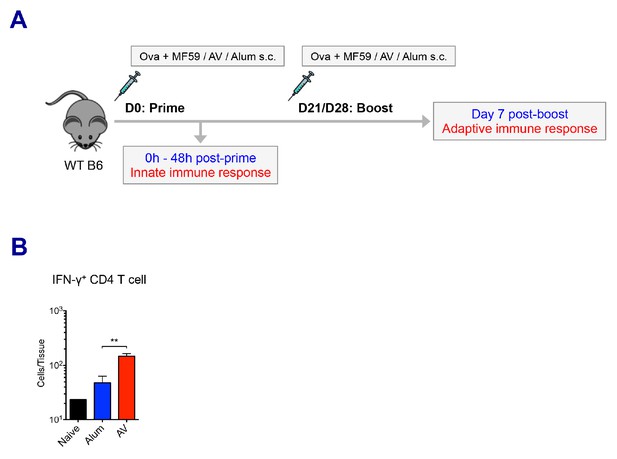
Experimental design and adoptive immune responses by alum, MF59 and AV.
(A) A cartoon depicts the prime-boost immunization strategy. (B) Numbers of IFN-γ+ CD4 T cells in dLNs. **p<0.01 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
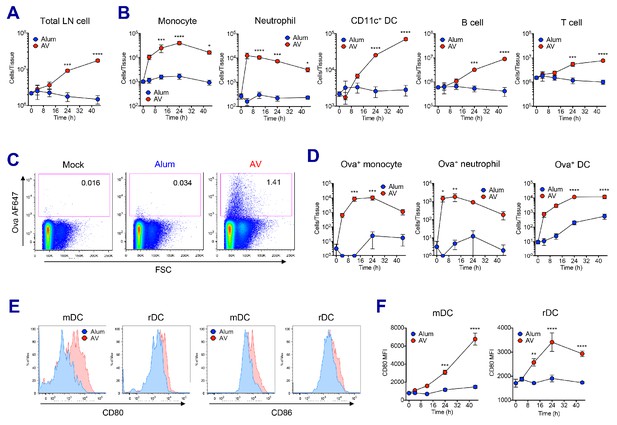
AV elicits robust innate immune responses.
WT B6 mice were vaccinated with Ova mixed with AV or alum, and innate immune responses were assessed in dLNs during the first 48 hr period. (A) Total cell numbers of inguinal LNs. (B) Absolute numbers of different immune cell populations. (C) At 12 hr post-vaccination, the uptake of antigen was measured using AF647-conjugated Ova. (D) Total numbers of Ova+ immune cell subsets were plotted. (E) Histograms show surface expression of CD80 and CD86 on migratory DCs and resident DCs. (F) Kinetic analysis of CD80 expression. Data are representative of two independent experiments (mean and s.e.m.). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (ANOVA).
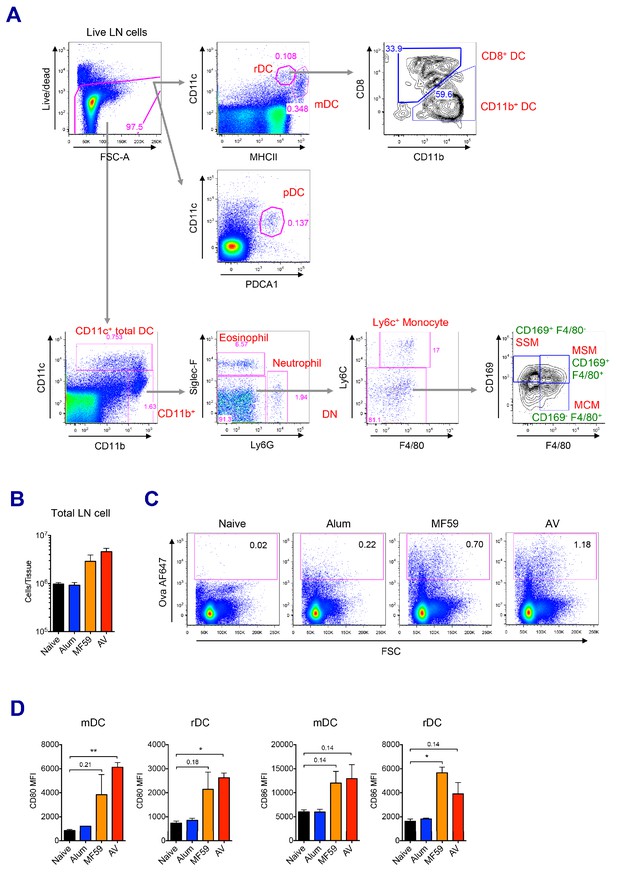
Analysis of innate immune response.
(A) Gating strategy of various innate immune cells in mouse LNs. (B–D) WT B6 mice were vaccinated with Ova mixed with MF59, AV or alum, and innate immune responses were assessed. (B) Total cell numbers of inguinal LNs at 24 hr post-vaccination. (C) At 12 hr post-vaccination, the uptake of antigen was measured using AF647-conjugated Ova. (D) Bar graphs show surface expression of CD80 and CD86 on migratory DCs and resident DCs at 24 hr after immunization. *p<0.05, **p<0.01 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
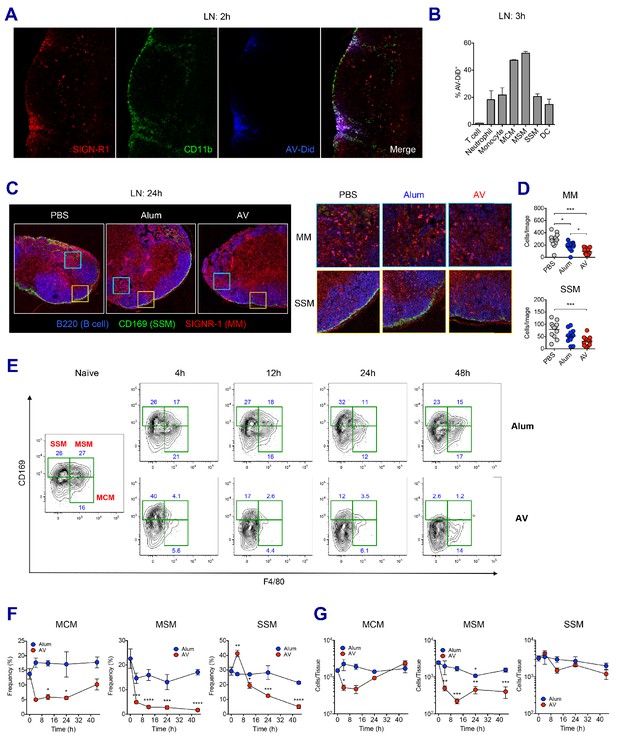
LN-resident macrophages uptake SE adjuvant and are eliminated.
(A and B) WT B6 mice were vaccinated with Ova together with AV-Did, and the uptake of AV-Did was measured in dLNs by immunofluorescence at 2 hr (A) and flow cytometry at 3 hr (B). (C and D) Immunofluorescence of dLNs at 24 hr post-vaccination with PBS, alum or AV. (D) Quantification of MMs (upper) and SSMs (lower) from the acquired confocal images using CellProfiler software. *p<0.05, ***p<0.001 (ANOVA). (E–G) Kinetic changes of dLN-resident macrophage subsets were analyzed by flow cytometry. FACS plots are pre-gated on CD11b+CD11c- Siglec-F- Ly6G- Ly6C- population. Frequencies (F) and absolute numbers (G) of each macrophage subset were plotted. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (ANOVA). Data are representative of two to three independent experiments (mean and s.e.m.).
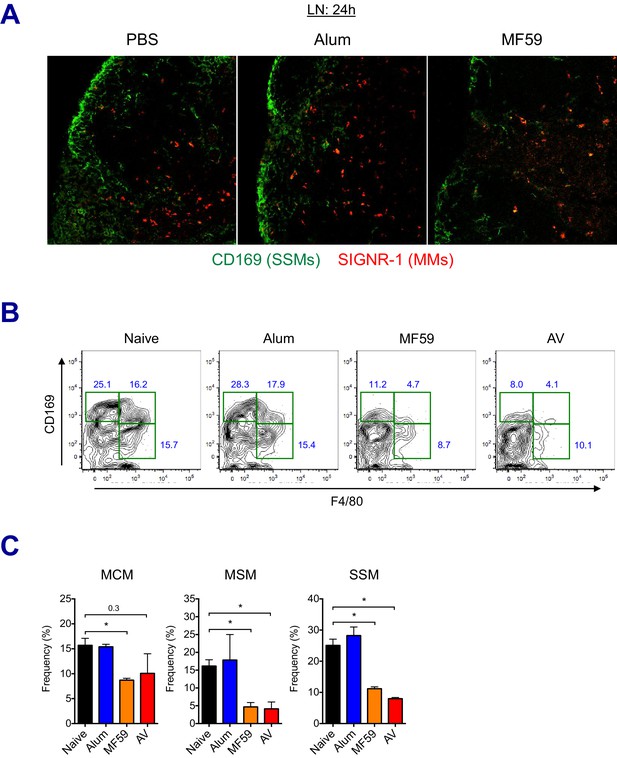
LN-resident macrophages uptake MF59 and undergo elimination.
(A) Immunofluorescence of dLNs at 24 hr post-vaccination with PBS, alum or MF59. (B–C) Frequencies of dLN-resident macrophage subsets were analyzed by flow cytometry at 24 hr post-immunization. FACS plots are pre-gated on CD11b+CD11c- Siglec-F- Ly6G- Ly6C- population. *p<0.05 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
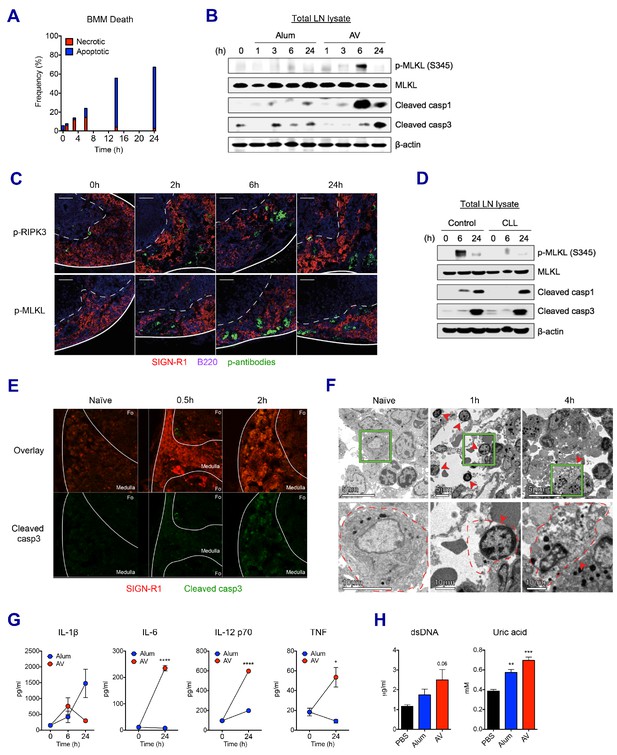
SE adjuvant triggers sequential waves of regulated necrosis and apoptosis in LN macrophages.
(A) Bone marrow-derived macrophages were incubated with AV for different time periods. Using co-staining of AnnexinV and Propidium Iodide, kinetic changes of necrotic and apoptotic cells were determined by flow cytometry. (B) After WT B6 mice were immunized with alum or AV, dLNs from each group were collected, and total dLN lysates were subjected to western blot analysis at indicated time points. (C) Immunofluorescence of dLNs at indicated time points to detect necroptosis signaling. (D) Western blot analysis with dLN lysates from control and CLL-treated WT B6 mice. (E) Immunofluorescence of dLNs at 0 hr, 0.5 hr and 2 hr post-vaccination. (F) Electron micrographs displaying AV-vaccinated dLNs at different time points. Red arrows represent cells with necrotic phenotype. (G) Serum levels of different cytokines were assessed by ELISA. *p<0.05, ****p<0.0001 (ANOVA). (H) At 24 hr post-immunization, the release of dsDNA and uric acid was determined in serum. **p<0.01, ***p<0.001 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
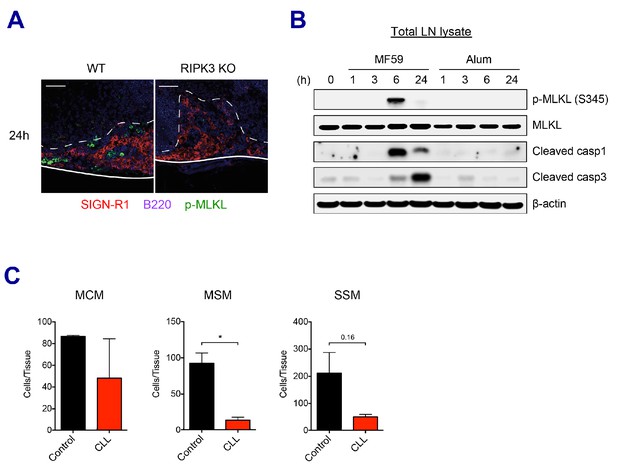
SE adjuvants triggers cell death signaling pathways.
(A) Immunofluorescence of dLNs from WT B6 and RIPK3 KO mice at 24 hr post-vaccination with AV. (B) Mice were immunized with MF59 or alum. dLNs were harvested at indicated time points and analyzed by western blot. (C) 5 days post-injection of control liposome or CLL, the depletion of each macrophage subset was assessed in dLNs. *p<0.05 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
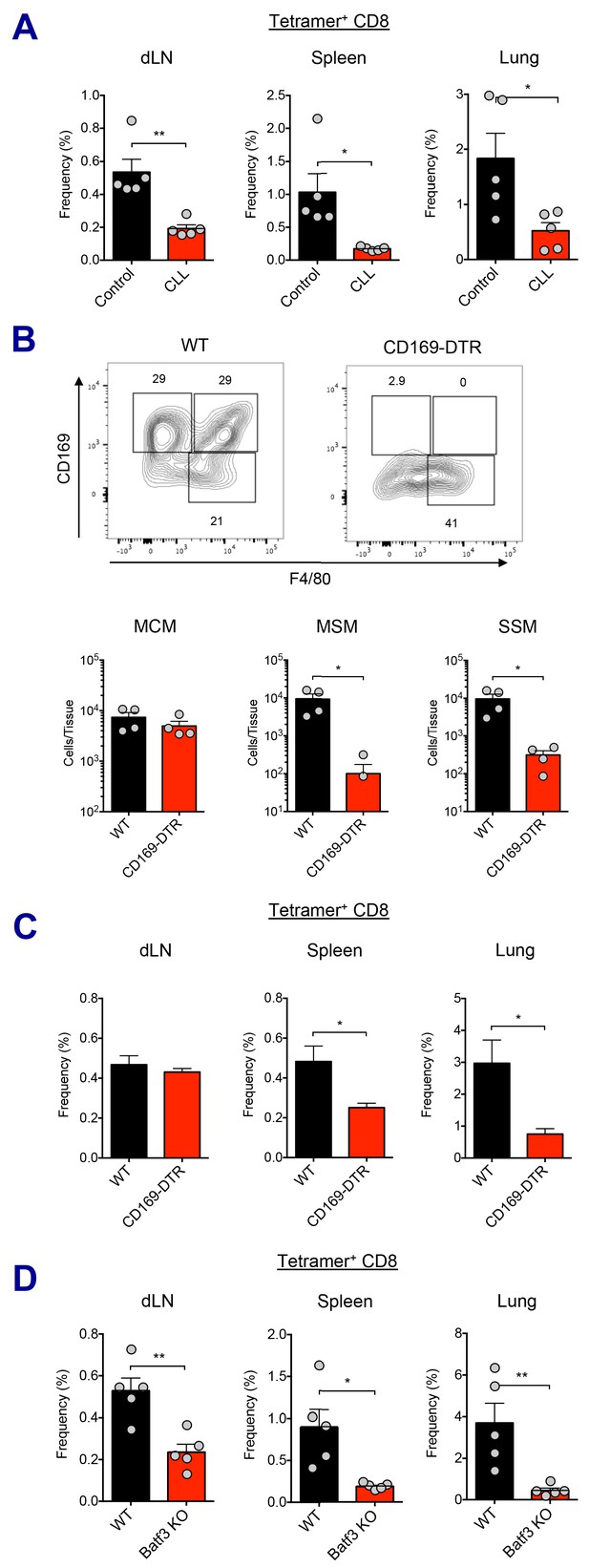
Macrophages and Batf3+ DCs cooperatively stimulate CD8 T cell response.
(A) WT B6 mice were injected with control or clodronate-loaded liposomes. 5 days later, mice were subsequently immunized with Ova and AV. Ova-specific CD8 T cell response at day 7 post-boost in different tissues. *p<0.05, **p<0.01 (t-test). (B and C) Mice got intraperitoneal injection with 400 ng of diphtheria toxin per mouse, and two days later, they were subsequently immunized by Ova plus AV. (B) The depletion of LN-resident macrophages was measured in CD169-DTR mice two days after the DT injection. *p<0.05 (t-test). (C) Ova-specific CD8 T cell responses in WT B6 and CD169-DTR mice at day 7 post-boost. *p<0.05 (t-test). (D) Ova-specific CD8 T cell response in WT B6 and Batf3 KO mice. *p<0.05, **p<0.01 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
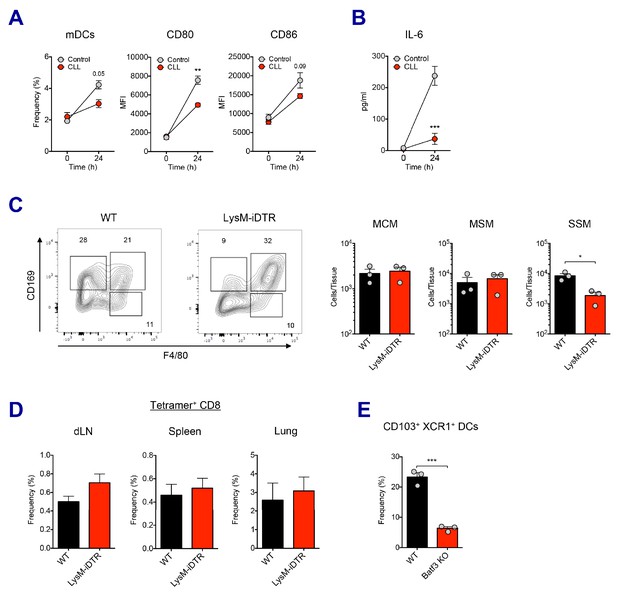
Association of macrophages and dendritic cells in SE adjuvant-mediated immune responses.
(A) 5 days after CLL injection, mice were immunized with Ova and AV. 24 hr post-immunization, mDC population and its activation status was measured. **p<0.01 (ANOVA). (B) Serum IL-6 level was determined at 24 hr post-immunization. ***p<0.001 (ANOVA). (C) The depletion of LN-resident macrophages was measured in LysM-iDTR mice two days after the DT injection. *p<0.05 (t-test). (D) CD8 T cell response in LysM-iDTR mice at day 7 post-boost. (E) Comparison of CD103+ XCR1+ DC levels in LNs between WT B6 and Batf3 KO mice. ***p<0.001 (t-test).
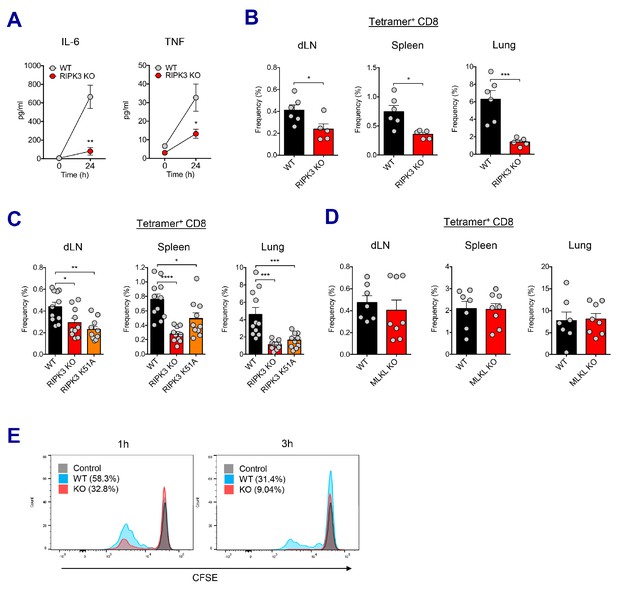
RIPK3 kinase-dependent signaling is critical for the induction of CD8 T cell response.
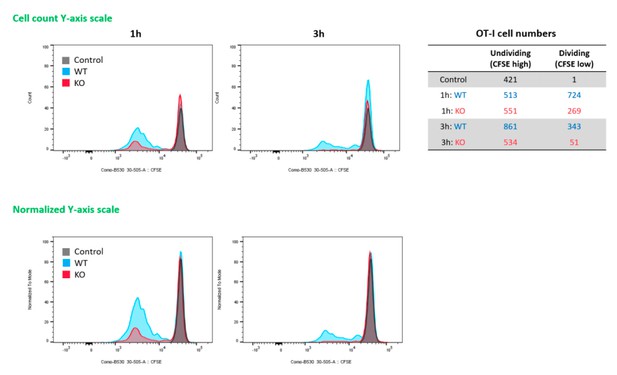
(A) WT B6 and RIPK3 KO mice were primed with Ova and AV, and serum cytokine levels were quantified at 24 hr. (B) At day 7 post-boost, Ova-specific CD8 T cell response was determined from different tissues in WT B6 and RIPK3 KO mice. *p<0.05, ***p<0.001 (t-test). (C) Ova-specific CD8 T cell responses in WT B6, RIPK3 KO and RIPK3 K51A kinase-dead mice. Data were pooled from two independent experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (t-test). (D) Ova-specific CD8 T cell responses in WT B6 and MLKL KO mice. (E) Histograms represent OT-I cell proliferation upon Ag cross-presentation (1h- or 3h-incubation of BMMs with AV). Gray, blue and red colors represent control, WT B6 BMM and RIPK3 KO BMM groups, respectively. Data are representative of two to three independent experiments (mean and s.e.m.).
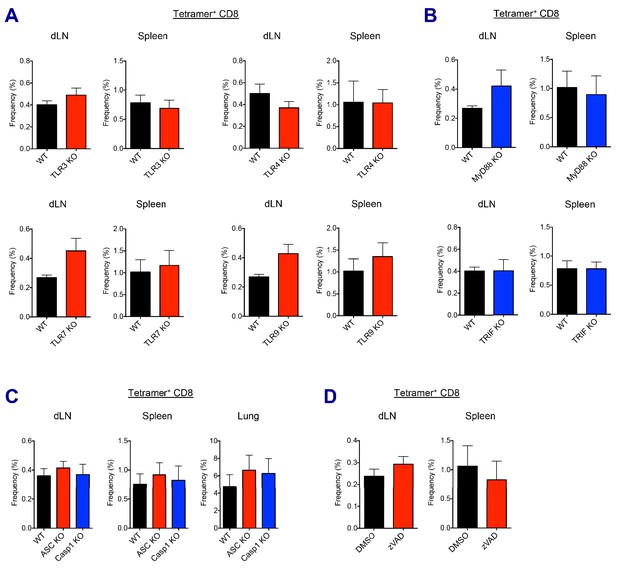
AV-induced CD8 T cell responses in different KO mice and drug treatment.
(A and B) Ova-specific CD8 T cell responses were determined at day 7 post-boost in different TLR KO, MyD88 or TRIF KO mice. (C) Ova-specific CD8 T cell responses were determined at day 7 post-boost in WT B6, ASC KO and Caspase 1 KO mice. (D) WT B6 mice were immunized in the presence or absence of z-VAD-fmk. At day 7 post-boost, CD8 T cell responses were analyzed. Data are representative of two independent experiments (mean and s.e.m.).
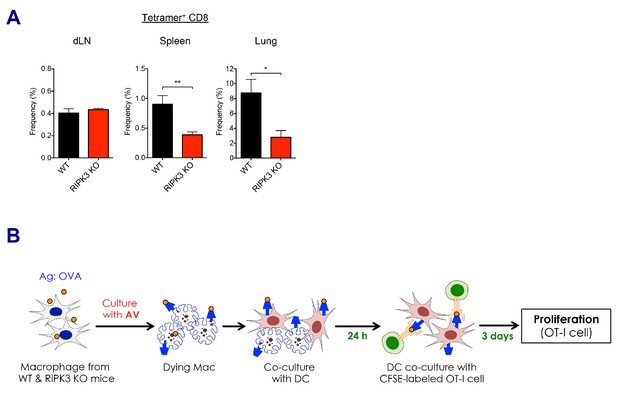
CD8 T cell responses in the absence of RIPK3 by SE adjuvants.
(A) After prime-boost vaccination with Ova and MF59, Ova-specific CD8 T cell responses were determined in WT B6 and RIPK3 KO mice. *p<0.05, **p<0.01 (t-test). Data are representative of two independent experiments (mean and s.e.m.). (B) Experimental procedure of in vitro cross-presentation assay. Briefly, BMMs from WT B6 or RIPK3 KO mice were incubated with AV and Ova, followed by co-culture with WT BMDCs. Subsequently, BMDCs were incubated with CFSE-labeled OT-I cells for 3 days, and numbers of cell division were analyzed by flow cytometer.
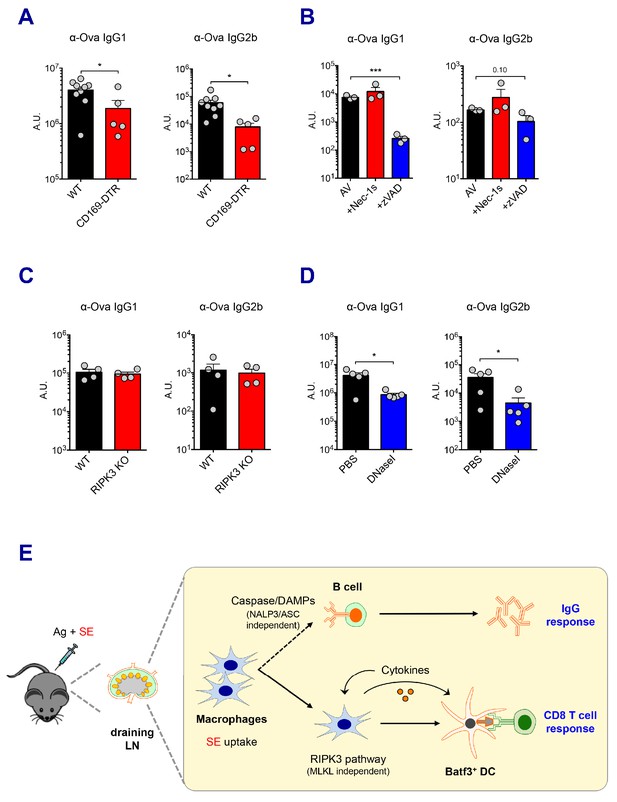
Caspase activity and DAMPs promote IgG responses.
Levels of IgG1 and IgG2b were assessed in serum samples at day 7 post-boost. (A) IgG levels in WT B6 and CD169-DTR mice at day 7 post-boost. (B) WT B6 mice were immunized with AV in the presence or absence of Nec-1s or z-VAD-fmk. (C) IgG levels in WT B6 and RIPK3 KO mice. (D) WT B6 mice were primed and boosted with AV in the presence or absence of DNaseI. (E) A working model describing distinctive mechanisms governing the IgG and CD8 T cell responses induced by the SE adjuvant. *p<0.05, ***p<0.001 (t-test). Data are representative of two to three independent experiments (mean and s.e.m.).
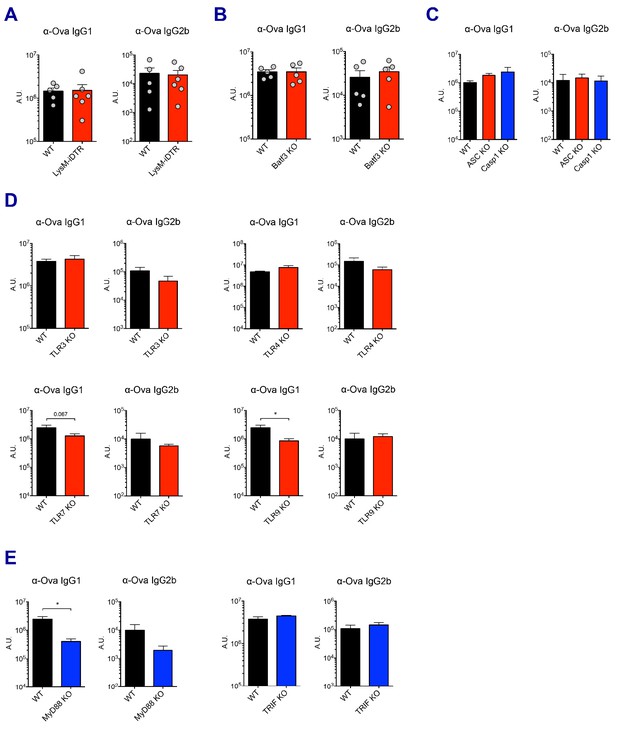
Ova-specific antibody responses.
(A–E) Ova-specific IgG1 and IgG2b levels in indicated experimental groups were determined in sera at day 7 post-boost. *p<0.05 (t-test). Data are representative of two independent experiments (mean and s.e.m.).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | C57BL/6J | The Jackson Laboratory | Stock No: 000664 | |
| Genetic reagent (M. musculus) | Batf3 KO | The Jackson Laboratory | Stock No: 013755 | |
| Genetic reagent (M. musculus) | Caspase 1 KO | The Jackson Laboratory | Stock No: 016621 | |
| Genetic reagent (M. musculus) | TLR3 KO | The Jackson Laboratory | Stock No: 005217 | |
| Genetic reagent (M. musculus) | TRIF KO | The Jackson Laboratory | Stock No: 005037 | |
| Genetic reagent (M. musculus) | RIPK3 KO | PMID:14749364 | ||
| Genetic reagent (M. musculus) | RIPK3K51A/K51A | PMID:25459880 | ||
| Genetic reagent (M. musculus) | CD169-DTR | PMID:23601688 | ||
| Genetic reagent (M. musculus) | LysM-iDTR | PMID:20176743 | ||
| Genetic reagent (M. musculus) | ASC KO | PMID:15190255 | ||
| Genetic reagent (M. musculus) | MLKL KO | PMID:24012422 | ||
| Genetic reagent (M. musculus) | TLR4 KO | PMID:16461338 | ||
| Genetic reagent (M. musculus) | TLR7 KO | PMID:16461338 | ||
| Genetic reagent (M. musculus) | TLR9 KO | PMID:20962088 | ||
| Genetic reagent (M. musculus) | MyD88 KO | PMID:16461338 | ||
| Antibody | CD11c (Hamster monoclonal) | BioLegend | 117330 | FCM 1:400 |
| Antibody | CD169 (Rat monoclonal) | BioLegend | 142413 | FCM 1:400 |
| Antibody | CD19 (Rat monoclonal) | BioLegend | 115541 | FCM 1:800 |
| Antibody | TCR-b (Hamster monoclonal) | BioLegend | 109224 | FCM 1:400 |
| Antibody | CD4 (Rat monoclonal) | BioLegend | 100557 | FCM 1:800 |
| Antibody | CD44 (Rat monoclonal) | BioLegend | 103047 | FCM 1:800 |
| Antibody | CD45R (B220) (Rat monoclonal) | BioLegend | 103244 103229 | FCM 1:800 IF 1:400 |
| Antibody | CD80 (Hamster monoclonal) | BioLegend | 104729 | FCM 1:800 |
| Antibody | CD86 (Rat monoclonal) | BioLegend | 105006 | FCM 1:800 |
| Antibody | CD8a (Rat monoclonal) | BioLegend | 100750 | FCM 1:800 |
| Antibody | IFN-g (Rat monoclonal) | BioLegend | 505806 | FCM 1:800 |
| Antibody | Ly6G (Rat monoclonal) | BioLegend | 127624 | FCM 1:800 |
| Antibody | Rabbit IgG AF647 (Donkey polyclonal) | BioLegend | 406421 | IF 1:400 |
| Antibody | Fc block (Rat monoclonal) | BD Biosciences | 553142 | FCM 1:800 |
| Antibody | CD103 (Rat monoclonal) | BD Biosciences | 564322 | FCM 1:400 |
| Antibody | CD11b (Rat monoclonal) | BD Biosciences | 563402 | FCM 1:800 |
| Antibody | CD279 (PD-1) (Hamster monoclonal) | BD Biosciences | 563059 | FCM 1:200 |
| Antibody | CD45 (Rat monoclonal) | BD Biosciences | 563053 | FCM 1:800 |
| Antibody | CD95 (Hamster monoclonal) | BD Biosciences | 563646 | FCM 1:800 |
| Antibody | CXCR5 (Rat monoclonal) | BD Biosciences | 551961 | FCM 1:100 |
| Antibody | GL7 (Rat monoclonal) | BD Biosciences | 562967 | FCM 1:400 |
| Antibody | F4/80 (Rat monoclonal) | eBioscience | 25-4801-82 | FCM 1:400 |
| Antibody | Ly6C (Rat monoclonal) | eBioscience | 45-5932-82 | FCM 1:800 |
| Antibody | MHC II (I-A/I-E) (Mouse monoclonal) | eBioscience | 56-5321-82 | FCM 1:800 |
| Antibody | CD209b (SIGN-R1) (Hamster monoclonal) | eBioscience | 16-2093-82 | IF 1:200 |
| Antibody | Anti-Rabbit IgG (Goat polyclonal) | Life Technologies | A-11070 | FCM 1:1000 |
| Antibody | Granzyme B (Mouse monoclonal) | Invitrogen | GRB05 | FCM 5 μl |
| Antibody | p-RIP3 (T231/S232) (Rabbit monoclonal) | Cell Signaling Technology | 91702 | IF 1:200 |
| Antibody | p-MLKL (S345) (Rabbit monoclonal) | Cell Signaling Technology | 37333 | IF 1:200 |
| Antibody | Cleaved caspase-3 (Rabbit polyclonal) | Cell Signaling Technology | 9661 | IF 1:400 |
| Antibody | MLKL (Rabbit monoclonal) | Cell Signaling Technology | 37705 | WB 1:1000 |
| Antibody | Cleaved caspase-1 (Rabbit polyclonal) | Santa Cruz Biotechnology | sc-514 | WB 1:500 |
| Antibody | Beta-actin (Rabbit polyclonal) | Cell Signaling Technology | 4967 | WB 1:1000 |
| Antibody | p-MLKL (S345) (Rabbit monoclonal) | Abcam | ab196436 | WB 1:1000 |
| Antibody | mouse IgG1-HRP (Goat polyclonal) | Southern Biotech | 1070–05 | ELISA 1:5000 |
| Antibody | mouse IgG2b-HRP (Goat polyclonal) | Southern Biotech | 1090–05 | ELISA 1:5000 |
| Antibody | mouse IgG2c-HRP (Goat polyclonal) | Southern Biotech | 1079–05 | ELISA 1:5000 |
| Peptide, recombinant protein | K(b)/Ova.SIINFEKL tetramer | NIH Tetramer Core Facility | FCM 1:400 | |
| Peptide, recombinant protein | EndoGrade Ovalbumin | Hyglos | 321001 | For injection |
| Peptide, recombinant protein | Ova-AF647 | Life Technologies | O34784 | |
| Peptide, recombinant protein | Albumin, chicken egg white (Ovalbumin) | Sigma-Aldrich | A2512 | For ELISA |
| Peptide, recombinant protein | Uricase from Candida sp. | Sigma-Aldrich | U0880-250UN | |
| Peptide, recombinant protein | DNase I | Roche | 10104159001 | |
| Peptide, recombinant protein | Murine GM-CSF | PeproTech | 315–03 | |
| Peptide, recombinant protein | Murine M-CSF | PeproTech | 315–02 | |
| Peptide, recombinant protein | Collagenase IV | Worthington Biochemical | LS004189 | |
| Peptide, recombinant protein | Streptavidin-PE | BD Biosciences | 554061 | |
| Chemical compound, drug | OptEIA TMB Substrate | BD Biosciences | 555214 | |
| Peptide, recombinant protein | Annexin V | Biolegend | 640906 | FCM 5 μl |
| Chemical compound, drug | MF59 | Novartis | ||
| Chemical compound, drug | AddaVax | InvivoGen | vac-adx-10 | |
| Chemical compound, drug | Alhydogel | InvivoGen | vac-alu-250 | |
| Chemical compound, drug | z-VAD-fmk | Cayman Chemical | 14463 | |
| Chemical compound, drug | Nec-1s | BioVision | 2263–5 | |
| Chemical compound, drug | Live/Dead Aqua stain kit | Invitrogen | L34957 | FCM 1:1000 |
| Chemical compound, drug | Blotting-grade blocker | Bio-Rad | 1706404 | |
| Chemical compound, drug | Protease/phosphatase inhibitor cocktail | Cell Signaling Technology | 5872 | |
| Chemical compound, drug | SuperSignalWest Femto/Dura chemiluminescent substrate | Thermo Scientific | 34094/34075 | |
| Commercial assay or kit | Mouse IL-1b ELISA kit | BD Biosciences | 559603 | |
| Commercial assay or kit | Mouse IL-6 ELISA kit | BD Biosciences | 555240 | |
| Commercial assay or kit | Mouse IL-12 (p70) ELISA Set | BD Biosciences | 555256 | |
| Commercial assay or kit | Mouse TNF ELISA Set II | BD Biosciences | 558534 | |
| Commercial assay or kit | Quanti-iT PicoGreen dsDNA assay kit | Invitrogen | P11496 | |
| Commercial assay or kit | Uric Acid Assay kit | Abcam | ab65344 | |
| Commercial assay or kit | Standard macrophage depletion kit | Encapsula NanoSciences | 8901 | Clodronate liposome |
| Software, algorithm | FlowJo | BD | ||
| Software, algorithm | Prism | GraphPad | ||
| Software, algorithm | CellProfiler | Broad Institute |