A native prokaryotic voltage-dependent calcium channel with a novel selectivity filter sequence
Figures
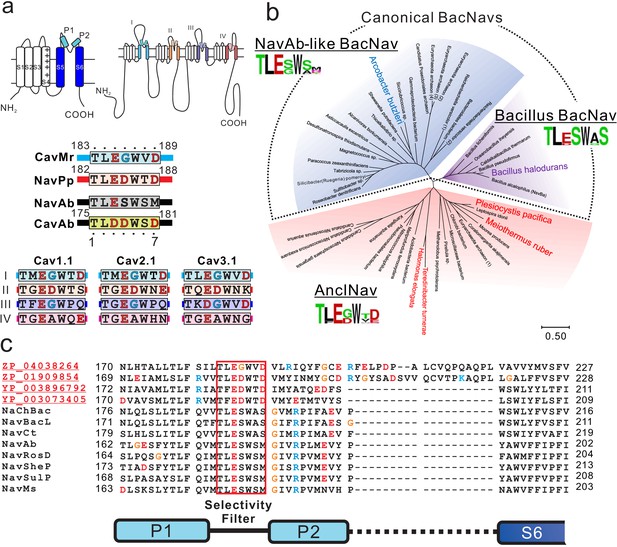
Sequence analysis of ancestor-like BacNavs.
(a) Schematic secondary structure and selectivity filter sequence of BacNavs and 24TM channels. A cylinder indicates an α-helix. The selectivity filter sequences are indicated using single-letter codes. Negatively charged residues are colored in red. Glycine residues in the position four are colored in cyan. The straight lines indicate the other parts of the pore domain. The selectivity filter sequences of hCav1.1 (UniProt ID: Q13698), hCav2.1 (O00555) and hCav3.1 (O43497) were used. (b) Phylogenetic tree of canonical BacNavs and ancestor-like BacNavs (AnclNavs). The MUSCLE program was used to align the multiple protein sequences of the channels (Figure 1—source data 1). The phylogenetic tree was generated using MEGA X. The branch lengths are proportional to the sequence divergence, with the scale bar corresponding to 0.5 substitutions per amino acid position. Three phylogenetically distinct groups are shown in different background colors (purple, Bacillus BacNavs; blue, NavAb-like BacNavs; red, AnclNavs). Four homologs with the taxon name colored in red in the AnclNav group were cloned and expressed to check the channel activity. Two of those, which are shown in larger and bold text, generated the detectable currents. The appearance frequency of amino acids in each of the selectivity filter sequences is shown under the respective group names. (c) Alignment of the deduced amino-acid sequences of the P1 helix to P2 helix domain of novel cloned homologs of AnclNavs with well characterized BacNavs.
-
Figure 1—source data 1
Amino-acid sequences used for making phylogenetic tree.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig1-data1-v1.fas
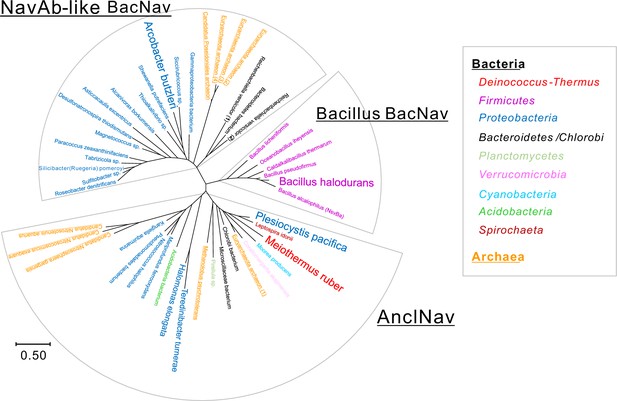
Distribution of the bacterial phylum and archaea in the phylogenetic tree of BacNavs.
Bacterial species that have a BacNav homolog are separately colored according to their different phyla, plus the archaea colored in orange. The branch lengths are proportional to the sequence divergence, with the scale bar corresponding to 0.5 substitutions per amino acid position.
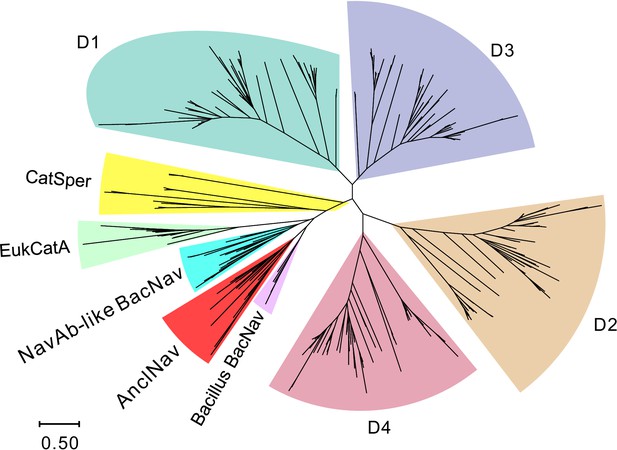
Phylogenetic analysis of BacNavs with eukaryotic voltage-gated cation channels.
Protein sequences of eukaryotic Cavs and Navs (Figure 1—figure supplement 2—source data 1) were divided into four subdomains and phylogenetically analyzed (D1–D4) with other single-domain channel types, including CatSper (yellow) and diatom EukCatA (dark green) channels. The branch lengths are proportional to the sequence divergence, with the scale bar corresponding to 0.5 substitutions per amino acid position.
-
Figure 1—figure supplement 2—source data 1
Amino-acid sequences used for making phylogenetic tree.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig1-figsupp2-data1-v1.fas
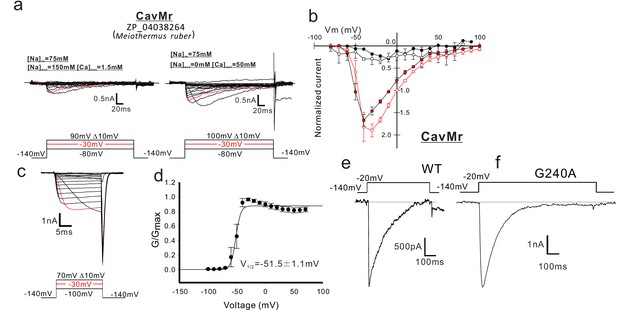
Functional expression of CavMr in SF-9 insect cells.
(a) Representative current traces used to describe the current-voltage relationships of CavMr in SF9 cells. The horizontal lines are superimposed to indicate the zero-current level in the representative current traces. Currents were generated in the bath solutions containing high Na+ (left) and high Ca2+ (right), by a series of step-pulses (shown at the bottom of the panel). (b) Current-voltage relationships of CavMr measured in the different bath solutions [filled black, 150 mM NaCl (n = 4); open black, 75 mM NaCl and 75 mM NMDG-HCl (n = 4); open red, 75 mM NaCl and 50 mM CaCl2 (n = 7); filled red, 50 mM CaCl2 and 75 mM NMDG-HCl (n = 6)] (Figure 2—source data 1). Currents of CavMr were normalized to that invoked by 0 mV depolarization stimuli under 75 mM NaCl and 50 mM CaCl2 bath solution. (c) Deactivation tail currents of CavMr. After prepulses of varying depolarization (bottom), tail currents were measured at −140 mV. (d) G/Gmax curve of CavMr generated by tail currents (n = 6) (Figure 2—source data 2). (e, f) Whole-cell currents in CavMr wild type [WT; (e) ] and a G240A mutant (f) when a pulse of −20 mV was given for 500 ms and 1 s, respectively, in a high Ca2+ bath solution.
-
Figure 2—source data 1
The values of the currents generated by each voltage stimulation.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig2-data1-v1.csv
-
Figure 2—source data 2
The values of G/Gmax of CavMr derived from the tail currents generated by each voltage stimulation.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig2-data2-v1.csv
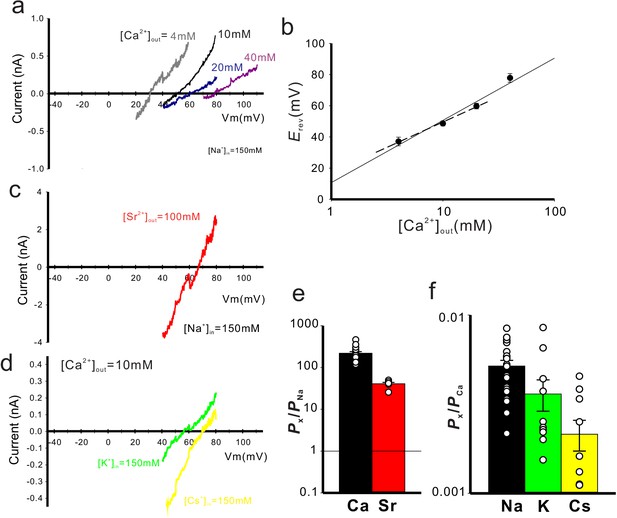
Cation selectivity of CavMr.
(a) Current-voltage relationship plot generated by ramp pulses in various [Ca2+]out and 150 mM [Na+]in. (b) The plot of the reversal potential to [Ca2+]out. Each value was obtained using the ramp pulse protocol shown in panel (a) (Figure 3—source data 1). The relationship was fitted by a line with the slope of 39.89 ± 3.31 mV per decade (n = 7). (c) Current-voltage relationship plot generated by ramp pulses in 100 mM [Sr2+]out and 150 mM [Na+]in. (d) Current-voltage relationship plots generated by ramp pulses in 10 mM [Ca2+]out and 150 mM [K+]in or [Cs+]in. (e) The relative permeability of Ca2+ or Sr2+ to Na+ in CavMr, calculated from the reversal potentials that were obtained by the ramp pulses shown in Figure 3—figure supplement 1 (Figure 3—source data 2). (f) The relative permeability of each monovalent cation to Ca2+ in CavMr, derived from the data shown in Figure 3—figure supplement 1 (Figure 3—source data 3).
-
Figure 3—source data 1
The reversal potentials to each extracellular Ca2+concentration.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig3-data1-v1.csv
-
Figure 3—source data 2
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig3-data2-v1.csv
-
Figure 3—source data 3
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig3-data3-v1.csv
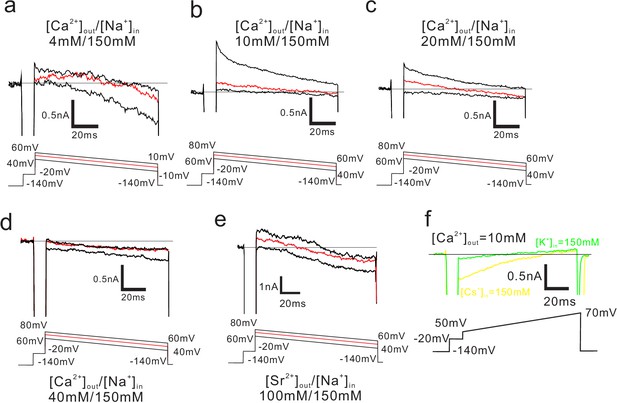
Representative current traces of CavMr generated by the ramp protocol.
(a–d) Recordings of the reversal potential of CavMr currents obtained using the ramp protocol. Currents were generated by the step pulse of −20 mV from −140 mV holding potential, followed by the ramp pulses with different voltage values (shown at the bottom). The values of the reversal potential recorded with three different ramp pulses were averaged and used in Figure 3. Currents were measured in the bath solution containing 4 mM (a), 10 mM (b), 20 mM (c) and 40 mM (d) CaCl2 and with a pipette solution containing 150 mM [Na+]. (e) Representative current traces of ramp pulses used to obtain the reversal potential under conditions of 100 mM [Sr2+]out and 150 mM [Na+]in. Currents were generated using the protocol shown in the lower part. (f) Representative current traces investigating the PCs/PCa and PK/PCa, the pipette solutions contained 150 mM [Cs+] for PCs/PCa and 150 mM [K+] for PK/PCa, while the bath solution contained 10 mM [Ca2+] in both cases.
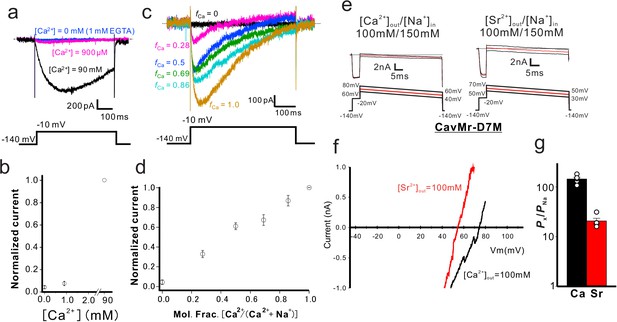
Characterization of the selectivity filter of CavMr.
(a) Examination of anomalous mole fraction effects in CavMr. CavMr currents were recorded in a bath solution containing the following ratios of Na+ and Ca2+ ([Na+]:[Ca2+]) — 0:90, 133.7:0.9 and 135:0 (mM), respectively. The 0 mM Ca2+ solution also contains 1 mM EGTA. (b) Plot of the normalized current amplitude of CavMr obtained from panel (a) (n = 3) (Figure 4—source data 1). (c) Representative current traces of CavMr under different mole fractions of Ca2+. fCa indicates [Ca2+]out / ([Ca2+]out + [Na+]out). (d) Plot of the normalized current amplitude to each mole fraction, as measured in c (n = 5) (Figure 4—source data 2). (e) For the evaluation of the relative permeability of Ca2+ and Sr2+ to Na+ of CavMr-D7M, Ca2+ solution [100 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted with Ca(OH)2) and 10 mM glucose] and Sr2+ solution [100 mM SrCl2, 10 mM HEPES (pH 7.4 adjusted by Sr[OH]2) and 10 mM glucose] were used as bath solutions. High-Na+ pipette solution [115 mM NaF, 35 mM NaCl, 10 mM EGTA, and 10 mM HEPES (pH 7.4 adjusted by NaOH)] was used. Currents were generated by the step pulse of −20 mV from −140 mV holding potential, followed by ramp pulses with different voltage values. The time courses of the change of membrane potentials are shown at the bottom of each current traces. (f) Current-voltage relationship plots generated by ramp pulses in 150 mM [Na+]in and 100 mM [Ca2+]out or [Sr2+]out. (g) The relative permeability of divalent cations to Na+ in CavMr-D7M, whose position 7 residue in the selectivity filter was neutralized by the corresponding residue of NavAb (Figure 4—source data 3).
-
Figure 4—source data 1
The values of the normalized current amplitude of CavMr.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig4-data1-v1.csv
-
Figure 4—source data 2
The values of the normalized current amplitude to each mole fraction.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig4-data2-v1.csv
-
Figure 4—source data 3
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig4-data3-v1.csv
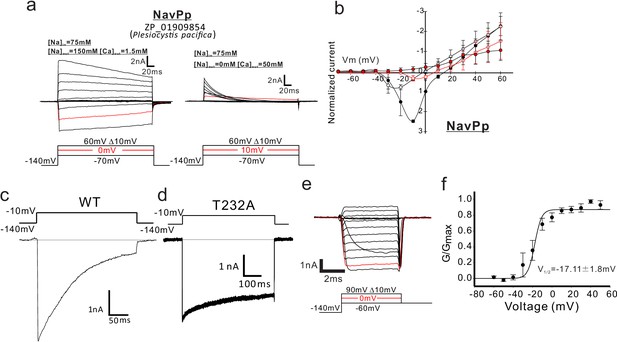
Functional expression of NavPp in SF-9 insect cells.
(a) Representative current traces used to obtain the current-voltage relationships of NavPp in SF9 cells. The horizontal lines are superimposed to indicate the zero-current level in the representative current traces. Currents were generated, in bath solutions containing high Na+ (left) and high Ca2+ (right), by a series of step-pulses shown at the bottom of the panel. (b) Current-voltage relationships of NavPp measured in the different bath solutions [filled black, 150 mM NaCl (n = 8), open black, 75 mM NaCl and 75 mM NMDG-HCl (n = 8); open red, 75 mM NaCl and 50 mM CaCl2 (n = 6); filled red, 50 mM CaCl2 and 75 mM NMDG-HCl (n = 8)] (Figure 5—source data 1). Currents of NavPp were normalized to that induced by 0 mV depolarization stimuli in a 150 mM NaCl bath solution. (c, d) Whole-cell recordings of wild-type NavPp [WT; (c)] and the NavPp T232A mutant (d) when a pulse of −10 mV was given for 250 ms and 500 ms in a high-Na+ bath solution, respectively. (e) Deactivation tail currents of NavPp T232A. After prepulses of varying depolarizing currents (bottom), tail currents were measured at −60 mV. (f) G/Gmax curve for NavPp T232A derived from the tail currents (n = 4) (Figure 5—source data 2).
-
Figure 5—source data 1
The values of the currents generated by each voltage stimulation.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig5-data1-v1.csv
-
Figure 5—source data 2
The values of G/Gmaxof NavPp T232A derived from the tail currents generated by each voltage stimulation.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig5-data2-v1.csv

Characterization of the selectivity filter of NavPp.
(a) Representative current traces for NavPp T232A generated by +20 mV stimulation pulses in various extracellular Ca2+ concentration solutions. (b) Current-voltage relationships of NavPp measured in various extracellular Ca2+ concentration solutions (n = 3) (Figure 6—source data 1). All values were normalized by the peak current amplitude in the 4 mM extracellular Ca2+ condition. (c) G/Gmax curve for NavPp T232A derived from the tail currents in various extracellular Ca2+ concentration solutions (n = 4) (Figure 6—source data 2). The maximum tail current amplitude in the 4 mM extracellular calcium condition was used as Gmax. (d) The permeability of different cation species relative to Na+ permeability in NavPp, calculated from the reversal potential that was obtained from the current traces of Figure 6—figure supplement 1b–e (Figure 6—source data 3). (e) The extracellular-calcium-inhibition in the single-point mutants of NavPp. The selectivity filter of NavPp was changed to the Ca2+-selective canonical-BacNavs mutants (T6S; TLEDWSD and T6A; TLEDWAD).
-
Figure 6—source data 1
The values of the currents generated by each voltage stimulation under each extracellular Ca2+concentration.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig6-data1-v1.csv
-
Figure 6—source data 2
The values of G/Gmax of NavPp T232A derived from the tail currents generated by each voltage stimulation under each extracellular Ca2+concentration.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig6-data2-v1.csv
-
Figure 6—source data 3
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig6-data3-v1.csv
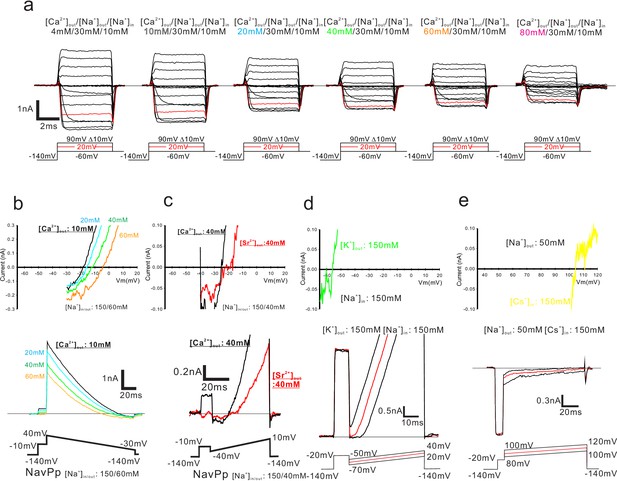
Representative current traces of NavPp generated by the step-up pulses and the ramp protocol.
(a) Representative current traces used to obtain the peak current and the deactivation tail current for evaluation of the current-voltage relationship and the voltage-dependent activation of NavPp T232A. (b) Current-voltage relationship plots (upper) and representative currents (bottom) used to obtain the reversal potential in NavPp for PCa/PNa. Currents were generated by the ramp protocol shown at the bottom of the panel. [Ca2+]out was varied from 10 mM to 60 mM with the [Na+] fixed in both the bath (60 mM) and pipette (150 mM). (c) Current-voltage relationship plots (upper) and representative currents (bottom) used to obtain the reversal potential in NavPp for PSr/PNa. Currents were generated by the ramp protocol shown at the bottom of the panel. [Sr2+]out was 40 mM with the[Na+] fixed in both the bath (40 mM) and pipette (150 mM). (d, e) Current-voltage relationship plots (upper) and representative currents (bottom) used to evaluate the permeability of K+ and Cs+ relative to that of Na+ of NavPp. K+ solution [150 mM KCl, 2 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted by KOH) and 10 mM glucose] and low Na+ solution [50 mM NaCl, 100 mM NMDG-HCl, 2 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted by NaOH) and 10 mM glucose] were used as bath solution. High-Na+ pipette solution [115 mM NaF, 35 mM NaCl, 10 mM EGTA, and 10 mM HEPES (pH 7.4 adjusted by NaOH)] and Cs+ pipette solution [115 mM CsF, 35 mM CsCl, 10 mM EGTA, and 10 mM HEPES (pH 7.4 adjusted by CsOH)] were used for K+ and Cs+ selectivity, respectively. Currents were generated by the step pulse of −20 mV from −140 mV holding potential, followed by the ramp pulses with different voltage values. The time courses of the change of membrane potentials were shown at the bottom of the respective current traces.

The cation selectivity of the channel mutants in which the selectivity filter is swapped between CavMr and NavPp.
(a) Amino acid sequences of the selectivity filter in the swapped mutants, CavMr-Pp, Nav-Pp, NavAb-Mr, and CavMr-Ab. The selectivity filter sequences of CavMr, NavPp and NavAb are indicated using single-letter codes with cyan, red, and gray shade, respectively. Negatively charged residues are colored in red. Glycine residues are colored in cyan. The straight lines of cyan, red, and black indicate the other part of pore domain of CavMr, NavPp, and NavAb, respectively. (b) Pore domains of crystal structure of NavAb (PDB code:5YUA). The selectivity filter, which corresponds to the sequences shown in panel (a), was indicated in red. (c) The relative permeability of divalent cations to Na+ (left) and that of monovalent cations to Ca2+ (right) in NavPp-Mr (Figure 7—source data 1). (d) The relative permeability of different cation species to Na+ in CavMr-Pp (Figure 7—source data 2).
-
Figure 7—source data 1
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig7-data1-v1.csv
-
Figure 7—source data 2
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig7-data2-v1.csv
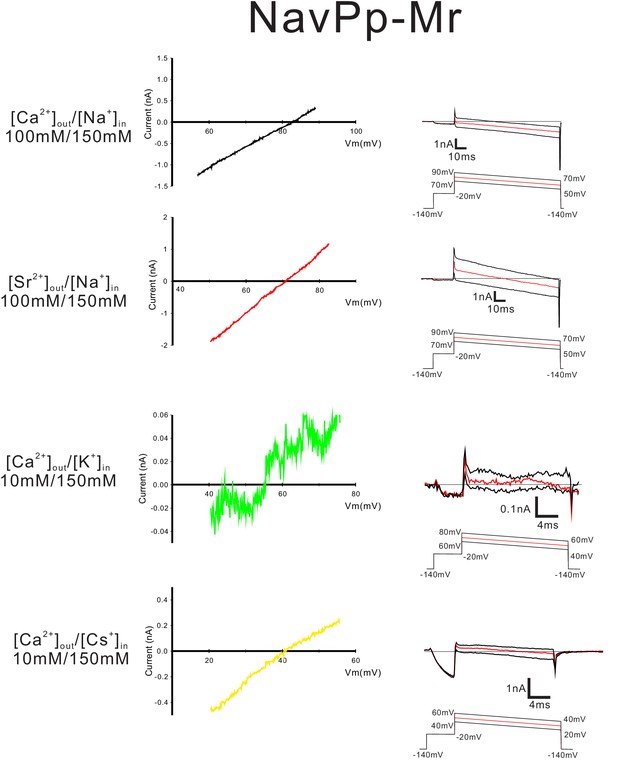
Current-voltage relationship plots and representative current traces of the ramp pulse of the CavMr-Pp selectivity-filter-swapped mutants.
Current-voltage relationship plots for CavMr-Pp using the averaged currents generated with three different ramp pulses in the solutions indicated on the left side. For the evaluation of the permeability of Ca2+, Sr2+, K+ and Cs+ relative to Na+, Ca2+ solution [100 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted with Ca(OH)2) and 10 mM glucose], Sr2+ solution [100 mM SrCl2, 10 mM HEPES (pH 7.4 adjusted by Sr(OH)2) and 10 mM glucose], K+ solution [150 mM KCl, 2 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted by KOH) and 10 mM glucose] and Cs solution [150 mM CsCl, 2 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted by CsOH) and 10 mM glucose] were used as bath solution, respectively. High-Na+ pipette solution [115 mM NaF, 35 mM NaCl, 10 mM EGTA and 10 mM HEPES (pH 7.4 adjusted by NaOH)] was used for the measurements of the relative permeability of Ca2+ and Sr2+. The permeability of K+ and Cs+ relative to Ca2+ was evaluated as an alternative to their permeability relative to Na+, because of the high Ca2+ selectivity of NavPp-Mr. For the evaluation, high-K+ pipette solution [115 mM KF, 35 mM KCl, 10 mM EGTA and 10 mM HEPES (pH 7.4 adjusted by KOH)] and high-Cs+ pipette solution [115 mM CsF, 35 mM CsCl, 10 mM EGTA and 10 mM HEPES (pH 7.4 adjusted by CsOH)] were used. As bath solution, Ca2+ solution [100 mM CaCl2, 10 mM HEPES (pH 7.4 adjusted with Ca(OH)2) and 10 mM glucose] was used. Currents were generated by the step pulse of −20 mV from −140 mV holding potential, followed by ramp pulses with different voltage values. The time courses of the change in membrane potentials are shown at the bottom of the respective current traces.
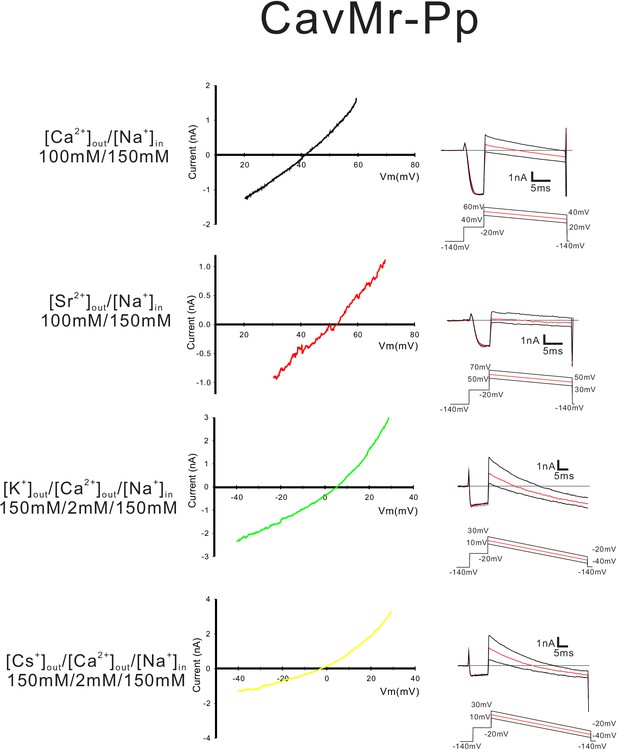
Current-voltage relationship plots and representative current traces for the ramp pulse of NavPp-Mr selectivity-filter-swapped mutants.
Current-voltage relationship plots of NavPp-Mr using the averaged currents generated with three different ramp pulses in the solutions indicated on the left side. To evaluate the relative permeability of Ca2+, Sr2+, K+ and Cs+ to Na+, Ca2+ solution, Sr2+ solution, K+ solution and Cs+ solution were used as the bath solution, respectively. High-Na+ pipette solution was used as the pipette solution. The solution contents are described in the 'Materials and methods' and in Figure 7—figure supplement 1. The time courses of the membrane potentials are shown at the bottom of each trace.
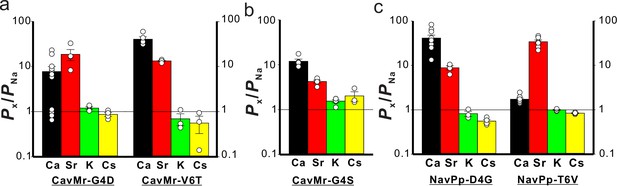
The single-point mutations that cause loss and acquistion of Ca2+ selectivity in CavMr and NavPp, respectively.
(a) The permeability of each cation species relative to Na+ permeability in the single-point mutants of CavMr. The selectivity filter of CavMr was changed to the corresponding residues of NavPp at position 4 (G4D) or position 6 (V6T) (Figure 8—source data 1). (b) The permeability of each cation species relative to Na+ permeability in the G4S mutant of CavMr, whose position 4 residue of the selectivity filter was mutated to the corresponding residue of canonical BacNavs (Figure 7—source data 1). (c) The permeability of each cation species relative to Na+ permeability in the single-point mutants of NavPp. The selectivity filter of NavPp was changed by swapping in the corresponding residues of CavMr at position 4 (D4G) or position 6 (T6V) (Figure 8—source data 3).
-
Figure 8—source data 1
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig8-data1-v1.csv
-
Figure 8—source data 2
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig8-data2-v1.csv
-
Figure 8—source data 3
The values of relative permeability.
- https://cdn.elifesciences.org/articles/52828/elife-52828-fig8-data3-v1.csv
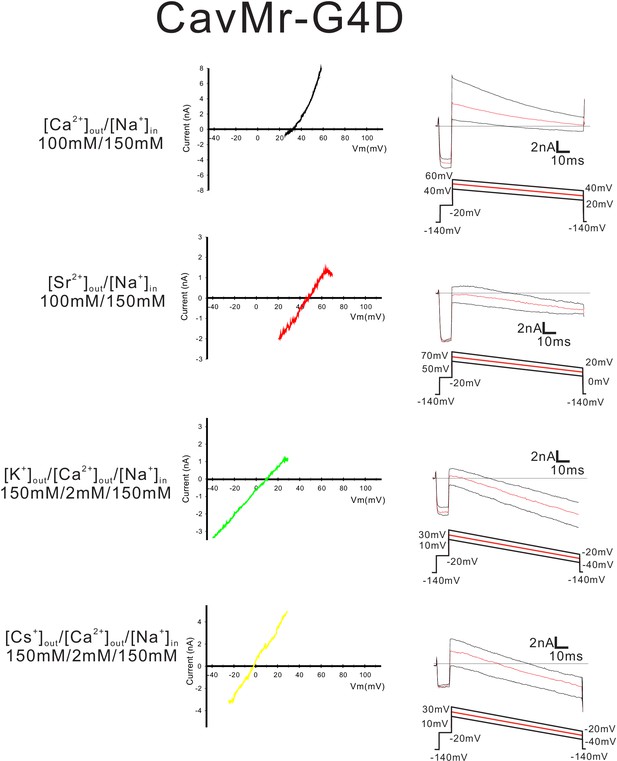
Current-voltage relationship plots and representative current traces for the ramp pulse of CavMr-G4D selectivity-filter-swapped mutants.
Current-voltage relationship plots of CavMr-G4D using the averaged currents generated with three different ramp pulses in the solutions indicated on the left side. To evaluate the permeability of Ca2+, Sr2+, K+ and Cs+ relative to that of Na+, Ca2+ solution, Sr2+ solution, K+ solution and Cs+ solution, respectively, was used as the bath solution. High-Na+ pipette solution was used as the pipette solution. The solution contents were described in the 'Materials and methods' and in Figure 7—figure supplement 1. The time courses of the changes in membrane potentials are shown at the bottom of the current traces.
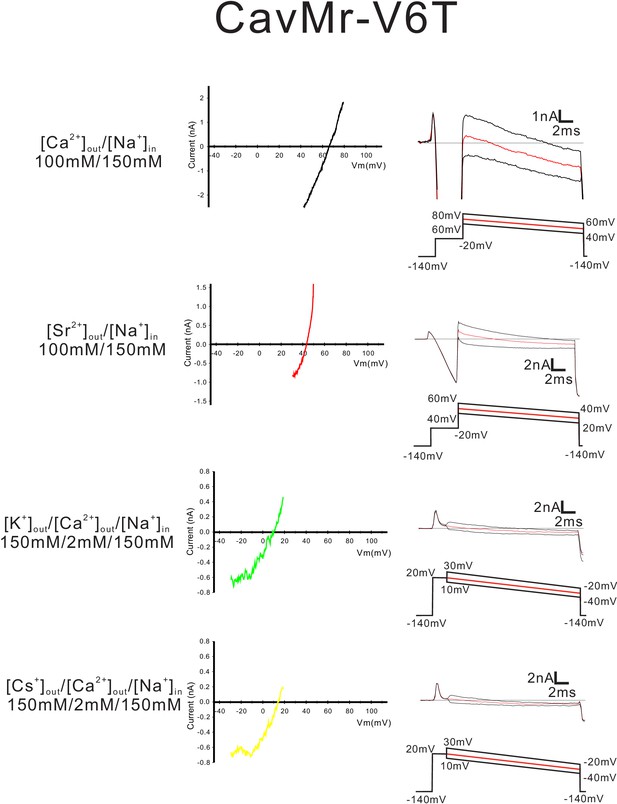
Current-voltage relationship plots and representative current traces for the ramp pulse of CavMr-V6T selectivity-filter-swapped mutants.
Current-voltage relationship plots for CavMr-V6T using the averaged currents generated with three different ramp pulses in the solutions indicated on the left side. To evaluate the permeability of Ca2+, Sr2+, K+ and Cs+ relative to that of Na+, Ca2+ solution, Sr2+ solution, K+ solution and Cs+ solution, respectively, were used as the bath solution. High-Na+ pipette solution was used as the pipette solution. The solution contents are described in the 'Materials and methods' and in Figure 7—figure supplement 1. The time courses of the changes in membrane potentials are shown at the bottom of the current traces.
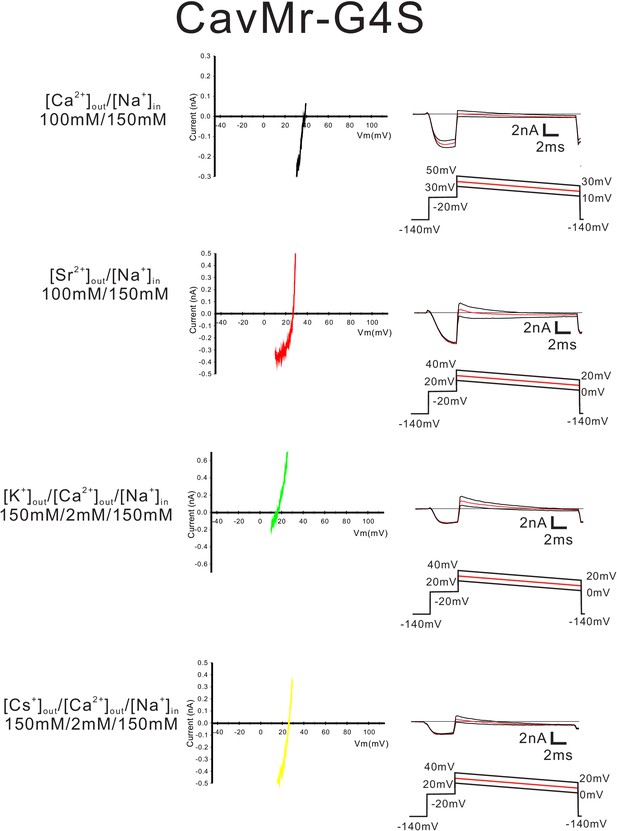
Current-voltage relationship plots and representative current traces for the ramp pulse of CavMr-G4S selectivity-filter-swapped mutants.
Current-voltage relationship plots for CavMr-G4S using the currents generated with the ramp pulse (red line) in the solutions indicated on the left side. To evaluate the permeability of Ca2+, Sr2+, K+ and Cs+ relative to that of Na+, Ca2+ solution, Sr2+ solution, K+ solution and Cs+ solution, respectively, were used as the bath solution. High-Na+ pipette solution was used as the pipette solution. The solution contents were described in the 'Materials and methods' and in Figure 7—figure supplement 1. The time courses of the changes in membrane potential are shown at the bottom of the current traces.
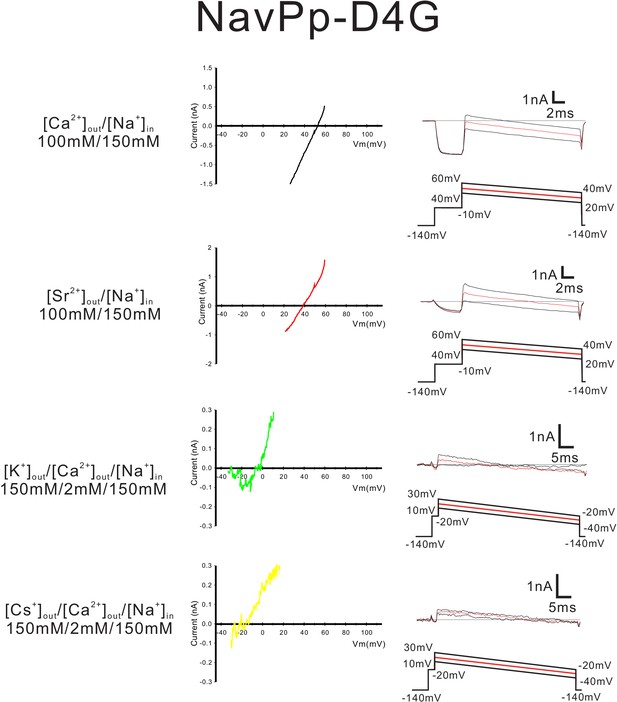
Current-voltage relationship plots and representative current traces for the ramp pulse of NavPp-D4G selectivity-filter-swapped mutants.
Current-voltage relationship plots for NavPp-D4G using the averaged currents generated with three different ramp pulses in the solutions indicated on the left side. To evaluate the permeability of Ca2+, Sr2+, K+ and Cs+ relative to that of Na+, Ca2+ solution, Sr2+ solution, K+ solution and Cs+ solution, respectively, were used as the bath solution. High-Na+ pipette solution was used. The solution contents are described in the 'Materials and methods' and in Figure 7—figure supplement 1. The time courses of the change in membrane potentials are shown at the bottom of the current traces.
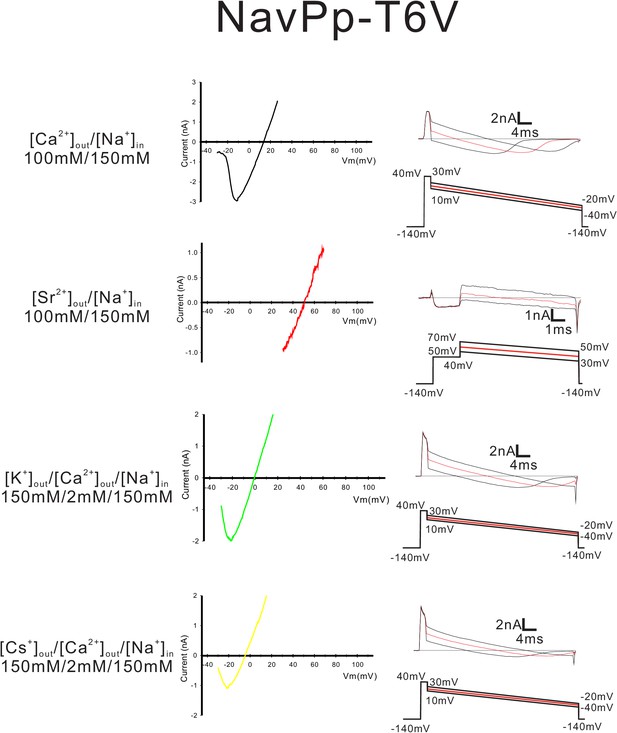
Current-voltage relationship plots and representative current traces for the ramp pulse of NavPp-T6V selectivity-filter-swapped mutants.
Current-voltage relationship plots of NavPp-T6V using the averaged currents generated with three different ramp pulses in the solutions indicated on the left side. To evaluate the permeability of Ca2+, Sr2+, K+ and Cs+ relative to that of Na+, Ca2+ solution, Sr2+ solution, K+ solution and Cs+ solution, respectively, were used as the bath solution. High-Na+ pipette solution was used. The solution contents are described in the 'Materials and methods' and in Figure 7—figure supplement 1. The time courses of the changes in membrane potentials are shown at the bottom of the current traces.
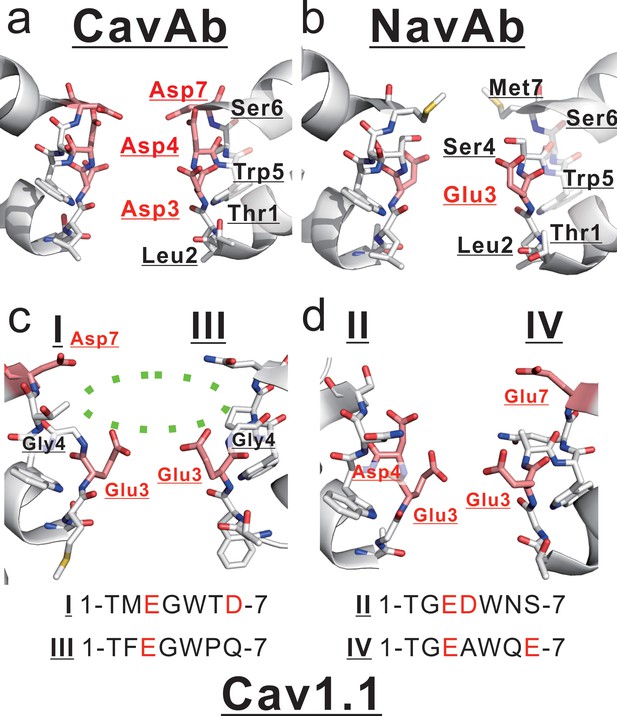
Comparison between mammalian and prokaryotic Cav.
(a, b) Structures of the selectivity filter in CavAb (PDB code: 4MVZ) and NavAb (PDB code: 5YUA). (c, d) Structure of the rabbit Cav1.1 selectivity filter (PDB code: 5GJV). The subdomains I and III (c), and II and IV (d) are shown separately. The carbon atoms of negatively charged residues are indicated in pink. A dashed green circle indicates the wide entrance of the selectivity filter.
Tables
Relative permeability of CavMr and NavPp.
All values are indicated as mean ± S.E.
| PCa/PNa | Sr/PNa | PK/PNa | PCs/PNa | |
|---|---|---|---|---|
| CavMr G240A | 218 ± 38 | 40.6 ± 3.4 | 0.0036 ± 0.00072a | 0.0021 ± 0.00042b |
| (n = 20) | (n = 6) | (n = 10) | (n = 10) | |
| Pp | 13.8 ± 2.0 | 24.5 ± 0.3 | 0.95 ± 0.04 | 0.57 ± 0.05 |
| (n = 7) | (n = 5) | (n = 4) | (n = 3) | |
| G4D | 7.73 ± 2.24 | 18.6 ± 6.1 | 1.20 ± 0.28 | 0.87 ± 0.21 |
| (n = 11) | (n = 4) | (n = 4) | (n = 4) | |
| G4S | 11.9 ± 1.5 | 4.23 ± 0.27 | 1.54 ± 0.12 | 2.02 ± 0.48 |
| (n = 5) | (n = 5) | (n = 5) | (n = 3) | |
| V6T | 40.1 ± 9.7 | 13.3 ± 2.5 | 0.69 ± 0.26 | 0.54 ± 0.60 |
| (n = 5) | (n = 5) | (n = 3) | (n = 3) | |
| D7M | 144 ± 12 | 20.7 ± 2.7 | N.D. | N.D. |
| (n = 5) | (n = 5) | |||
| NavPp T232A | 0.308 ± 0.028 | 0.38 ± 0.027 | 0.16 ± 0.026 | 0.0052 ± 0.0006 |
| (n = 18) | (n = 9) | (n = 9) | (n = 7) | |
| Mr | 215 ± 33 | 86.3 ± 12.2 | 0.0045 ± 0.00072a | 0.0135 ± 0.0039b |
| (n = 7) | (n = 4) | (n = 4) | (n = 8) | |
| D4G | 41.4 ± 6.7 | 8.85 ± 0.95 | 0.81 ± 0.11 | 0.56 ± 0.05 |
| (n = 10) | (n = 4) | (n = 3) | (n = 4) | |
| T6V | 1.72 ± 0.19 | 33.9 ± 5.0 | 0.99 ± 0.03 | 0.84 ± 0.02 |
| (n = 10) | (n = 8) | (n = 4) | (n = 4) |
-
a Because of high Ca2+ selectivity, PK/PCa are indicated.
b Because of high Ca2+ selectivity, PCs/PCa are indicated.





