Brain states govern the spatio-temporal dynamics of resting-state functional connectivity
Figures
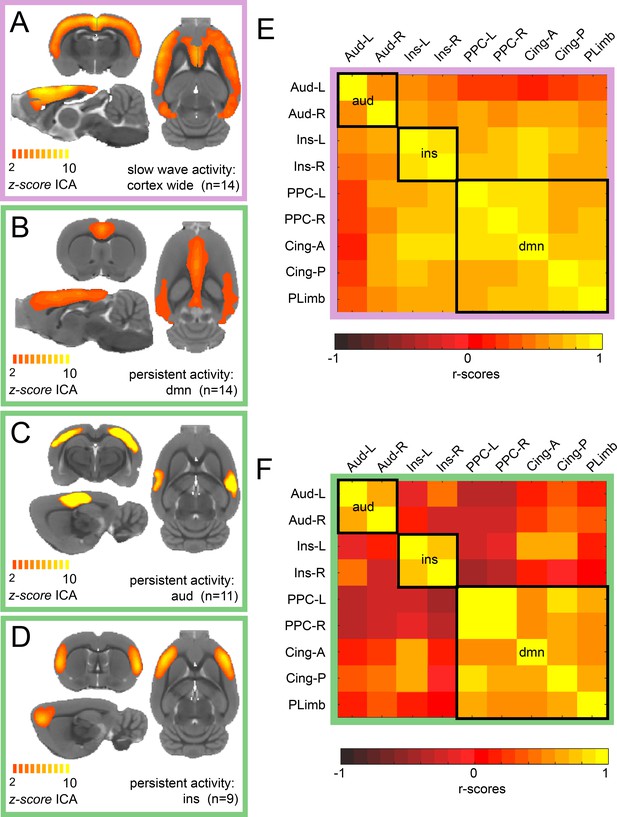
ICA reveals distinct components for slow wave and persistent activity.
(A) Average z-score maps of the cortex-wide component during slow wave activity. B-D. Average z-score maps of two components found in 14, 11 and 9 animals respectively (B Default mode network, C auditory component and D insula activation) during persistent activity. A detailed diagram with all the z-scores maps subject by subject can be found in Figure 1—figure supplement 1 (E) Resting-state functional connectivity matrix during slow wave activity from the nine regions of interest (ROIs) found in the ICA of persistent activity (B–D). Black squares outline the three networks. The ROIs are auditory cortex left and right (Aud-L, Aud-R), Insula left and right (Ins-L, Ins-R), posterior parietal cortex left and right (PPC-L and PPC-R), andterior and posterior cingulate cortex (Cing-A and Cing-P) and orbital/prelimbic cortex (PLimb). F. Same analysis as in E but for the signal obtained during persistent activity.
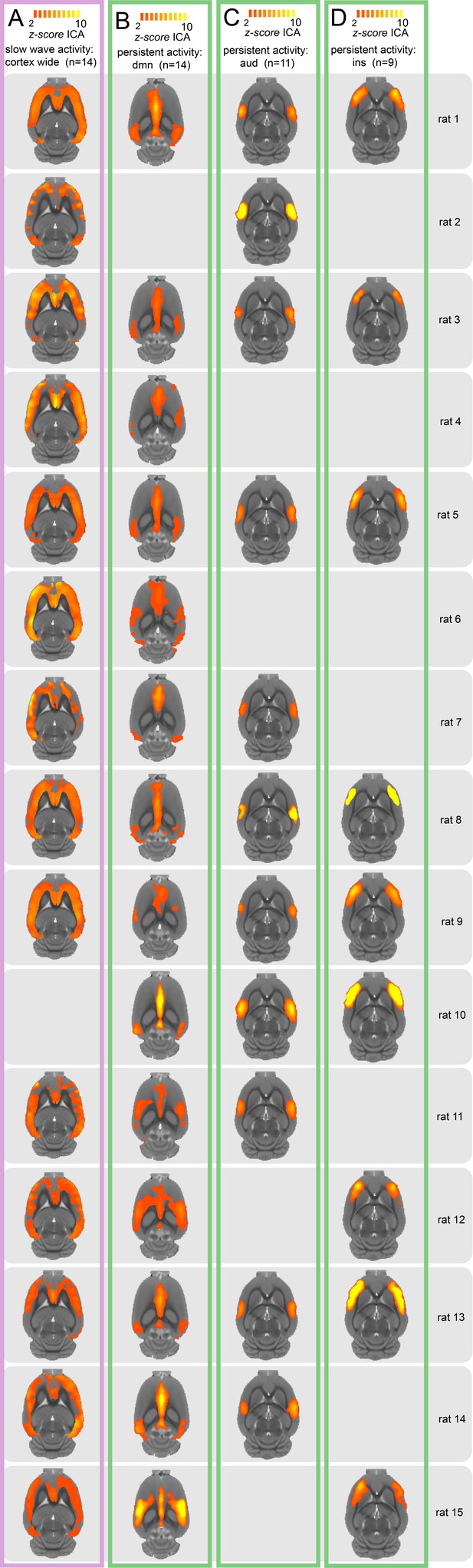
Individual z-scores obtained from the ICA for each animal shown in transversal view of an atlas template.
Components of slow wave activity are shown in magenta and those found during persistent activity are shown in green. (A) Cortex wide component. (B) Default mode network. (C) Auditory network. (D) Insular activation. Last column on the right indicates the animal.
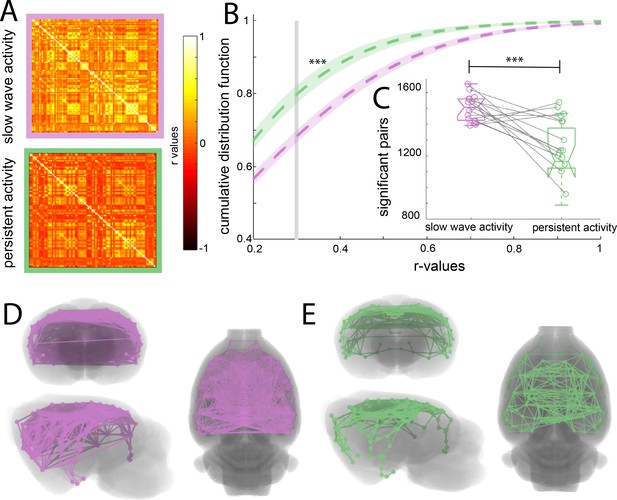
Increased functional connectivity during isoflurane-induced slow wave activity compared to persistent activity related to medetomidine sedation.
(A) Average of the correlation matrices (n = 8) of the mean BOLD signal in 96 cortical regions for slow wave activity (magenta) and persistent activity (green). (B) Cumulative distribution curve for the r-values of the correlations. The vertical gray line lies at the mean of the FDR values used as a cutoff to identify significant correlations, ***p<0.001, paired t-test. (C) Box plot of the amount of significant correlations for each individual animal in both activity states ***p<0.001, paired t-test, vertical dashed line represents the data distribution, central horizontal bar the median and the two extremes horizontal bars point the interquartile range. (D) Diagram of the significant cortical connections during slow wave activity. Each line represents significant connections between cortical nodes (96 in total). Circles signaling the nodes are plotted in the center of the ROI. (E) Similar than D but for the persistent activity networks.
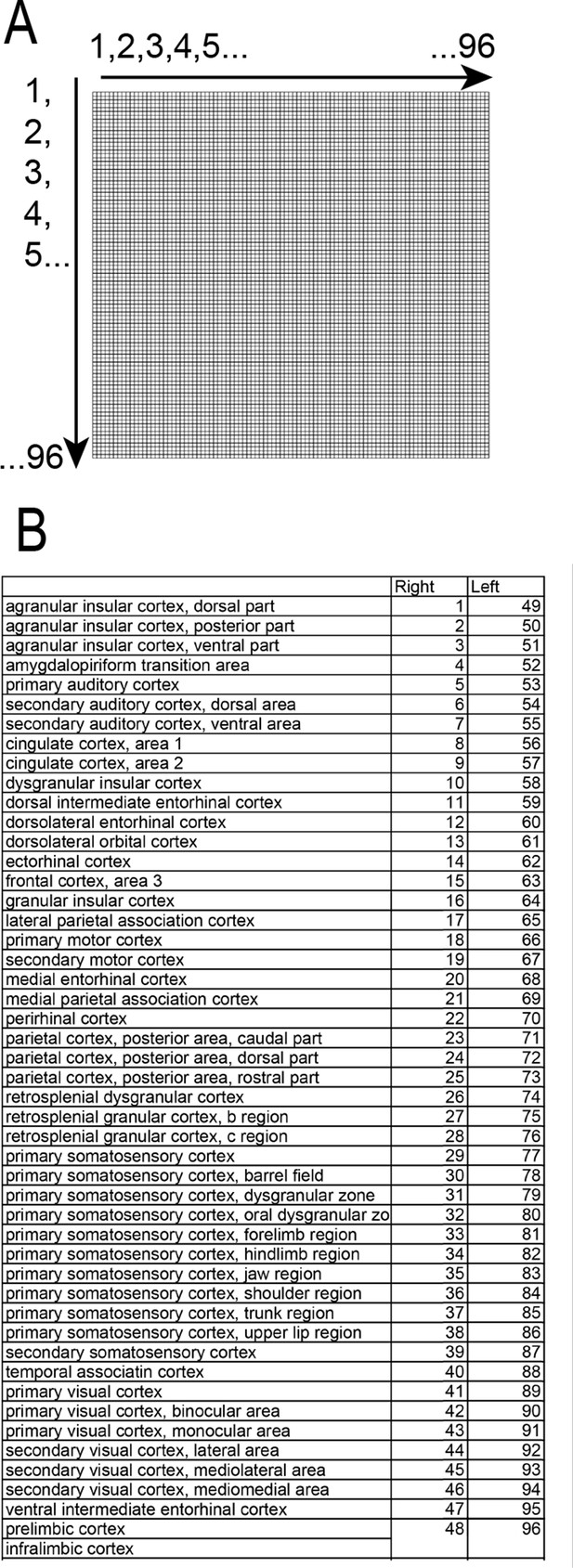
Cortical areas used for connectivity analysis.
(A) Configuration of the distribution of cortical areas within the connectivity maps. (B) Corresponding localization of each area used in the connectivity analyses. The area’s names and distribution are taken from the Valdes-Hernandez et al atlas (Valdés-Hernández et al., 2011).
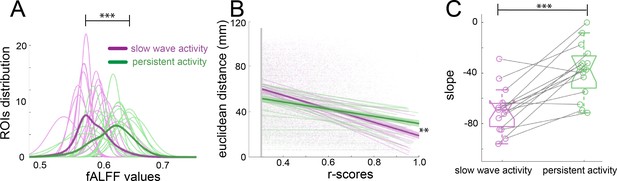
Network dynamics differ for slow wave and persistent activity.
(A) Plot of the fALFF analysis. The fALFF value was obtained for each region of interest and then a normal distribution was fit with the values of the 96 cortical ROIs for each individual during slow wave (n = 15, magenta) and persistent activity (n = 15, green), ***p<=0.001. The average values of the distribution for each condition is represented by the thick line. (B) Correlation between the r-scores of each pair of cortical ROIs and their Euclidean distance, **p<=0.01. Each point corresponds to an r-score of a particular pair of ROIs. Linear correlation between ROIs and distance is plotted for persistent (n = 15, green) and slow wave activity (n = 15, magenta). The means of the correlations for each condition and the standard error of the mean are plotted in thicker lines. (C) Box plot of the slopes for each individual plotted in B. ***p<=0.001, paired t-test (n = 15). vertical dashed line represents the data distribution, central horizontal bar the median and the two extremes horizontal bars point the interquartile range.
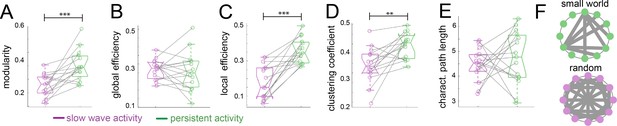
Graph theory shows a random network signature during slow wave activity.
Legend: *=p < 0.05; **=p < 0.01; ***=p < 0.001 (paired t-test n = 15); slow wave activity (magenta), persistent activity (green). vertical dashed line represents the data distribution, central horizontal bar the median and the two extremes horizontal bars point the interquartile range. (A) Modularity values. (B) Global efficiency values. (C) Local efficiency values. (D) Clustering coefficients. (E) Characteristic path lengths. (F) Diagram representing a characteristic configuration of small world (green) and randomized networks (magenta).
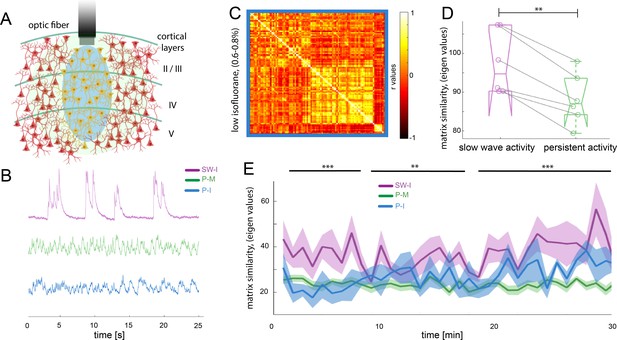
Different activity states of the brain result in characteristic functional connectivity matrices.
(A) Calcium fiber photometry schematic. OGB-1 was bolus-injected and an optic fiber with a diameter of 200 µm was implanted at a cortical depth of about 300 μm. Blue light was used for excitation of OGB-1. Emitted fluorescence, comprising the changes in cytosolic calcium concentration of the local neural population was collected by the same fiber, and recorded by the fiber optometer. (B) Characteristic signal traces of photometry recordings during slow wave activity induced by high isoflurane (SW-I, magenta), and persistent activity induced either by medetomidine (P-M, green) or by lower concentrations of isoflurane (blue, P–I). (C) Average of the correlation matrices (n = 6) of the mean BOLD signal in 96 cortical regions under low isoflurane concentration. (D) Matrix similarity analysis of the low isoflurane (P-I n = 6) persistent activity experiments compared with the average matrix generated under high isoflurane related slow wave activity (SW-activity, magenta) and the medetomidine-induced persistent activity (P-M, green), **p<0.01, (n = 6, paired t-test). (E) Matrix similarity observed under the three conditions under a dynamic connectivity analysis. Lines represent the average of individual variability for slow wave activity (SW-I, n = 15, magenta), persistent activity induced with medetomidine (P-M, n = 15, green) and persistent activity induced with isoflurane (P-I, n = 6 blue), the semitransparent stripe correspond to the standard error of the mean for each condition. The matrices were generated for a 5-min period with steps of 1 min and were compared with the average matrix generated in the first 5 min for each individual animal, **p<0.01 (paired t-test n = 15 in SW-I and P-M conditions).
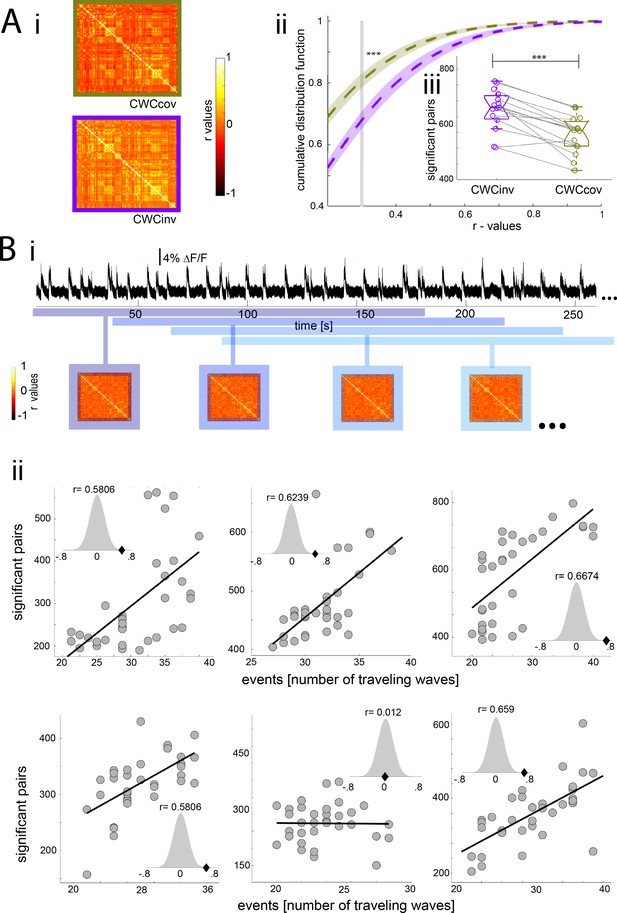
Population down-up transitions drive functional connectivity during slow wave activity.
(A) Functional connectivity of the BOLD signal using the cortex wide component of the ICA as covariable (CWCcov): i- Average of the correlation matrices (n = 8) of the mean BOLD signal in 96 cortical regions during slow wave activity using the pan-cortical component of the ICA as covariable (CWCcov, purple frame) or the flipped values of the same component as control (CWCinv, brown frame). ii- Cumulative distribution curve for the r-values of the correlations during slow wave activity using the cortex-wide component of the ICA as regressor (CWCcov), or similarly using the flipped values of the same component (CWCinv). The vertical gray line lies at the mean of the FDR values used as cutoff to identify significant correlations, ***p<0.001 (paired t-test, n = 15). iii- Plot of the number of significant correlations for each individual in both conditions **p<0.01. (B) Correlation between the number of significant pairs in the functional connectivity matrix and the number of down-up transitions. i- Correlation scheme. The number of down-up transitions was quantified for the respective time period (upper panel) and then correlated with the number of significant pairs obtained from the functional connectivity matrix (lower panel). ii- 5 out of 6 animals analyzed showed a significant positive correlation between number of down-up transitions and number of significant functionally connected pairs. Histogram subplots indicate the results of the permutation test. This analysis corroborates the Pearson correlation showing the r-scores of the five significant cases to lie beyond 5% of significance in the normal distribution built with 10000 surrogates of shuffled data.
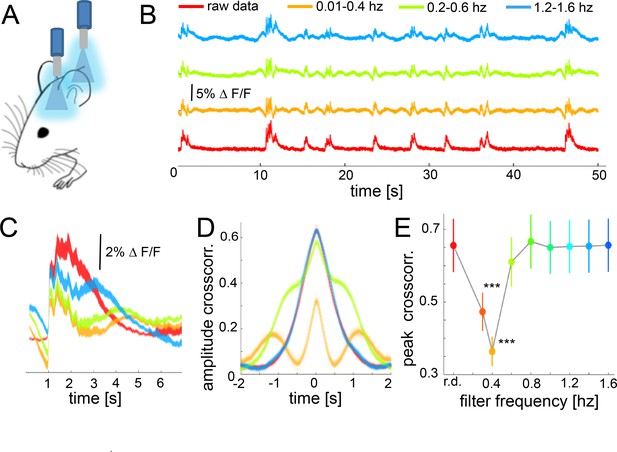
During slow wave activity, the correlation between distant optical recordings is driven by slow-wave-associated calcium waves.
(A) Schematic of fiber photometry in cortical areas S1 and V1. (B) Exemplary fluorescent signal trace recorded from V1 filtered at different band-stop intervals, excluding those particular intervals from the recordings. (C) Mean of the down-up transitions for a trace (∆f/f) at different band stop filters. It can be observed how the amplitude, length and size of the slow waves are modified at each filtered bandwidth. (D) Cross-correlation results of a single recording filtered at different band-stop intervals (color code is the same than in subplot B). (E) Average of the cross-correlation peak values (n = 6) at different band-stop intervals, ***p<0.001 (Paired t-test, n = 6).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Rattus norvegicus) | Lewis rat | Janvier labs | LEW/OrlRj | Female 160–200 gr |
| Chemical compound, drug | PBS tabletts | gibco | 18912–014 | |
| Chemical compound, drug | xylocaine | AstraZeneca | PUN080440 | 2% |
| Chemical compound, drug | isoflurane | AbbVie | 8506 CHEBI:6015 | 1–1.55% |
| Chemical compound, drug | Oregon-Green BAPTA1 AM | Invitrogen Thermo Fisher scientific | O6807 | 1 mM |
| Chemical compound, drug | Glucose | B. Brown | 2355740 | 10% |
| Chemical compound, drug | Medetomidine hydrochloride | Dorbene vet. Zoetis | 08164–43 | 0.08 mg/kg/hr |
| Chemical compound, drug | Carbomer | Vidisic | 74013T296/52-DE | 2 mg/gr |
| Chemical compound, drug | NaCl | Fresenius Kabi France | 13KLP183 | 0.9% |
| Chemical compound, drug | Agarose | Sigma-Aldrich | CAS: 9012-36-6 | 2% |
| Software, algorithm | Brain Voyager 20.6 | Brain Innovation, Maastricht, Netherlands | ||
| Software, algorithm | Matlab R2018a | (The Mathworks, Inc, Natick, MA, USA) | Codes available at https://github.com/Strohlab/connectivityelife copy archived athttps://github.com/elifesciences-publications/connectivityelife |





