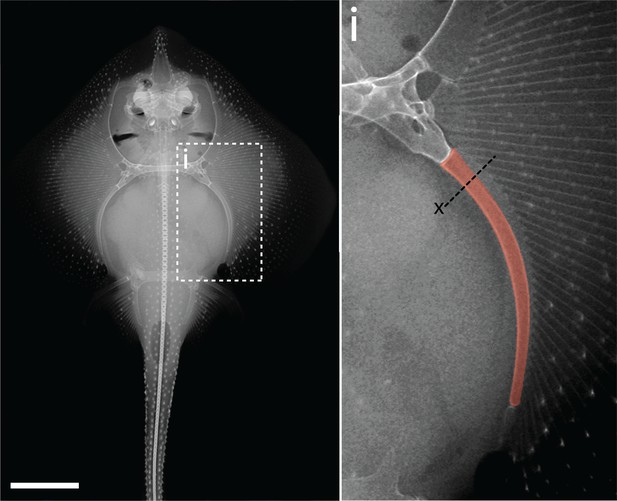
Adult chondrogenesis and spontaneous cartilage repair in the skate, Leucoraja erinacea
Figures
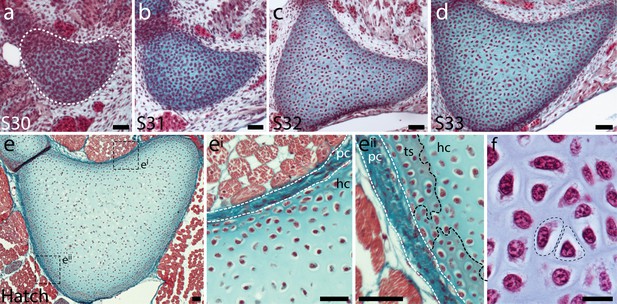
Embryonic development of the skate metapterygium.
Transverse histological sections through the centre of the developing metapterygium at (a) S30 (white dashed line indicates condensation boundary), (b) S31, (c) S32, (d) S33 and (e) hatching. By hatching, the metapterygium is (ei) bound by a fibrous perichondrium, and (eii) a surface layer of calcified cartilage in the form of mineralized ‘tesserae’ begins to develop beneath the perichondrium. (f) Cells in the hyaline cartilaginous core of the metapterygium adopt typical chondrocyte morphology, and are recessed within lacunae in the abundant extracellular matrix. All sections stained with modified Masson’s trichrome. Plane of section as indicated in Figure 1i. hc, hyaline cartilage; pc, perichondrium; ts, tesserae. Scale bars: (a-eii) 50 μm, (f) 20 μm.
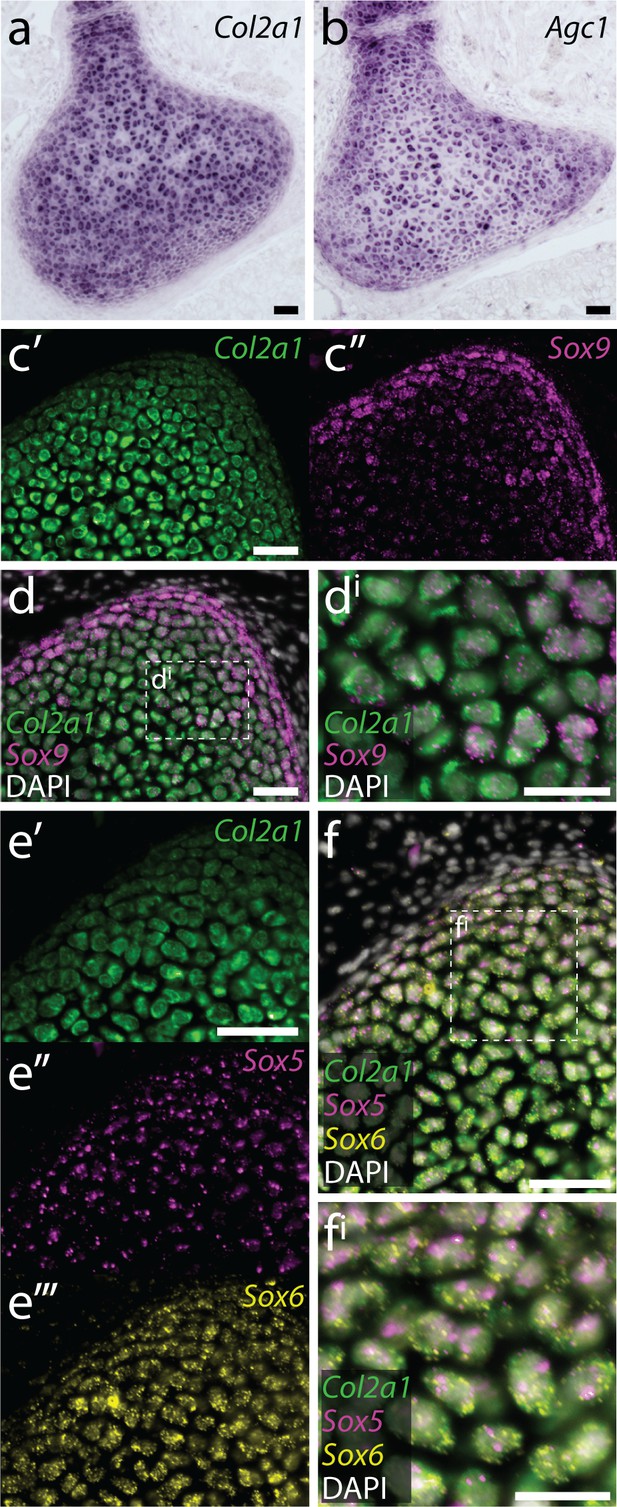
Conserved co-expression of genes encoding ECM components and upstream transcription factors in skate cartilage.
(a) At S32, chromogenic mRNA in situ hybridization reveals that chondrocytes within the developing metapterygium express Col2a1 and (b) Agc1. Multiplexed fluorescent mRNA in situ hybridization by chain reaction (HCR) reveals that skate chondrocytes co-expression (c-d) Col2a1 and Sox9, and (e-f) Col2a1, Sox5 and Sox6, pointing to conservation of transcriptional regulation of Col2a1 by SoxD- and E-class transcription factors in jawed vertebrates. Plane of section as indicated in Figure 1i. Scale bars: (a-d) 50 μm, (di) 30 μm, (e-f) 50 μm, (fi) 30 μm.
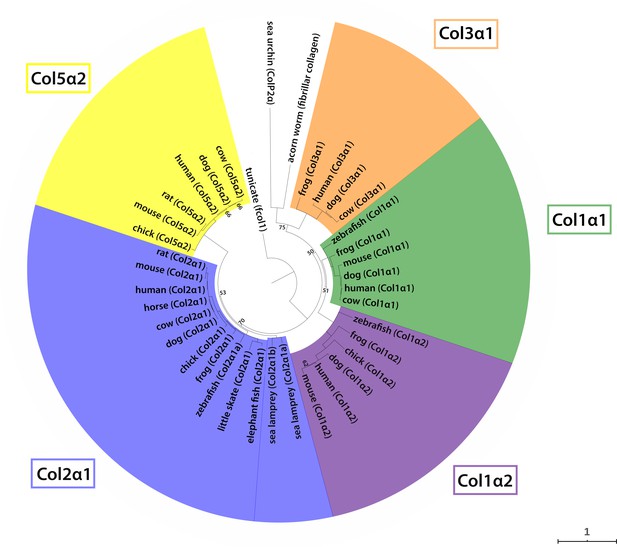
Phylogenetic analysis of vertebrate fibrillar collagens.
Phylogenetic analysis of selected vertebrate fibrillar collagen amino acid sequences resolves five clades (Col3a1, Col1a1, Col1a2, Col2a1 and Col5a2) and confirms orthology of our newly reported little skate Col2a1 sequence (GenBank MT254563).
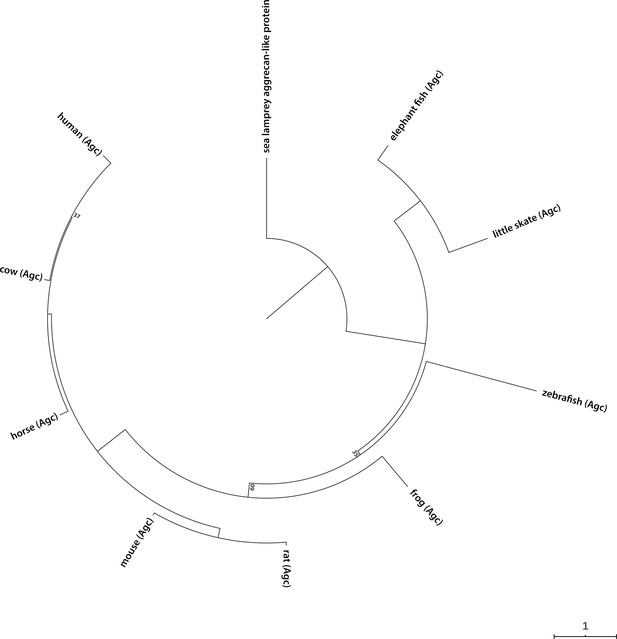
Phylogenetic analysis of vertebrate aggrecan.
Phylogenetic analysis of selected vertebrate aggrecan (Agc) amino acid sequences confirms orthology of our newly reported little skate Agc sequence (GenBank MT254564).
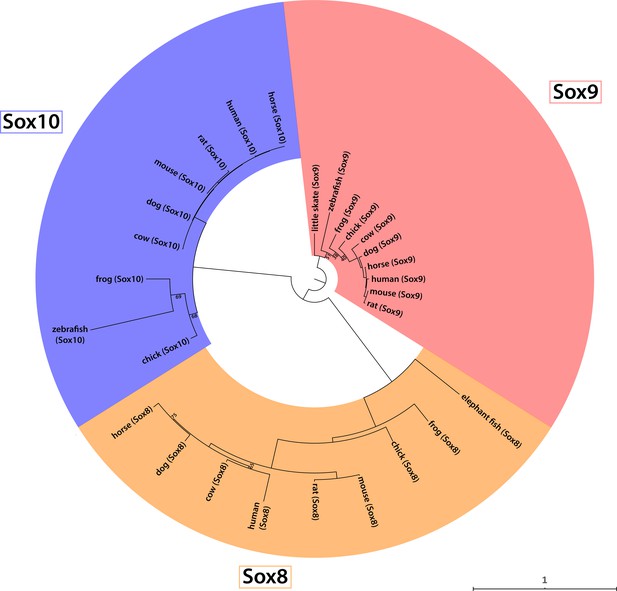
Phylogenetic analysis of the vertebrate SoxE family.
Phylogenetic analysis of amino acid sequences of selected vertebrate SoxE family members resolves three clades (Sox8, Sox9 and Sox10), and confirms orthology of our newly reported little skate Sox9 sequence (GenBank MT254560).
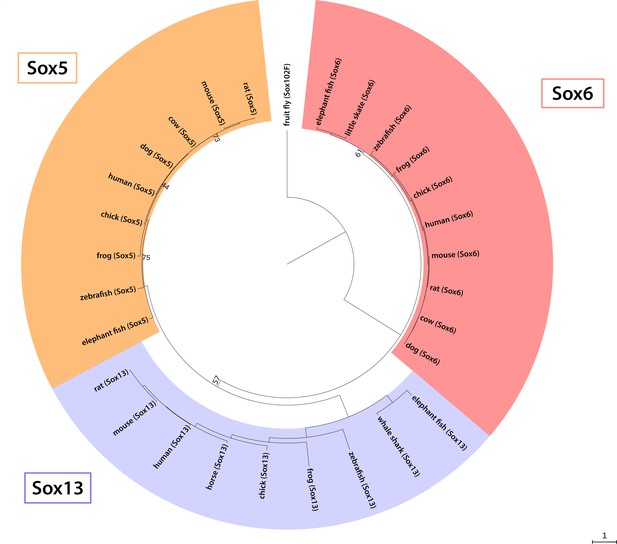
Phylogenetic analysis of the vertebrate SoxD family.
Phylogenetic analysis of amino acid sequences of selected vertebrate SoxD family members resolves three clades (Sox5, Sox6 and Sox13), and confirms orthology of our newly reported little skate Sox6 sequence (GenBank MT254562). We also report a new sequence fragment for little skate Sox5 (GenBank MT254561), which falls within the predicted 3’ UTR, and so was not included in this analysis.
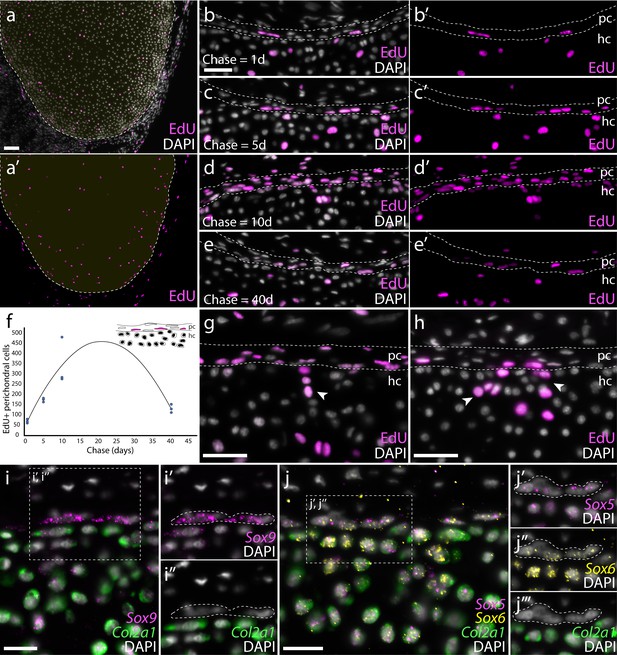
Proliferation of chondrocytes and recruitment of new chondrocytes from putative perichondral progenitors in skate hatchlings.
(a) Transverse sections through the metapterygium of skate hatchlings reveals a concentration of label-retaining (EdU+) cells around the periphery of the element, both in the cartilage and in the perichondrium, as well as sparse label-retaining cells in the center of the metapterygium. An EdU pulse-chase experiment reveals that the perichondrium is an expanding cell population. (b) 1 day chase reveals sporadic labelling of cells within the perichondrium, but an increase in the number of EdU+ perichondral cells after (c) 5- and (d) 10 day chases. (e) After a 40 day chase, reduction in the number EdU+ cells in the perichondrium points to label dilution over several rounds of cell division. (f) Sum total EdU+ perichondral cells from three adjacent 8 μm transverse paraffin sections through the metapterygium after EdU pulse and 1-, 5-, 10- and 40 day chase (n = 3 individuals per time point). (g) The occurrence of clusters of EdU+ chondrocytes immediately adjacent to EdU+ perichondral cells after 10 day and (h) 40 day chase points to the likely perichondral origin of these cells. mRNA in situ hybridisation by HCR for (i) Sox9 and Col2a1, and for (j), Sox5, Sox6 and Col2a1 reveals a population of perichondral cells that sit at the cartilage-perichondral boundary, and that co-express Sox9, Sox5 and Sox6 but not Col2a1. These cells (white dashed outline) are morphologically similar to the label-retaining perichondral cells identified in (b-e). In all images, plane of section as indicated in Figure 1i. hc, hyaline cartilage; pc, perichondrium. Scale bars: (a) 100 μm, (b-e), (g-h) 50 μm, (i-j) 25 μm.
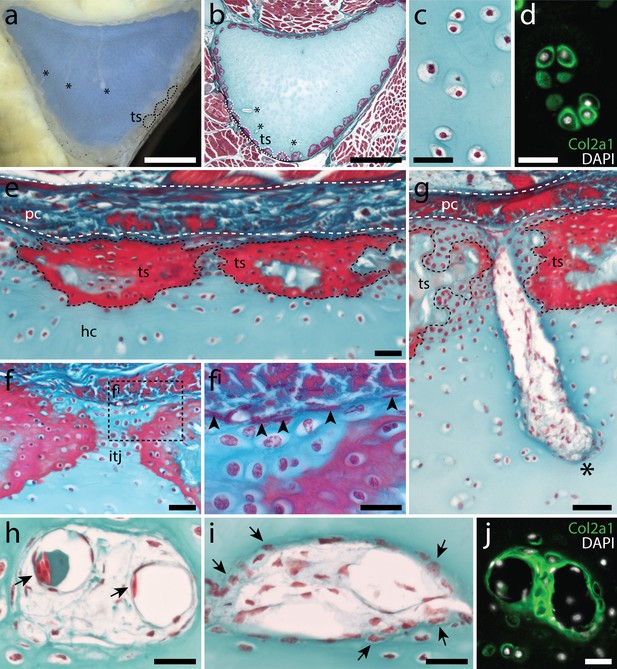
Histological features of the metapterygium in the adult skate.
(a) Transverse vibratome and (b) histological sections through the adult skate metapterygium reveal cartilage canals (asterisks) originating in the perichondrium and extending into the cartilaginous core of the element. The surface of the metapterygium is covered by calcified tesserae (dashed outlines). (c) Cells within the hyaline cartilage core of the metapterygium exhibit typical chondrocyte morphology, and (d) are surrounded by abundant pericellular type II collagen. (e) Mineralized tesserae sit between the hyaline cartilage core and an overlying fibrous perichondrium. (f) Examination of the unmineralized hyaline cartilage of the intertesseral joint region reveals a population of flattened, spindle-shaped cells (black arrowheads in fi) sitting at the boundary between the cartilage and the perichondrium. (g) Cartilage canals (asterisk) can be seen entering the hyaline cartilage of the metapterygium through the intertesseral joint region. These canals originate in the perichondrium, and extend into the core cartilage of the metapterygium. (h) Cartilage canals are not lined by an epithelium, and contain some red blood cells (black arrows), but predominantly (i) connective tissue-like cells – many of which appear to be invading adjacent cartilage ECM (black arrows). (j) Cartilage canals are sites of active type collagen secretion, as indicated by positive immunostaining for Col2a1. (b-c) and (e-i) stained with modified Masson’s trichrome. Plane of section as indicated in Figure 1i. hc, hyaline cartilage; itj, intertesseral joint region; pc, perichondrium; ts, tesserae. Scale bars: (a-b) 2 mm, (c-d) 30 μm, (e-f) 50 μm, (fi) 30 μm, (g) 50 μm, (h-j) 30 μm.
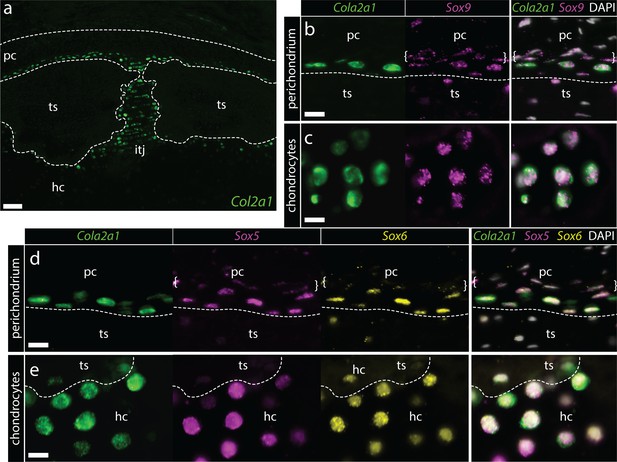
Conserved co-expression of Col2a1, Sox9, Sox5 and Sox6 in peripheral chondrocytes and inner perichondral cells of the adult metapterygium.
(a) Col2a1 is highly expressed by peripheral chondrocytes of the metapterygium, in supratesseral chondrocytes and in the hyaline cartilage around the tesserae (b) Supratesseral and (c) peripheral chondrocytes co-express Col2a1 and Sox9, as well as (d-e) Col2a1, Sox5 and Sox6. In (b) and (d) white brackets indicate inner perichondral cells that co-express Sox9, Sox5 and Sox6 but not Col2a1. Plane of section as indicated in Figure 1i. hc, hyaline cartilage; pc, perichondrium; ts, tesserae. Scale bars: (a) 100 μm, (b-e) 15 μm.
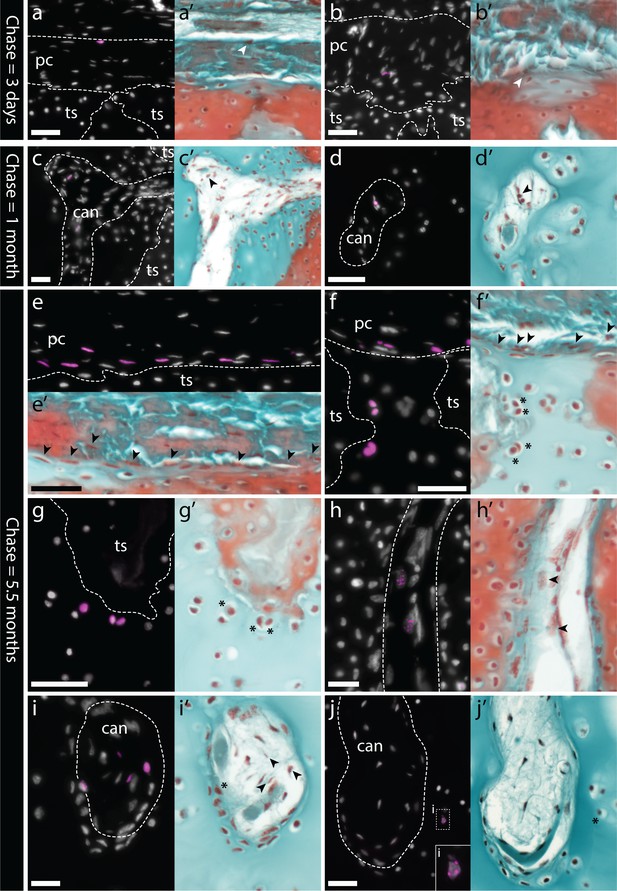
Label-retaining perichondral cells are cartilage progenitors in the adult metapterygium.
(a) After a 3 day chase, EdU retention is detected in cells of the outer and (b) inner perichondrium. (c-d) After a 1 month chase, EdU-retaining cells are additionally detected inside cartilage canals. (e) After a 5.5 month chase, EdU-retaining cells are detected in abundance in the inner perichondrium, and also in peripheral chondrocytes, including (f) in the hyaline cartilage of the intertesseral joint region and (g) in hyaline cartilage beneath tesserae. (h) EdU-retaining cells are detected in greater abundance inside cartilage canals, and (i) can also be seen migrating from inside cartilage canals into adjacent ECM. (j) EdU-retaining chondrocytes are also detected in the core of the metapterygium, adjacent to the blind end of cartilage canals. For each panel, the same section was imaged for EdU detection (counterstained with DAPI), and subsequently stained with modified Masson’s trichrome. In histochemical images, EdU+ nuclei in the perichondrium or in cartilage canals are indicated with arrowheads, while EdU+ chondrocytes are indicated with an asterisk. Plane of section as indicated in Figure 1i. can, canal; pc, perichondrium; ts, tesserae. Scale bars: (a-c) 50 μm, (d) 30 μm, (e-g) 50 μm, (h-j) 30 μm.
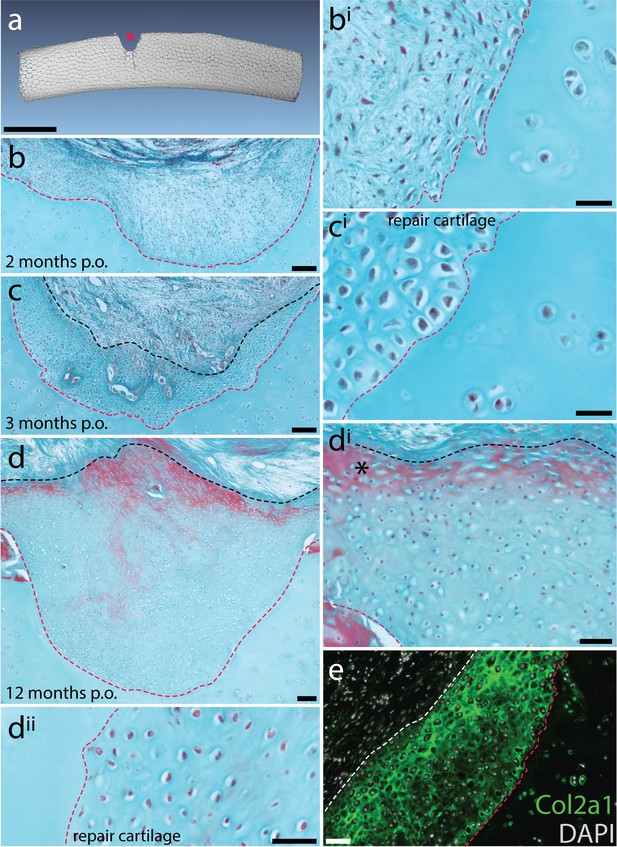
Spontaneous repair of hyaline cartilage in the skate.
(a) 3D reconstruction of a dissected metapterygium 2 weeks following experimental cartilage injury. Note the biopsy (red asterisk) has left a void of ~1/3 the diameter of the metapterygium. (b, bi) By 2 months post-operation (p.o.), the injury site has been filled with a fibrous connective tissue, and (c, ci) by 3 months p.o., this connective tissue begins to differentiate into cartilage. Note that the cells of the repair tissue adopt chondrocyte morphology, and the ECM of the repair tissue is integrated with adjacent cartilage. (d) By 12 months p.o., the injury site has been completely filled with repair cartilage. (di) Red staining of ECM at the surface of the repair tissue (*) could indicate the re-appearance of tissue with a perichondral-like nature, or the re-establishment of tesserae at the injured surface of the metapterygium. However, (dii) the vast majority of repair tissue is composed of typical hyaline cartilage. (e) Immunofluorescence reveals abundant type II collagen (Col2a1) in the ECM of repair cartilage. In (b-d), the red dashed line indicates the boundary of the biopsy, and the black dashed line indicates the extent of repair cartilage. In (e) the red dashed line indicates the boundary of the biopsy, and the white dashed line indicates the extent of repair cartilage. hc, hyaline cartilage; pc, perichondrium; ts, tesserae. Scale bars: (a) 1 cm, (b) 100 μm, (bi) 30 μm, (c) 100 μm, (ci) 30 μm, (d) 100 μm, (di) 50 μm, (dii) 50 μm, (e) 50 μm.
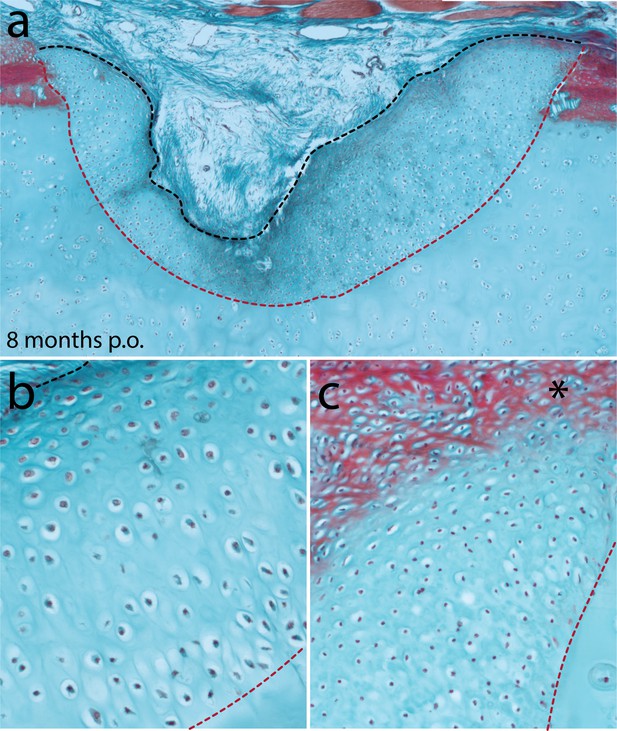
Cartilage repair in the metapterygium of the skate 8 months post-operation.
(a) Eight months after cartilage biopsy, the injury site has largely filled with repair cartilage. (b) The vast majority of repair cartilage resembles typical hyaline cartilage, though (c) we also observe some superficial red staining of the ECM (*), which could indicate the re-appearance of tissue with a perichondral-like nature, or the re-establishment of superficial tesserae at the surface of the metapterygium. In all panels, the red dashed line indicates the boundary of the biopsy, and the black dashed line indicates the extent of repair cartilage.
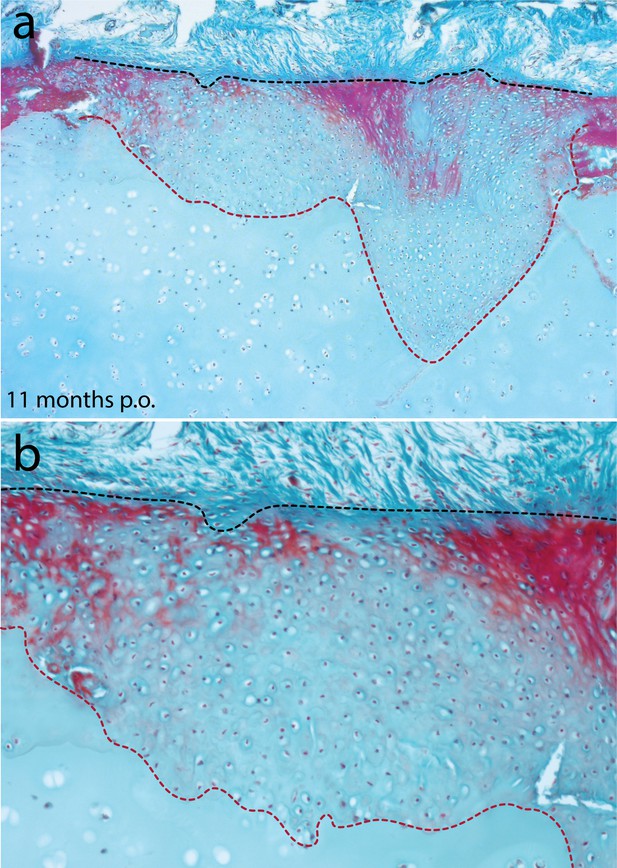
Cartilage repair in the metapterygium of the skate 11 months post-operation.
(a) Eleven months after cartilage biopsy, the injury site has completely filled with repair cartilage. (b) The repair cartilage consists largely of typical hyaline cartilage, though with some superficial red staining of the ECM, which could indicate the re-appearance of tissue with a perichondral-like nature, or the re-establishment of superficial tesserae at the surface of the metapterygium. In all panels, the red dashed line indicates the boundary of the biopsy, and the black dashed line indicates the extent of repair cartilage.
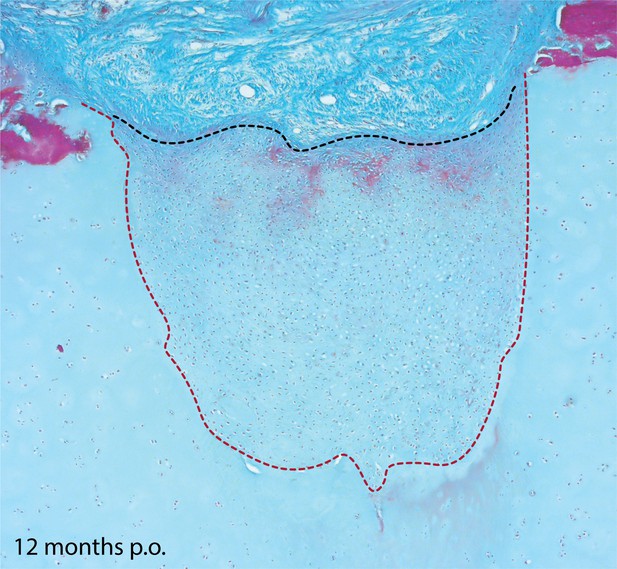
Cartilage repair in the metapterygium of the skate 12 months post-operation.
Twelve months after cartilage biopsy, the injury site has nearly completely filled with repair cartilage. The repair cartilage consists largely of typical hyaline cartilage, though with some superficial red staining of the ECM, which could indicate the re-appearance of tissue with a perichondral-like nature, or the re-establishment of superficial tesserae at the surface of the metapterygium. The red dashed line indicates the boundary of the biopsy, and the black dashed line indicates the extent of repair cartilage. Note that this image is from a different individual than that figure in Figure 7d.
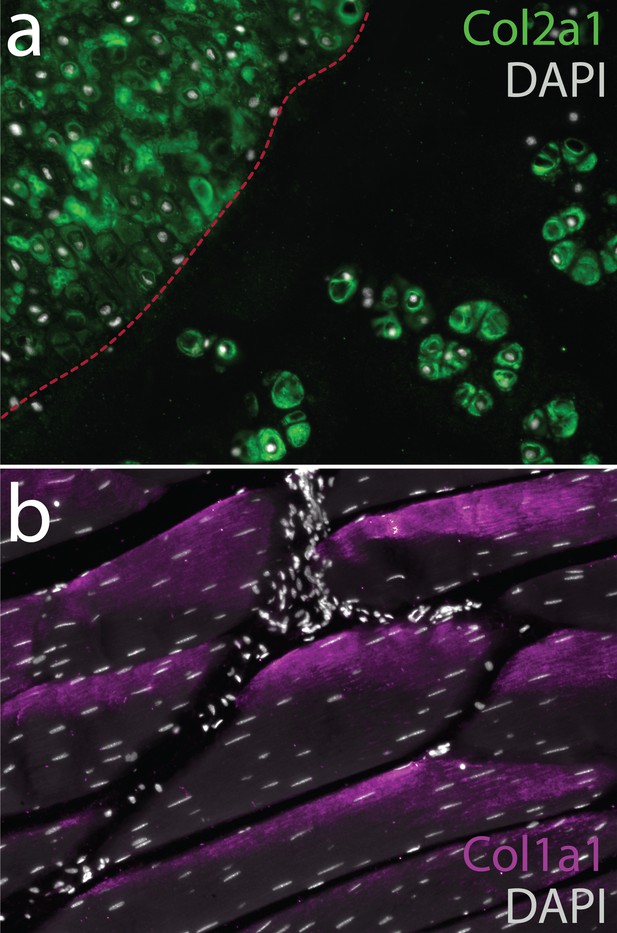
Collagen composition of ECM in uninjured and repair cartilage in the skate metapterygium 8 months post-operation.
(a) Immunodetection of type II collagen reveals localization to the pericellular ECM of both uninjured and repair cartilage in the metapterygium. Conversely, we detected no type I collagen in the ECM of uninjured or repair cartilage, despite (b) positive immunodetection of type I collagen in the ECM of adjacent skeletal muscle fibres. In (a) red dashed line indicates the boundary of the biopsy, with repair cartilage to the left.
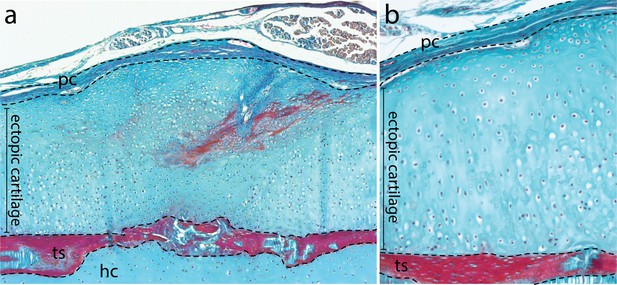
Ectopic cartilage following perichondral disruption in the metapterygium of the skate.
(a–b) In two animals, failed biopsies resulted in damage to the perichondrium without removal of cartilage. In both instances, this resulted in the induction of large masses of ectopic cartilage beneath the perichondrium, and above the tesserae. hc, hyaline cartilage; pc, perichondrium; ts, tesserae.
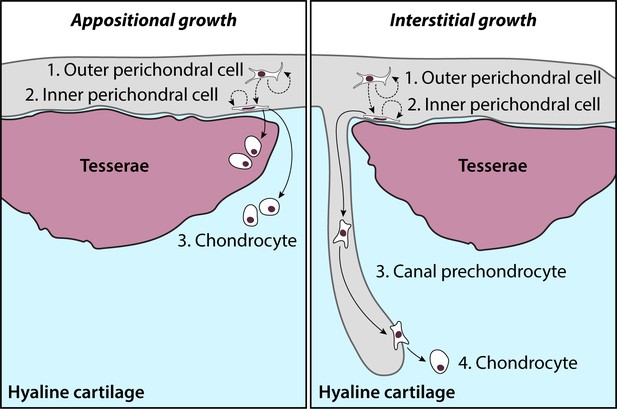
Model of adult chondrogenesis in the skate.
Adult skate cartilage grows both appositionally (i.e. through peripheral addition of new chondrocytes and expansion of ECM) and interstitially (i.e. through incorporation of new chondrocytes deeper in the hyaline cartilage core). In both cases, progenitor cells reside within the perichondrium. Label-retention experiments indicate that cells cycle in both the outer and inner perichondrium (possibly with the former giving rise to the latter, along with self-renewal), and that cells of perichondral origin give rise to new chondrocytes. In appositional growth, chondrocyte progeny of perichondral progenitors resides in peripheral hyaline cartilage (or, occasionally, become incorporated into growing tesserae). In interstitial growth, prechondrocyte progeny of perichondral progenitors is transported to the cartilage core, where they ultimately invade the cartilage ECM and differentiate into chondrocytes.
Tables
Recovery of EdU-retaining cells within the metapterygium of adult skates after pulse and 3 day, 1-, 2- and 5.5 month chase.
| Number of EdU-retaining cells in… | ||||
|---|---|---|---|---|
| Chase time | Perichondrium (outer) | Perichondrium (inner) | Cartilage canals | Chondrocytes |
| 3 days (1) | 22 | 3 | 1 | 0 |
| 3 days (2) | 6 | 0 | 0 | 0 |
| 1 month (1) | 9 | 7 | 3 | 0 |
| 1 month (2) | 9 | 0 | 2 | 0 |
| 2 months (1) | 16 | 0 | 2 | 2 |
| 2 months (2) | 107 | 31 | 4 | 0 |
| 5.5 months (1) | 271 | 259 | 20 | 25 |
| 5.5 months (2) | 66 | 15 | 20 | 10 |