A mammalian Wnt5a–Ror2–Vangl2 axis controls the cytoskeleton and confers cellular properties required for alveologenesis
Figures
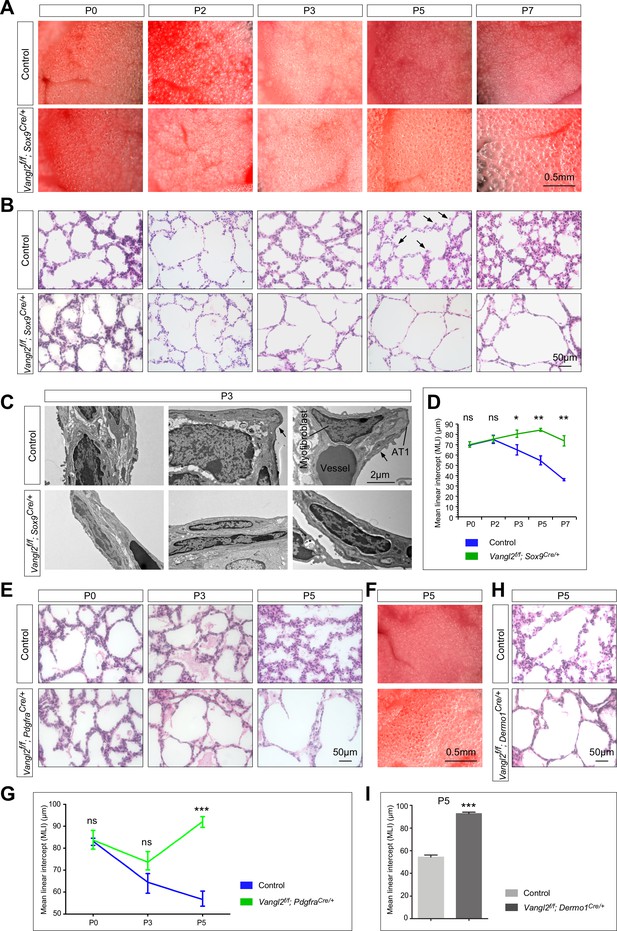
Vangl2 is required in both the lung epithelium and mesenchyme for alveolar formation.
(A) Surface view of dissected lungs from wild-type and Vangl2f/f; Sox9Cre/+ mice at different postnatal (P) stages as indicated. Enlarged saccules were discerned in Vangl2f/f; Sox9Cre/+ lungs at P3 and their size increased significantly as postnatal lung development proceeded. (B) Hematoxylin and eosin-stained lung sections of wild-type and Vangl2f/f; Sox9Cre/+ mice at different postnatal stages. Histological analysis confirmed the presence of enlarged saccules in Vangl2-deficient lungs starting at P3. Arrows point to rudimentary secondary septa. (C) Transmission electron micrographs of lungs from wild-type and Vangl2f/f; Sox9Cre/+ mice at P3. Rudimentary secondary septa (arrows), in which the alveolar type I (AT1) cells encased myofibroblasts and blood vessels, were seen in control but not Vangl2-deficient lungs. (D) Measurement of the mean linear intercept (MLI) in wild-type and Vangl2f/f; Sox9Cre/+ lungs (n = 3 for each group). The MLI was increased in Vangl2-deficient lungs, starting at P3. (E) Hematoxylin and eosin-stained lung sections of wild-type and Vangl2f/f; PdgfraCre/+ mice at different postnatal stages. Enlarged saccules were detected in Vangl2f/f; PdgfraCre/+ lungs at P3 and their size increased significantly as postnatal lung development proceeded. (F) Surface view of dissected lungs from wild-type and Vangl2f/f; PdgfraCre/+ mice at P5. Larger saccules were found in Vangl2 mutant lungs induced by PdgfraCre. (G) Measurement of the MLI in wild-type and Vangl2f/f; PdgfraCre/+ lungs (n = 3 for each group). The MLI was increased in Vangl2-deficient lungs, starting at P5. (H) Hematoxylin and eosin-stained lung sections of wild-type and Vangl2f/f; Dermo1Cre/+ mice at P5. Larger saccules were found in Vangl2 mutant lungs induced by Dermo1Cre. (I) Measurement of the MLI in wild-type and Vangl2f/f; Dermo1Cre/+ lungs (n = 3 for each group). The MLI was increased in Vangl2-deficient lungs. All values are mean ± SEM. (*) p<0.05; (**) p<0.01; ns, not significant (unpaired Student’s t-test).
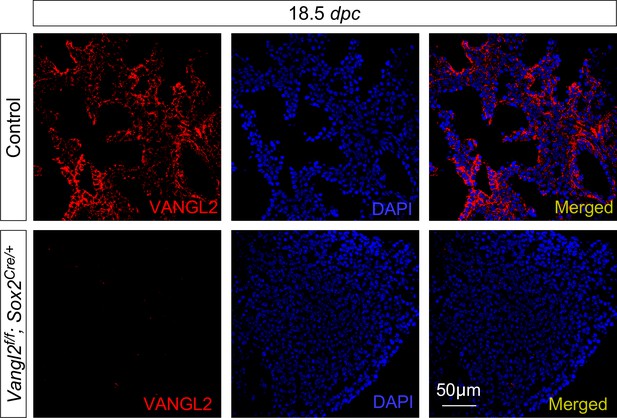
VANGL2 is broadly expressed in both the lung epithelium and mesenchyme.
Immunostaining of lung sections collected from control and Vangl2f/f; Sox2Cre/+ mice at 18.5 days post coitus (dpc). VANGL2 was detected in both the lung epithelium and mesenchyme in wild-type lungs. VANGL2 immunoreactivity was completely absent in the lungs of Vangl2f/f; Sox2Cre/+ mice, validating the VANGL2 immunoreactivity detected by VANGL2 antibodies. Note that early expression of Sox2-Cre in all epiblast cells by 6.5 dpc effectively converted Vangl2f into a null allele in all embryonic lineages. Vangl2f/f; Sox2Cre/+ is in essence equivalent to Vangl2–/–.
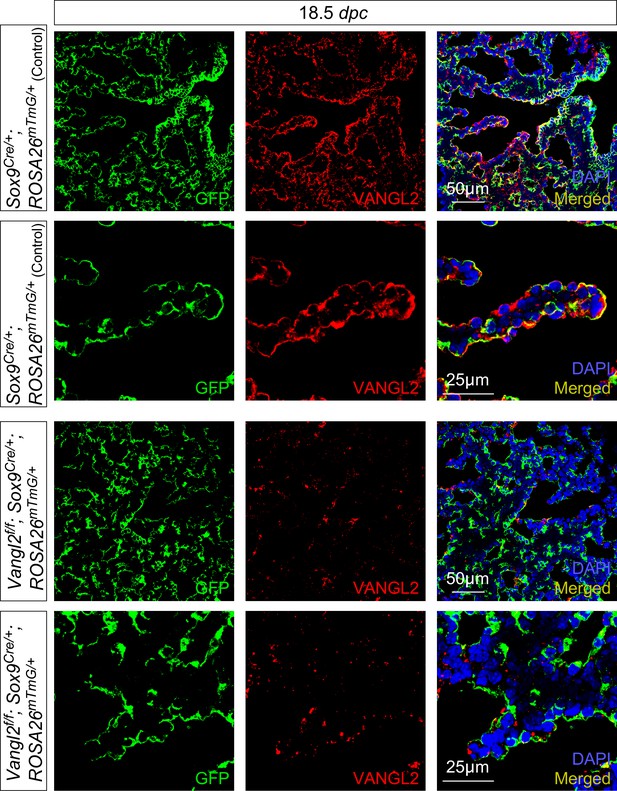
VANGL2 is selectively removed in the distal lung epithelium by Sox9-Cre.
Immunostaining of lung sections collected from Sox9Cre/+; ROSA26mTmG/+ (control) and Vangl2f/f; Sox9Cre/+; ROSA26mTmG/+ mice at 18.5 days post coitus (dpc). Sox9-Cre induced GFP expression from the ROSA26mTmG locus. VANGL2 immunoreactivity was selectively lost in the lung epithelium (GFP+) of Vangl2f/f; Sox9Cre/+; ROSA26mTmG/+ mice; VANGL2 expression was retained in the lung mesenchyme.
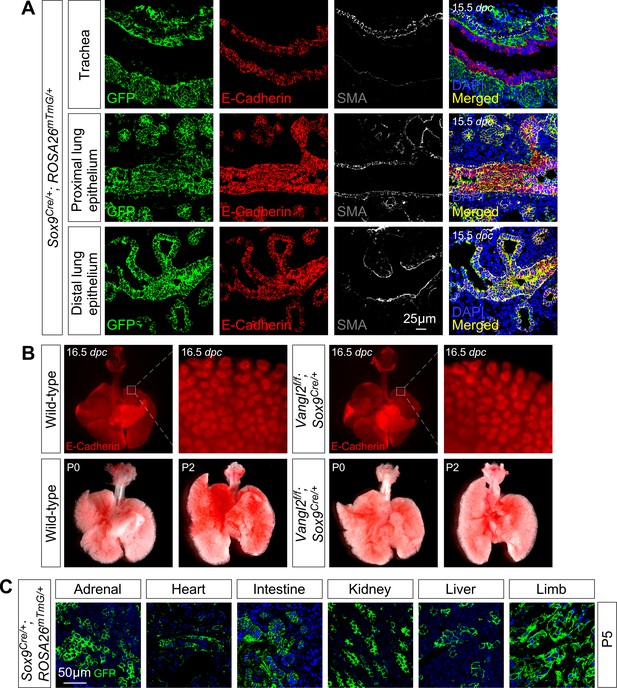
Sox9-Cre is broadly expressed but Vangl2f/f; Sox9Cre/+ mice do not display defects in lung branching or saccule formation.
(A) Immunostaining of lung sections collected from Sox9Cre/+; ROSA26mTmG/+ mice at 15.5 days post coitus (dpc). (B) Ventral views of dissected lungs from control or Vangl2f/f; Sox9Cre/+ mice at 16.5 dpc, postnatal (P) day 0 or 2. No apparent phenotypes in branching (likely due to the presence of Vangl1) or sacculation were discerned in the mutant lungs. (C) Immunostaining of sections of various tissues collected from Sox9Cre/+; ROSA26mTmG/+ mice at P5.
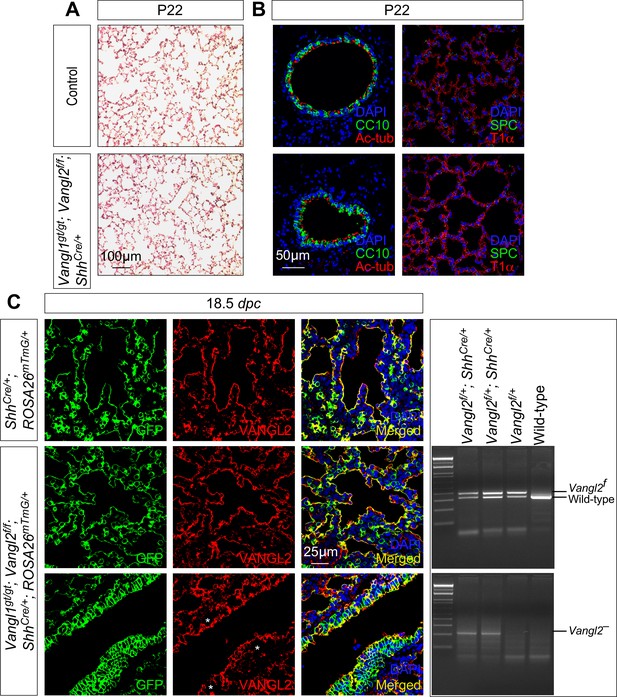
Shh-Cre fails to efficiently remove Vangl2 in the lung epithelium.
(A) Hematoxylin and eosin-stained lung sections of control and Vangl1gt/gt; Vangl2f/f; ShhCre/+ mice at postnatal (P) day 22. Alveoli formed properly in Vangl1gt/gt; Vangl2f/f; ShhCre/+ lungs, which were indistinguishable from control lungs. (B) Immunostaining of lung sections collected from control and Vangl1gt/gt; Vangl2f/f; ShhCre/+ mice at P22. There was no discernable difference in cell type specification and distribution between control and Vangl1gt/gt; Vangl2f/f; ShhCre/+ lungs. CC10 marked club (Clara) cells and Ac-tub marked ciliated cells in the airways. SPC labeled alveolar type II cells and T1α labeled alveolar type I cells in alveoli. (C) Immunostaining of lung sections collected from ShhCre/+; ROSA26mTmG/+ (control) and Vangl1gt/gt; Vangl2f/f; ShhCre/+; ROSA26mTmG/+ mice at 18.5 days post coitus (dpc). Shh-Cre induced epithelial GFP expression from the ROSA26mTmG locus. Only small pockets (*) of the proximal lung epithelium in Vangl1gt/gt; Vangl2f/f; ShhCre/+; ROSA26mTmG/+ mice displayed VANGL2 loss in comparison with controls. This is consistent with the presence of Vangl2– (null) by PCR.
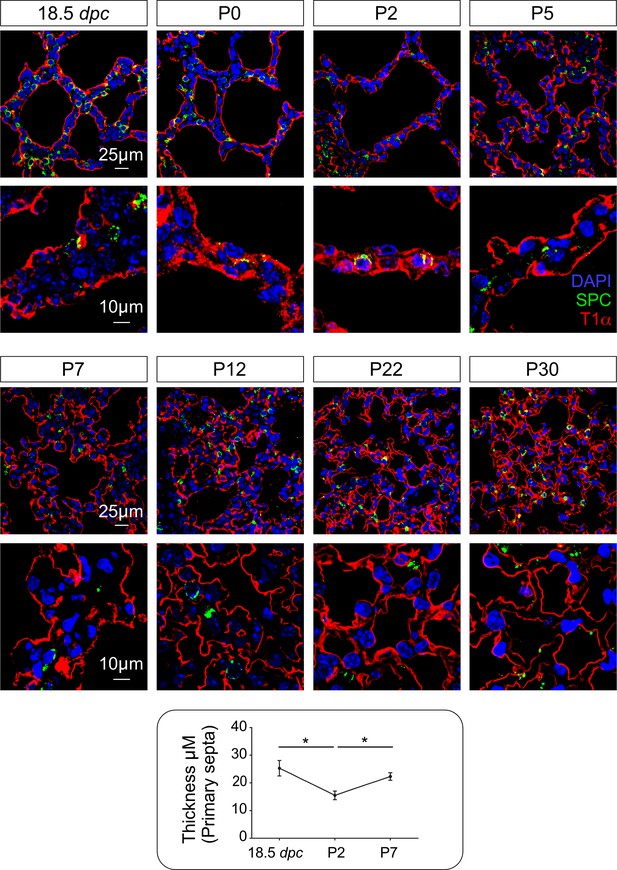
A time course of the development of the alveolar septa.
Immunostaining of lung sections collected from wild-type mice at 18.5 days post coitus (dpc) and various postnatal (P) days as indicated. The primary septa appeared to be visibly thinner during the first three days of postnatal life but its thickness increased subsequently. T1α labeled alveolar type I cells while SPC marked alveolar type II cells. All values are mean SEM. (*) p<0.05 (unpaired Student’s t-test).
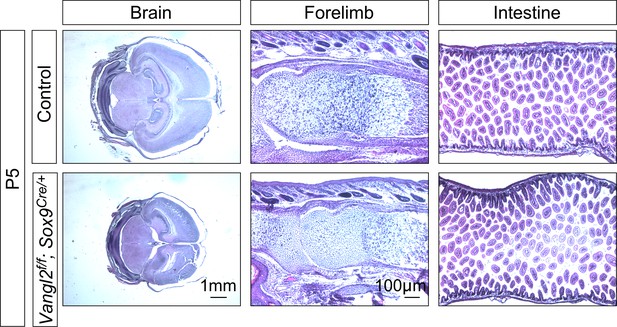
Histological analysis of tissues and organs in Vangl2f/f; Sox9Cre/+ mice.
Hematoxylin and eosin-stained tissue sections of control and Vangl2f/f; Sox9Cre/+ mice at postnatal (P) day 5. Histological analysis revealed no apparent defects in the brain, bone and small intestine where Sox9-Cre is expressed.
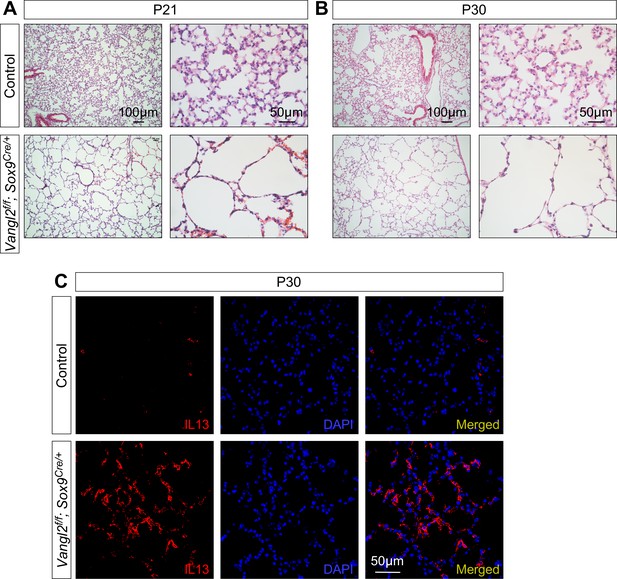
Loss of epithelial Vangl2 disrupts secondary septa and alveolar formation.
(A, B) Hematoxylin and eosin-stained lung sections of control and Vangl2f/f; Sox9Cre/+ mice at postnatal (P) day 21 and 30. Secondary septa and alveoli failed to form and only thin primary septa persisted in Vangl2f/f; Sox9Cre/+ lungs. (C) Immunostaining of lung sections collected from control and Vangl2f/f; Sox9Cre/+ mice at P30. IL13 expression levels were elevated in the mutant lungs. Note that these Vangl2f/f; Sox9Cre/+ animals were survivors since many of them succumbed to death prior to P10. No survivors beyond four weeks carry the genotype of Vangl1gt/+; Vangl2f/f; Sox9Cre/+, consistent with a minor role of Vangl1 in alveologenesis.
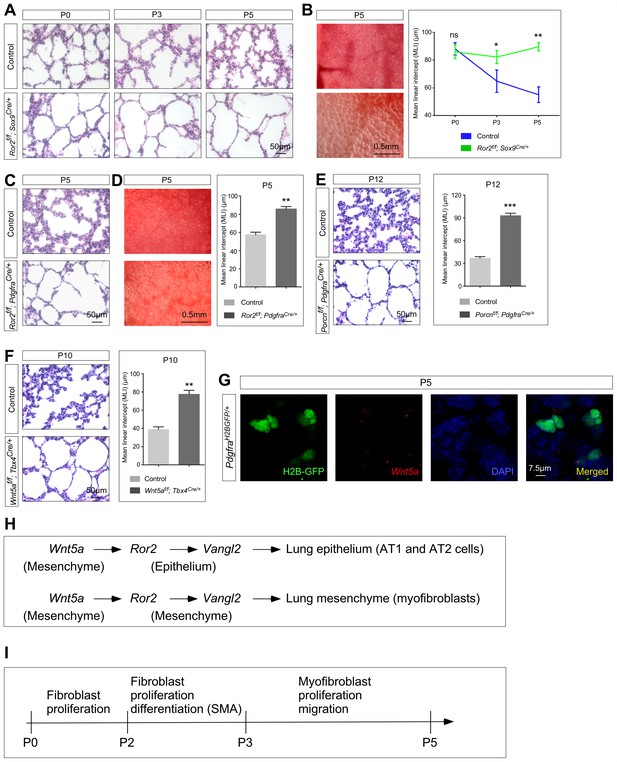
Ror2 is required in both the lung epithelium and mesenchyme while Wnt5a is required in the lung mesenchyme for alveolar formation.
(A) Hematoxylin and eosin-stained lung sections of wild-type and Ror2f/f; Sox9Cre/+ mice at different postnatal (P) stages. Histological analysis revealed the presence of enlarged saccules starting at P3 in Ror2-deficient lungs. (B) Surface view of dissected lungs from wild-type and Ror2f/f; Sox9Cre/+ mice at P5. Larger saccules were found in Ror2 mutant lungs induced by Sox9Cre and the MLI was correspondingly increased in Ror2-deficient lungs. (C) Hematoxylin and eosin-stained lung sections of wild-type and Ror2f/f; PdgfraCre/+ mice at P5. Histological analysis confirmed the presence of enlarged saccules starting at P3 in Ror2-deficient lungs. (D) Surface view of dissected lungs from wild-type and Ror2f/f; PdgfraCre/+ mice at P5. Larger saccules were found in Ror2 mutant lungs induced by PdgfraCre with an increased MLI. (E) Hematoxylin and eosin-stained lung sections of wild-type and Porcnf/f; PdgfraCre/+ mice at P12. Larger saccules were found in Porcn mutant lungs induced by Pdgfra-Cre with an increased MLI. (F) Hematoxylin and eosin-stained lung sections of wild-type and Wnt5af/f; Tbx4Cre/+ mice at P10. Larger saccules were found in Wnt5a mutant lungs induced by Tbx4-Cre with an increased MLI. (G) Combined in situ hybridization (PLISH)/immunohistochemistry on lung sections of PdgfraH2BGFP/+ mice to examine Wnt5a expression. Wnt5a mRNA was mainly detected in myofibroblasts (H2BGFP+) and not in the lung epithelium. (H) Schematic diagram of a Wnt5a–Ror2–Vangl2 axis that functions in both the lung epithelium and mesenchyme to regulate alveologenesis. (I) Schematic diagram of the temporal sequence of fibroblast/myofibroblast proliferation, differentiation and migration.
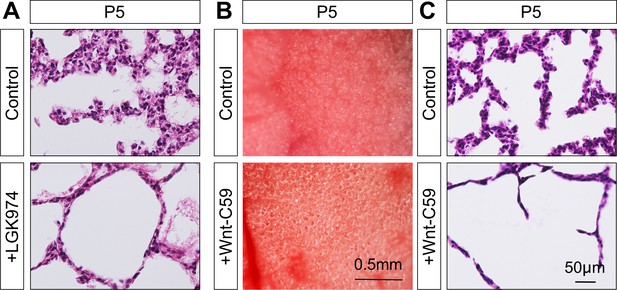
Inhibition of PORCUPINE activity leads to alveolar defects.
(A) Hematoxylin and eosin-stained lung sections of mice at postnatal (P) day 5, which were treated with vehicles (control) or LGK974 (PORCUPINE inhibitor) at birth. (B) Surface view of dissected lungs from mice at P5, which were treated with vehicles or Wnt-C59 (PORCUPINE inhibitor) at birth. Larger saccules were found in lungs treated with Wnt-C59 compared to controls. (C) Histological analysis confirmed the presence of enlarged saccules in Wnt-C59-treated lungs.
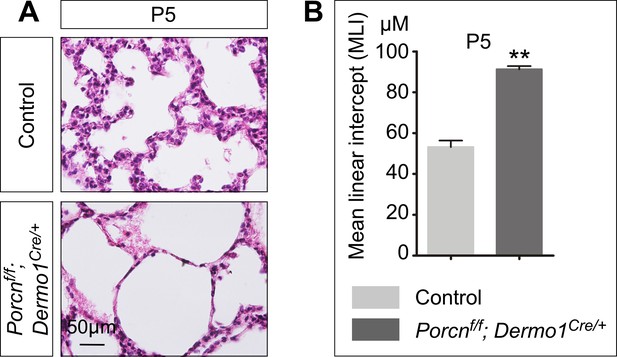
Elimination of mesenchymal Porcupine affects alveolar formation.
(A) Hematoxylin and eosin-stained lung sections of control and Porcnf/f; Dermo1Cre/+ mice at postnatal (P) day 5. Histological analysis revealed the presence of enlarged saccules in the absence of mesenchymal Porcn induced by Dermo1-Cre. (B) Measurement of the mean linear intercept (MLI) in control and Porcnf/f; Dermo1Cre/+ lungs at P5. The MLI was increased in the mutant lungs. All values are mean SEM. (**) p<0.01 (unpaired Student’s t-test).
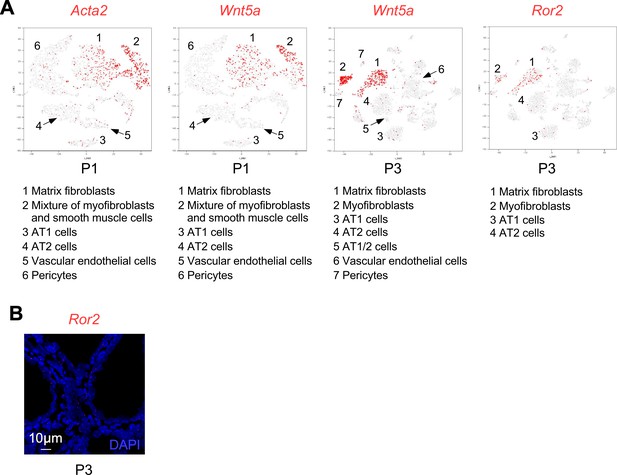
Single-cell RNA-Seq reveals expression of Wnt5a in myofibroblasts.
(A) Single-cell RNA-Seq data of mouse lung cells at postnatal (P) 1 and 3 were downloaded from LungMAP, which is an NIH-funded consortium to generate publicly available data. Expression of Acta2 (SMA), Wnt5a and Ror2 (red color) was superimposed with predicted mouse lung cell populations on a t-SNE plot. At P1, Wnt5a is expressed in airway/vascular smooth muscle cells (ACTA2+) in addition to alveolar fibroblasts (ACTA2–). At P3, Wnt5a is highly expressed in fibroblasts and myofibroblasts but not in alveolar epithelial cells, endothelial cells or pericytes. At P3, Ror2 is also highly expressed in fibroblasts and myofibroblasts with lower levels of expression in alveolar epithelial cells. (B) In situ hybridization (PLISH) on lung sections of wild-type mice to examine Ror2 expression. Ror2 was broadly expressed in both the lung epithelium and mesenchyme.
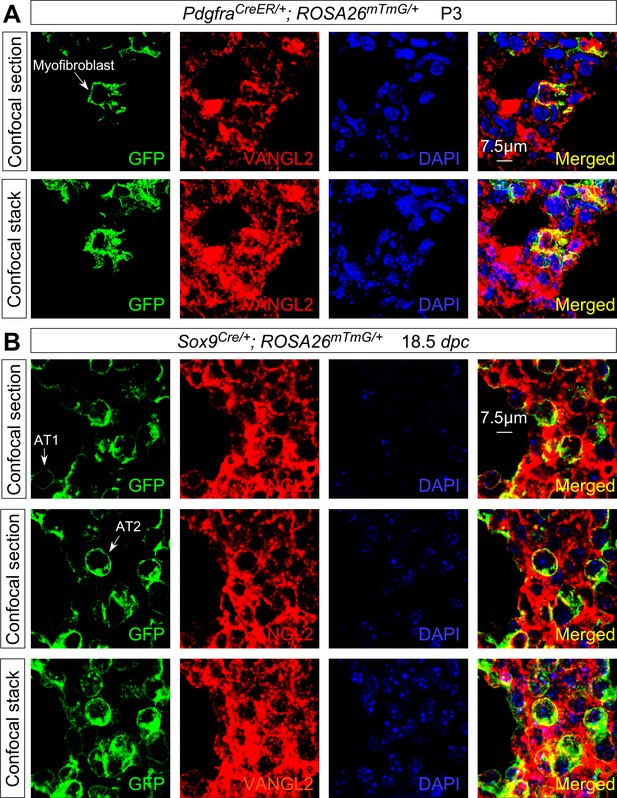
VANGL2 is not asymmetrically localized in lung epithelial and mesenchymal cells.
(A) Immunostaining of lung sections collected from PdgfraCreER/+; ROSA26mTmG/+ mice at postnatal (P) day 3. Leaky expression of CreER led to GFP-labeling (from the ROSA26mTmG/+ locus) of individual myofibroblasts. No apparent asymmetric distribution of VANGL2 was detected in myofibroblasts. This was revealed by examining each confocal section. The confocal stack represents the compilation of all confocal sections for a given myofibroblast examined. (B) Immunostaining of lung sections collected from Sox9Cre/+; ROSA26mTmG/+ mice at 18.5 days post coitus (dpc). Activation of GFP by Sox9-Cre resulted in labeling of alveolar type I (AT1) and type II (AT2) cells, which could be distinguished by morphology. No apparent asymmetric distribution of VANGL2 was detected in AT1 or AT2 cells. This was revealed by examining each confocal section. The confocal stack represents the compilation of all confocal sections for a given AT1 or AT2 cell examined. The extended morphology of AT1 cells placed an inherent limitation on the proportion of cell membrane that could be visualized in a given AT1 cell. Nevertheless, in regions that could be discerned, especially cell membranes surrounding the nucleus, no asymmetric distribution of VANGL2 was revealed.
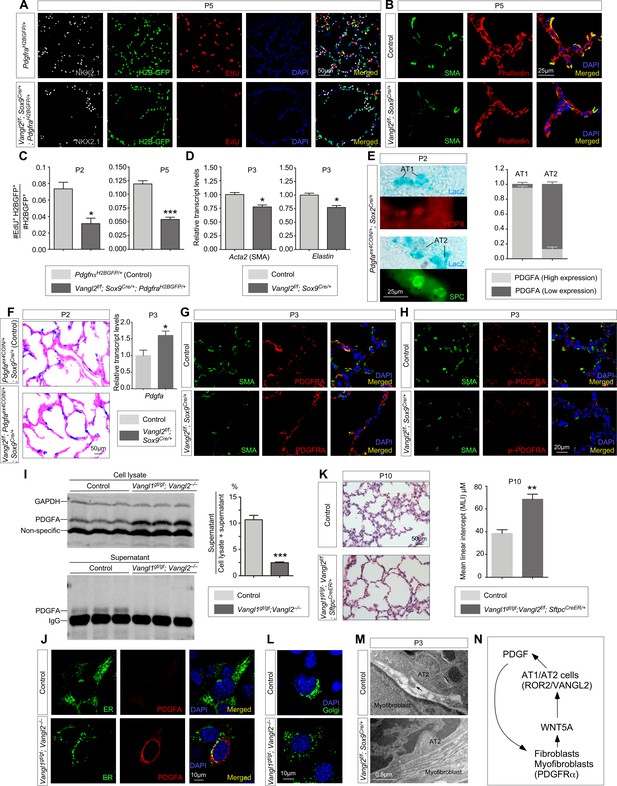
Vangl2 is required for PDGF ligand trafficking/release from PDGF-producing cells and subsequently PDGF signal reception.
(A) Immunostaining of lung sections collected from PdgfraH2BGFP/+ (control) and Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ mice injected with EdU at postnatal (P) day 5. Lung epithelial cells were distinguished by NKX2.1 staining while myofibroblasts were marked by H2B-GFP from the Pdgfra locus (PdgfraH2BGFP). The number of EdU+ cells was reduced in Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ lungs compared to controls. (B) Immunostaining of lung sections collected from control and Vangl2f/f; Sox9Cre/+ mice at P5. Organization of the cytoskeleton and stress fibers was disrupted and SMA at the prospective sites of secondary septation was sparse in Vangl2-deficient lungs. (C) Quantification of myofibroblast proliferation in PdgfraH2BGFP/+ (control) and Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ lungs at P2 and P5. The rate of myofibroblast proliferation was calculated as the ratio of the number of EdU+ myofibroblasts (EdU+H2BGFP+) to the number of myofibroblasts (H2BGFP+). An apparent reduction in the percentage of proliferating myofibroblasts was detected in Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ lungs compared to controls (n = 3 for each group) at P2 and P5. (D) qPCR analysis of Acta2 (SMA) and Elastin in control and Vangl2f/f; Sox9Cre/+ lungs at P3. The mRNA levels of Acta2 and Elastin were significantly reduced in the absence of epithelial Vangl2 induced by Sox9-Cre (n = 3 for each group). (E) Immunostaining of lung sections collected from Pdgfaex4COIN/+; Sox2Cre/+ mice at P2. β-galactosidase (LacZ) was induced in PDGFA-producing cells by Sox2-Cre. LacZ-staining (blue) was followed by immunostaining against HOPX (marker for AT1 cells) and SPC (marker for AT2 cells). LacZ-positive cells also expressed either HOPX or SPC. The number of AT1 or AT2 cells that harbored either high or low PDGFA (LacZ) was counted. (F) LacZ-staining (blue) of lung sections collected from Pdgfaex4COIN/+; Sox9Cre/+ (control) and Vangl2f/f; Pdgfaex4COIN/+; Sox9Cre/+ mice at P2. The slides were counterstained with eosin (red). No difference in the intensity of LacZ (+) cells in the lung was found in these two mouse lines. The mRNA levels of Pdgfa in control and mutant lungs were determined by qPCR. (G) Immunostaining of lung sections collected from control and Vangl2f/f; Sox9Cre/+ mice at P3. No difference in PDGFRA expression levels in individual myofibroblasts was noted between control and Vangl2f/f; Sox9Cre/+ lungs. (H) Immunostaining of lung sections collected from control and Vangl2f/f; Sox9Cre/+ mice at P3. A significant reduction in the levels of phosphorylated (p) PDGFRA in individual myofibroblasts was found in Vangl2f/f; Sox9Cre/+ lungs compared to controls. (I) Western blot analysis of cell lysates and supernatants from control and Vangl1gt/gt; Vangl2–/– cells lentivirally transduced with PDGFA-expressing constructs. The amount of PDGFA released into the media was significantly reduced in Vangl1gt/gt; Vangl2–/– cells compared to controls (n = 3 for each group). GAPDH served as a loading control. We noticed that the amount of secreted proteins (normalized to the cell number) from Vangl1/2 mutant cells was reduced compared to controls. This suggests a general defect in protein processing/secretion in the absence of Vangl1/2. In this case, it is possible that other secreted ligands could also impact alveolar development. (J) Immunostaining of controls and Vangl1gt/gt; Vangl2–/– cells lentivirally transduced with PDGFA-expressing constructs. Endoplasmic reticulum (ER) was marked by mEmerald-ER-5. (K) Hematoxylin and eosin-stained lung sections of wild-type and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+ mice injected with tamoxifen and collected at P10. Enlarged saccules were found in Vangl1gt/gt; Vangl2f/f; SftpcCreER/+ lungs in comparison with controls. (L) Immunostaining of controls and Vangl1gt/gt; Vangl2–/– cells. The Golgi stacks were dispersed in Vangl1gt/gt; Vangl2–/– cells compared to controls. Golgi was marked by mEmerald-Golgi-7. (M) Transmission electron micrographs of lungs from wild-type and Vangl2f/f; Sox9Cre/+ mice at P3. Cellular extension (arrow) from alveolar type II cells to myofibroblasts was observed in control lungs but were absent in Vangl2f/f; Sox9Cre/+ lungs. (N) Schematic diagram of a positive feedback loop between WNT5A and PDGF to generate a pool of fibroblasts/myofibroblasts for alveologenesis. All values are mean ± SEM. (*) p<0.05; (**) p<0.01; (***) p<0.001; ns, not significant (unpaired Student’s t-test).
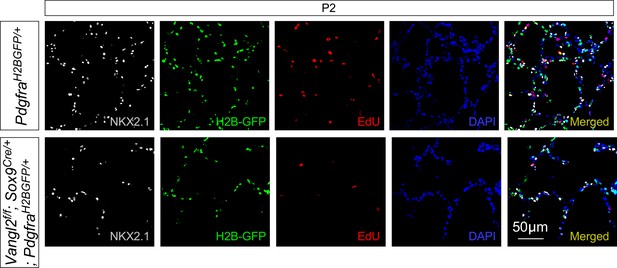
Loss of epithelial Vangl2 leads to reduced myofibroblast proliferation.
Immunostaining of lung sections collected from PdgfraH2BGFP/+ (control) and Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ mice injected with EdU at postnatal (P) day 2. Lung epithelial cells were distinguished by NKX2.1 staining while myofibroblasts were marked by H2B-GFP from the Pdgfra locus (PdgfraH2BGFP). The number of EdU+ cells was reduced in Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ lungs compared to controls. Note that quantification of proliferating myofibroblasts (EdU+H2BGFP+) is shown in Figure 3C.
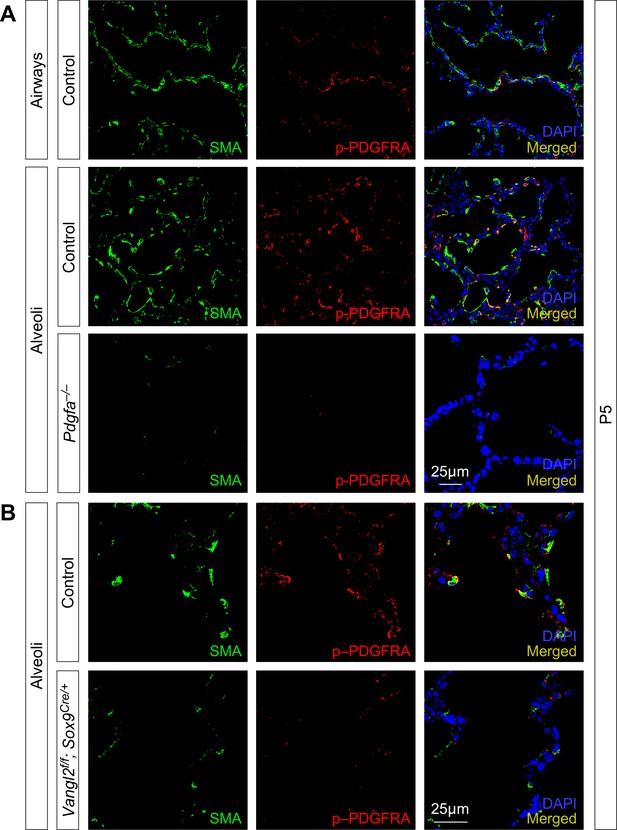
Removal of epithelial Vangl2 results in reduced levels of phosphorylated PDGFRA.
(A) Immunostaining of lung sections collected from control and Pdgfaex4COIN/ex4COIN; Sox2Cre/+ (abbreviated as Pdgfa–/–) mice at postnatal (P) day 5. PDGFRA+ myofibroblasts were absent in Pdgfa–/– lungs. Accordingly, phosphorylated (p) PDGFRA (p-PDGFRA) was barely detectable in Pdgfa–/– lungs. (B) Immunostaining of lung sections collected from control and Vangl2f/f; Sox9Cre/+ mice at P5. A significant reduction in the levels of p-PDGFRA in individual myofibroblasts was found in Vangl2f/f; Sox9Cre/+ lungs compared to controls. Smooth muscle actin (SMA) was primarily detected in smooth muscles and myofibroblasts.
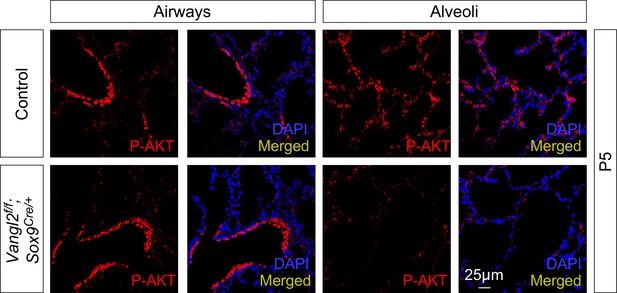
PDGF signal reception is reduced in lungs deficient in Vangl1/2 signaling.
Immunostaining of lung sections collected from control and Vangl2f/f; Sox9Cre/+ mice at postnatal (P) day 5. While phosphorylated (p) AKT (p-AKT) showed no difference between control and Vangl2f/f; Sox9Cre/+ lungs in the airways, p-AKT levels were significantly reduced in the alveoli of Vangl2f/f; Sox9Cre/+ mice.
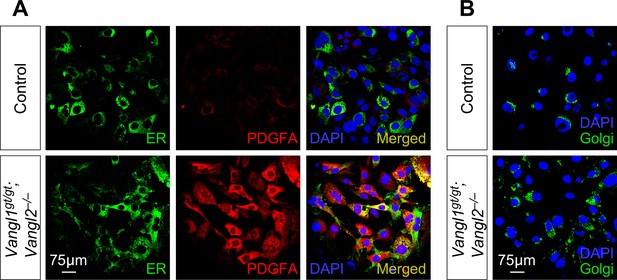
Accumulation of PDGF ligand in the secretory pathway in the absence of VANGL1/2.
(A) Immunostaining of controls and Vangl1gt/gt; Vangl2–/– cells lentivirally transduced with PDGFA-expressing constructs. Endoplasmic reticulum (ER) was marked by mEmerald-ER-5. The levels of PDGFA were significantly increased in the secretory pathway of Vangl1/2-deficient cells. (B) Immunostaining of controls and Vangl1gt/gt; Vangl2–/– cells lentivirally transduced with PDGFA-expressing constructs. Golgi was marked by mEmerald-Golgi-7. The Golgi stacks were dispersed in Vangl1/2-deficient cells.
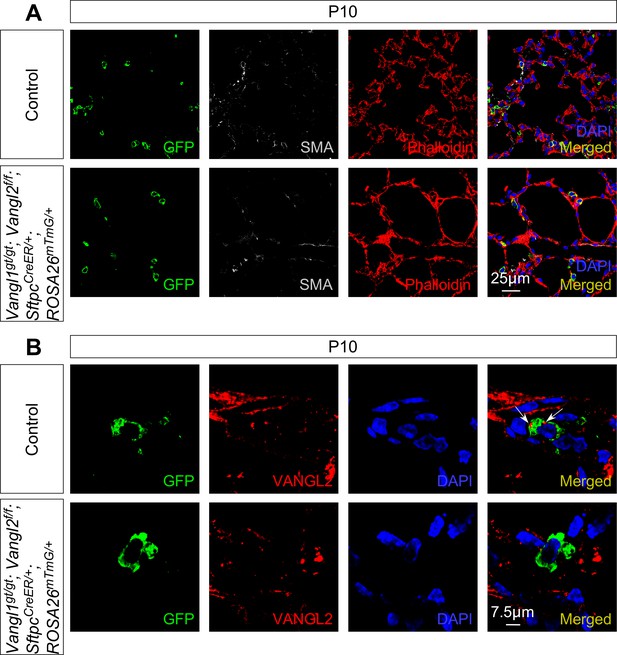
Loss of Vangl1/2 in alveolar type II cells results in alveolar defects.
(A) Immunostaining of lung sections collected from control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice at postnatal (P) day 10. Tamoxifen was administered at P0. Inactivation of Vangl1/2 in alveolar type II (AT2) cells led to alveolar defects. (B) Immunohistochemical analysis of lung sections collected from control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice revealed loss of VANGL2 in AT2 cells (GFP+) in the mutant lungs.
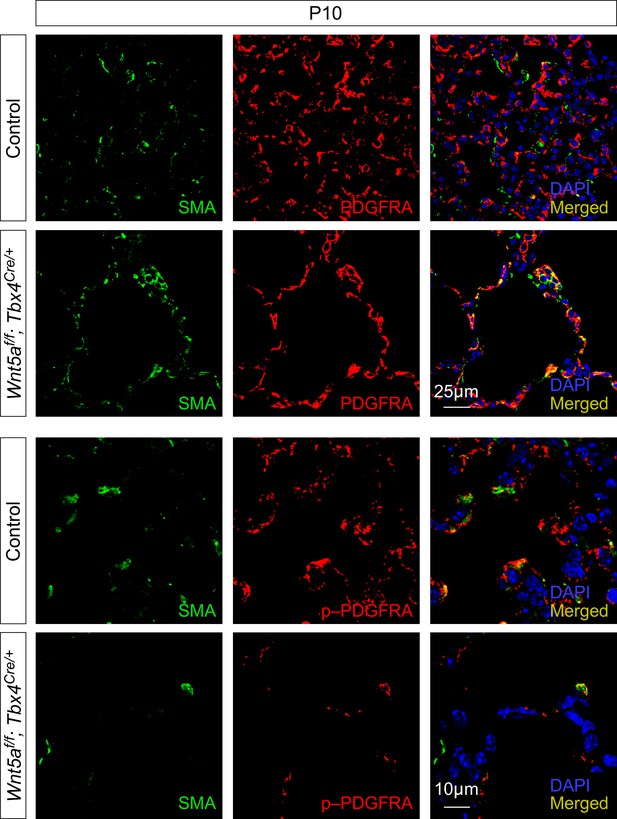
Removal of mesenchymal Wnt5a results in reduced levels of phosphorylated PDGFRA.
Immunostaining of lung sections collected from control and Wnt5af/f; Tbx4Cre/+ mice at postnatal (P) day 10. A significant reduction in the levels of phosphorylated (p) PDGFRA in individual myofibroblasts was found in Wnt5af/f; Tbx4Cre/+ lungs compared to controls. By contrast, the levels of PDGFRA in individual myofibroblasts were unaffected.
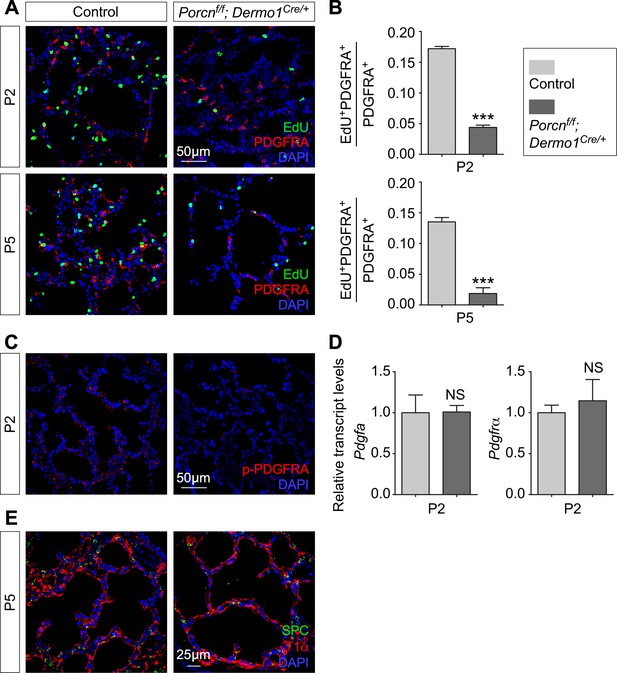
Mesenchymal Wnt signaling is required for fibroblast/myofibroblast proliferation but does not affect differentiation of alveolar epithelial cells.
(A, C, E) Immunostaining of lung sections from control and Porcnf/f; Dermo1Cre/+ mice at postnatal (P) day 2 and 5. The number of proliferating myofibroblasts (EdU+PDGFRA+) was reduced in the absence of mesenchymal Wnt signaling (shown in A). Quantification (n = 3 for each group) was shown in (B). Phosphorylated (p) PDGFRA was significantly reduced in the mutant lungs (shown in C) while the transcript levels of Pdgfa and Pdgfra were unaltered (n = 3 for each group) (shown in D). Loss of mesenchymal Wnt signaling had no effect on differentiation of alveolar epithelial cells (shown in E). All values are mean SEM. (***) p<0.001; ns, not significant (unpaired Student’s t-test).
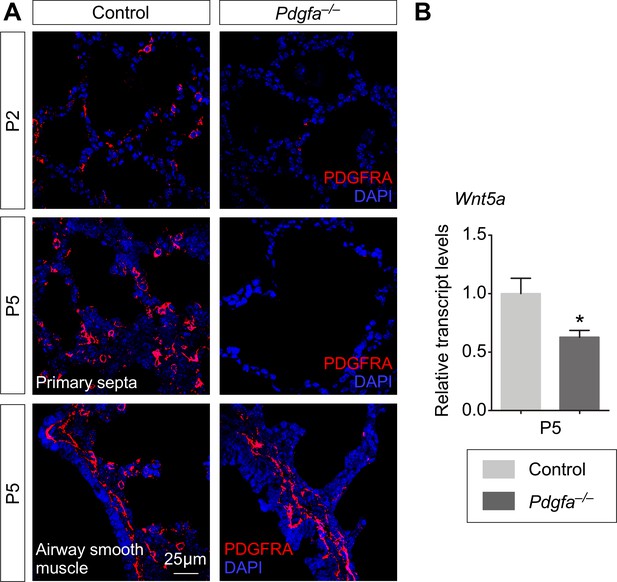
PDGF signaling is required for preserving a pool of WNT5A-secreting myofibroblasts.
(A) Immunostaining of lung sections collected from control and Pdgfaex4COIN/ex4COIN; Sox2Cre/+ (abbreviated as Pdgfa–/–) mice at postnatal (P) day 2 and 5. PDGFRA+ myofibroblasts were absent in Pdgfa–/– lungs. This indicates that the source of WNT5A (produced from myofibroblasts) for alveolar development was depleted. By contrast, PDGFRA+ smooth muscle cells in the airway were unaffected in the absence of PDGF signaling. (B) qPCR analysis of Wnt5a transcript levels in control and Pdgfa–/– lungs (n = 4) at P5. Wnt5a mRNA levels were reduced in the absence of PDGF signaling. All values are mean SEM. (*) p<0.05. (unpaired Student’s t-test).
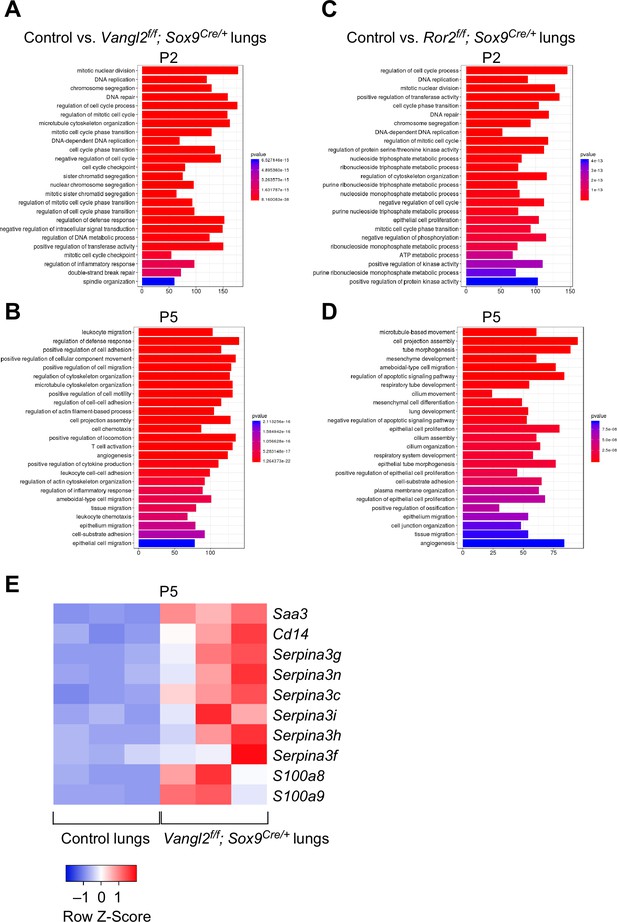
Vangl2 and Ror2 regulate similar pathways.
(A–D) Pathway analysis of transcriptomes derived from RNA-Seq of control, Vangl2f/f; Sox9Cre/+, and Ror2f/f; Sox9Cre/+ lungs at postnatal (P) day 2 and 5 (n = 3 for each group). The top 25 enriched terms in GO (gene ontology) biological processes were shown. Loss of Vangl2 or Ror2 revealed changes in similar pathways, suggesting that Vangl2 and Ror2 function in the same pathway. (E) Heatmap of selected mouse genes from control and Vangl2f/f; Sox9Cre/+ lungs at P5. Loss of epithelial Vangl2 in mouse lungs activated these genes. Interestingly, they are known to be elevated in lungs of human emphysema patients and are biomarkers for emphysema.
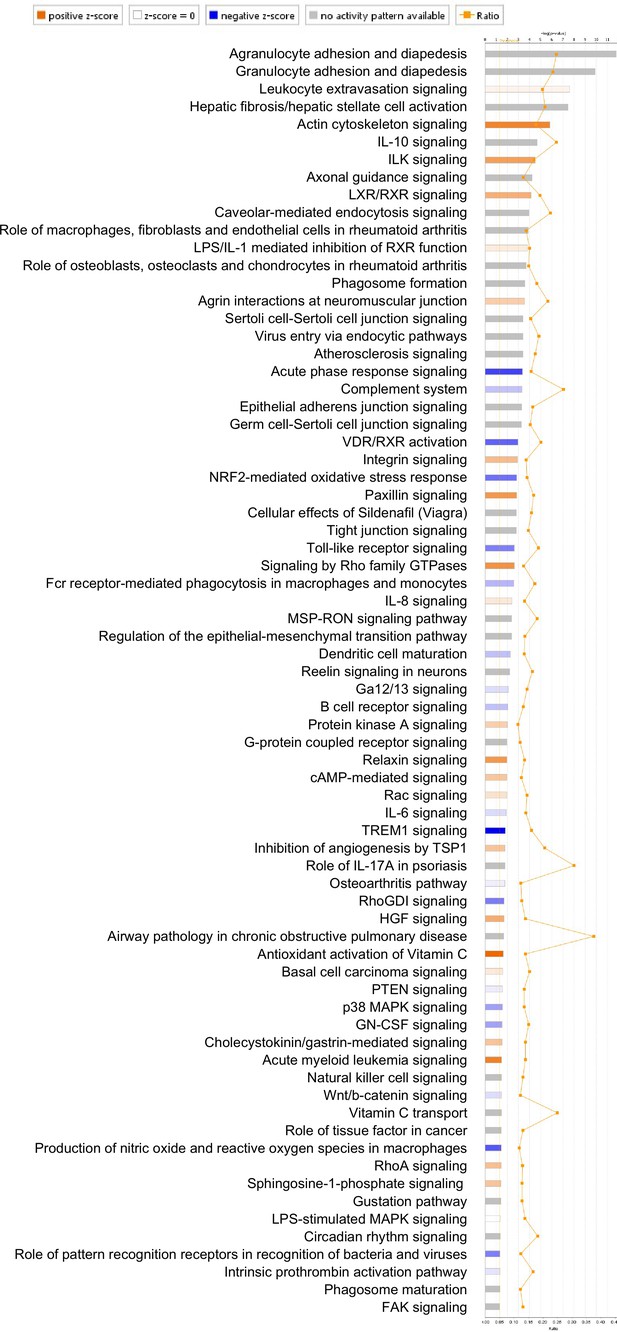
Pathway analysis of differentially expressed genes identified in RNA-Seq analysis of control and Vangl2f/f; Sox9Cre/+ lungs at postnatal (P) day 5.
p-value and z-score were shown. The calculated z-score indicates the prediction of overall increase or decrease in pathway activity. For a z-score >0, the pathway is expected to be activated; for a z-score <0, the pathway is expected to be inhibited. The ratio indicates the ratio of genes from the dataset that map to the pathway divided by the total number of genes that map to the same pathway. The orange line indicates a threshold of -log(p-value) =1.30 (p<0.05) and the cutoff was set at -log(p-value) =3 (p<0.001).
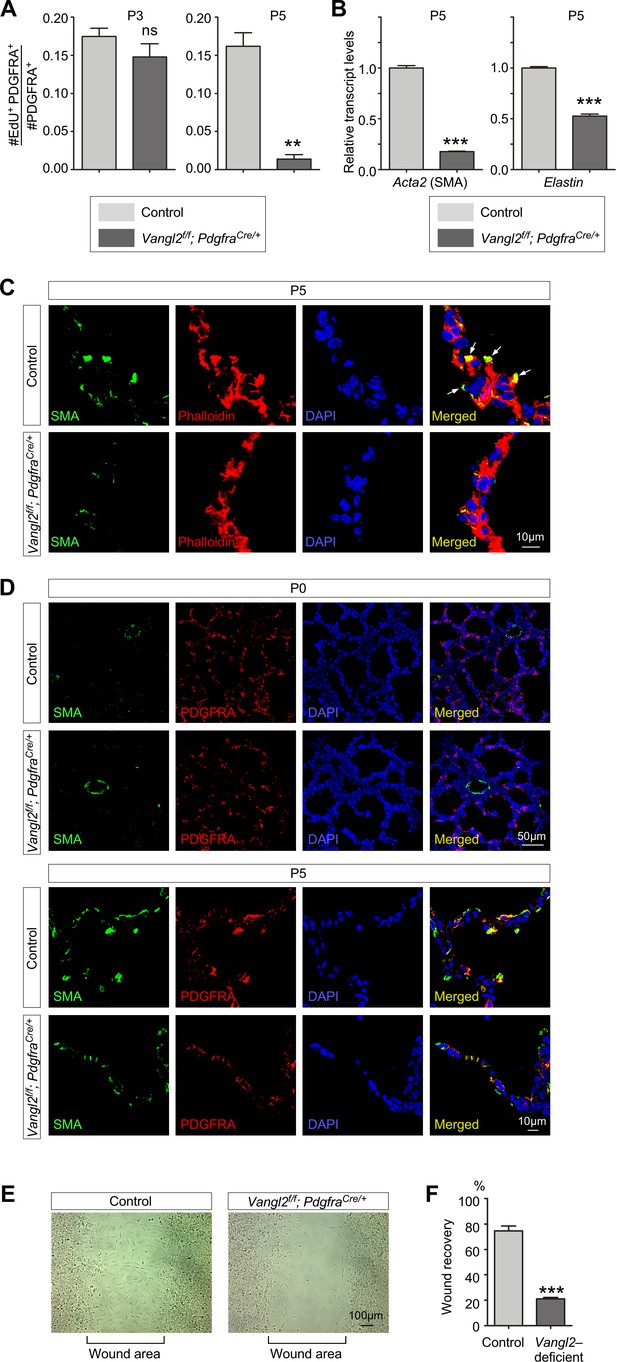
Vangl2 regulates the cytoskeleton of myofibroblasts and their migration.
(A) Quantification of myofibroblast proliferation in control and Vangl2f/f; PdgfraCre/+ lungs at postnatal (P) day 3 and 5. The rate of myofibroblast proliferation was calculated as the ratio of the number of EdU+ myofibroblasts (EdU+PDGFRA+) to the number of myofibroblasts (PDGFRA+). An apparent reduction in the percentage of proliferating myofibroblasts was detected in Vangl2f/f; PdgfraCre/+ lungs compared to controls (n = 3 for each group) at P5 but not at P3. (B) qPCR analysis of Acta2 (SMA) and Elastin in control and Vangl2f/f; PdgfraCre/+ lungs at P5. The mRNA levels of Acta2 and Elastin were significantly reduced in the absence of Vangl2 in myofibroblasts (n = 3 for each group). (C) Immunostaining of lung sections collected from control and Vangl2f/f; PdgfraCre/+ mice at P5. The actomyosin cytoskeleton (stained by phalloidin) failed to organize around the prospective sites of secondary septa formation in Vangl2f/f; PdgfraCre/+ lungs. In addition, smooth muscle actin (SMA) levels were significantly reduced and did not form stress fibers at the prospective sites of secondary septation. Arrows point to rudimentary secondary septa in control lungs. (D) Immunostaining of lung sections collected from control and Vangl2f/f; PdgfraCre/+ mice at P0 and P5. Migration of myofibroblasts (PDGFRA+/SMA+) to the prospective sites of secondary septa failed to occur in the mutant lungs. (E) Wound recovery assays to assess the migratory ability of myofibroblasts derived from control and Vangl2f/f; PdgfraCre/+ lungs. Within 36–48 hr, the wound area has been populated by migrating myofibroblasts derived from control lungs. By contrast, few myofibroblasts from Vangl2f/f; PdgfraCre/+ lungs reached the wound area within the same time frame. (F) Quantification of wound recovery by myofibroblasts derived from control and Vangl2f/f; PdgfraCre/+ lungs within 36–48 hr (n = 3 for each group). These results imply that mesenchymal Vangl2 controls subsequent myofibroblast proliferation after the initial expansion or the migration defect exerts a secondary effect on myofibroblast proliferation or both. All values are mean ± SEM. (***) p<0.001 (unpaired Student’s t-test).
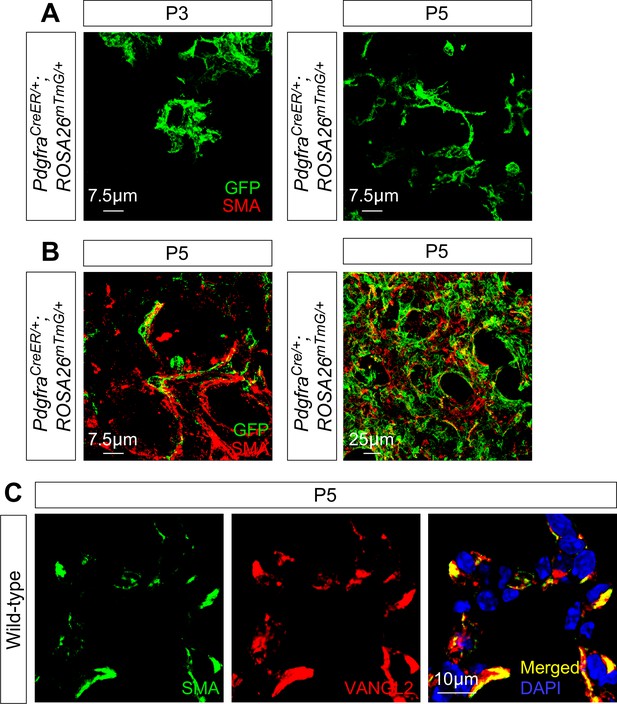
Myofibroblasts send out cellular extensions to form a network during alveologenesis.
(A) Immunostaining of lung sections from PdgfraCreER/+; ROSA26mTmG/+ mice at postnatal (P) day 3 and 5. Leaky CreER expression labeled myofibroblasts. Cellular extensions of myofibroblasts became apparent at P5. (B) Immunostaining of lung sections from PdgfraCreER/+; ROSA26mTmG/+ or PdgfraCre/+; ROSA26mTmG/+ mice at P5. Confocal stacks were shown to visualize the network of myofibroblasts. Smooth muscle actin (SMA) localized to cellular extensions of myofibroblasts. (C) Immunostaining of lung sections from wild-type mice at P5. VANGL2 and SMA colocalized at the cellular extensions of myofibroblasts.
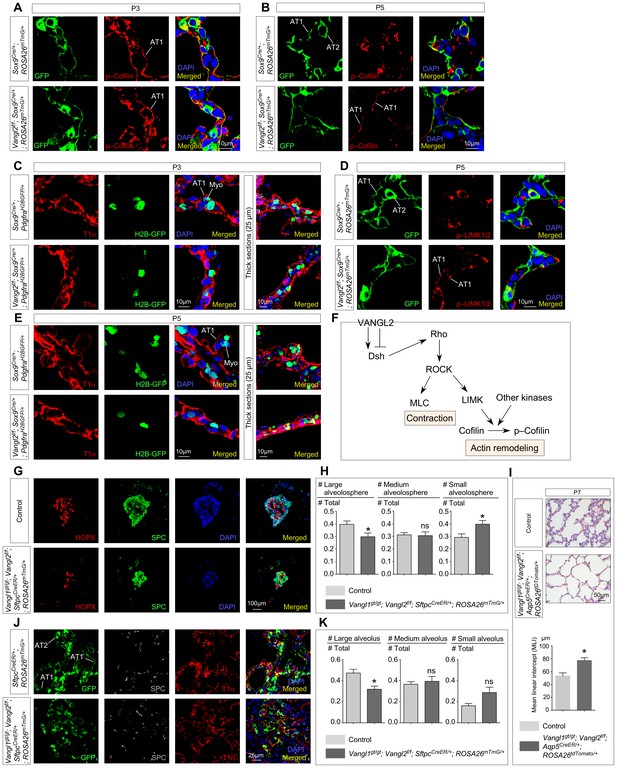
Vangl2 controls the cytoskeleton of alveolar type I cells, their cell shape changes and their ability in forming alveolospheres and new alveoli.
(A, B, D) Immunostaining of lung sections collected from Sox9Cre/+; ROSA26mTmG/+ (control) and Vangl2f/f; Sox9Cre/+; ROSA26mTmG/+ mice at postnatal (P) day 3 and 5. GFP from the ROSA26 locus (ROSA26mTmG) was activated in both alveolar type I (AT1) and type II (AT2) cells which could be distinguished by their morphology. The levels of phosphorylated (p) Cofilin and LIMK were significantly reduced in AT1 cells in control lungs by P5. By contrast, p-Cofilin and p-LIMK persisted in AT1 cells in Vangl2f/f; Sox9Cre/+; ROSA26mTmG/+ lungs. (C, E) Immunostaining of lung sections collected from Sox9Cre/+; PdgfraH2BGFP/+ (control) and Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ mice at P3 and P5. Cell shape change of AT1 cells (T1α+) was observed in control lungs at the prospective sites of secondary septation to encase myofibroblasts (H2BGFP+) (myo) that had migrated toward AT1 cells. AT1 cells in Vangl2f/f; Sox9Cre/+; PdgfraH2BGFP/+ lungs failed to undergo similar morphological changes. (F) Schematic diagram of a Vangl2-initiated signaling cascade that leads to phosphorylation of Cofilin and actin remodeling. (G) Immunostaining of alveolospheres derived from control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice injected with tamoxifen. Both SPC+ and HOPX+ cells were found in alveolospheres derived from control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice despite a difference in size and organization. (H) Quantification of the percentage of large (cross-sectional area >0.06mm2), medium (cross-sectional area between 0.015mm2 and 0.06mm2) and small (cross-sectional area <0.015mm2) alveolospheres derived from control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice (n = 5 for each group). The percentage of large alveolospheres derived from Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice was reduced. (I) Hematoxylin and eosin-stained lung sections of wild-type and Vangl1gt/gt; Vangl2f/f; Aqp5CreER/+; ROSA26tdTomato/+ mice injected with tamoxifen and collected at P7. Enlarged saccules were found in Vangl1gt/gt; Vangl2f/f; Aqp5CreER/+; ROSA26tdTomato/+ lungs with an increased MLI in comparison with controls (n = 3 for each group). (J) Immunostaining of lung sections collected from SftpcCreER/+; ROSA26mTmG/+ (control) and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice injected with tamoxifen and treated with bleomycin. Lungs were harvested at 30 days post-bleomycin administration. In control lungs, GFP-labeled AT2 cells proliferated and differentiated into AT1 cells to form new alveoli. By contrast, fewer alveoli were produced in Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ lungs. (K) Quantification of the percentage of large (cross-sectional area >0.6mm2), medium (cross-sectional area between 0.2mm2 and 0.6mm2) and small (cross-sectional area <0.2mm2) alveoli derived from SftpcCreER/+; ROSA26mTmG/+ (control) and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ mice (n = 5 for each group). The percentage of large alveoli in Vangl1gt/gt; Vangl2f/f; SftpcCreER/+; ROSA26mTmG/+ lungs was reduced. All values are mean ± SEM. (*) p<0.05; (**) p<0.01; (***) p<0.001; ns, not significant (unpaired Student’s t-test).
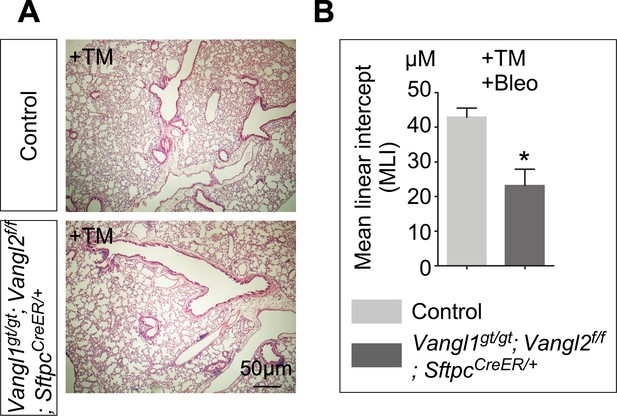
Bleomycin-induced lung injury results in a reduced MLI in the absence of Vangl1/2.
(A) Hematoxylin and eosin-stained lung sections of control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+ mice, which received tamoxifen (TM) injection. Lungs collected at 3 months post-TM (without bleomycin treatment) showed no evidence of lung fibrosis. (B) Measurement of the mean linear intercept (MLI) in control and Vangl1gt/gt; Vangl2f/f; SftpcCreER/+ mice, which received TM and subsequently bleomycin. Lungs collected at one month post-bleomycin showed a reduction in the MLI of the mutant lungs. The reduced MLI in Vangl1gt/gt; Vangl2f/f; SftpcCreER/+ lungs indicates a reduced distance between two primary or secondary septa in the regenerating alveoli, consistent with the definition of smaller alveoli. Smaller alveoli may be related to alterations in the cytoskeleton in the absence of Vangl1/2. All values are mean SEM. (*) p<0.05 (unpaired Student’s t-test).
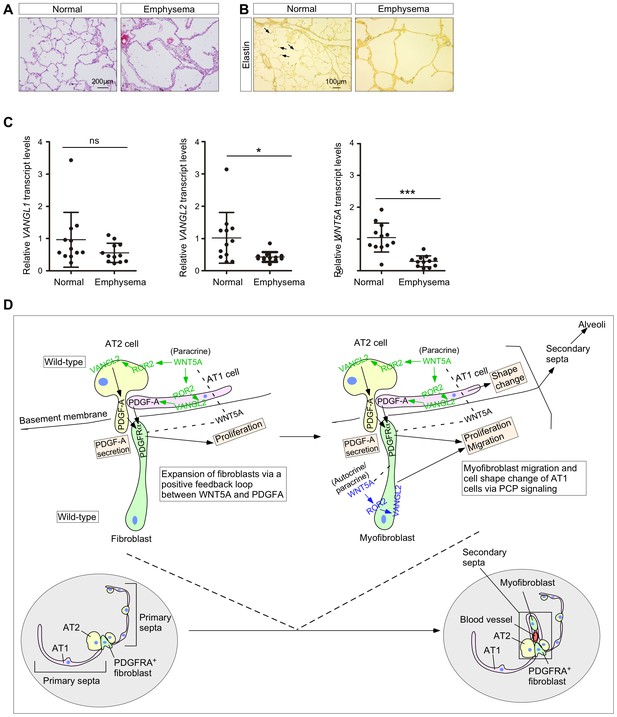
The WNT–VANGL axis is downregulated in the lungs of human emphysema patients.
(A) Hematoxylin and eosin-stained lung sections of normal and emphysema patients. Characteristic disruption of alveoli was observed in emphysema patients, resulting in increased airspace. (B) Elastin staining of lung sections of normal and emphysema patients. The slides were counterstained with tartrazine. Elastin, which was detected at the secondary septa in normal lungs (arrows), was greatly reduced in the lungs of emphysema patients. (C) qPCR analysis of VANGL1, VANGL2 and WNT5A in lungs from normal and emphysema patients (n = 12 for each group). The mRNA levels of VANGL2 and WNT5A were significantly reduced in emphysema patients. All values are mean ± SEM. (*) p<0.05; (**) p<0.01; (***) p<0.001; ns, not significant (unpaired Student’s t-test). (D) A new model of alveolar formation through control of cellular properties by PCP signaling. We propose that epithelial PCP signaling via the Wnt5a–Ror2–Vangl2 axis (colored green) controls PDGF secretion from alveolar type I and type II cells to promote proliferation of mesenchymal fibroblasts. In this process, a positive feedback loop between WNT5A and PDGF leads to expansion of the fibroblast pool required for subsequent alveologenesis. The expanded fibroblast population expresses SMA and becomes myofibroblasts, which continue to proliferate. In addition, myofibroblasts migrate to the prospective site of secondary septation in response to PCP signaling (colored blue). Likewise, epithelial PCP signaling (colored green) instructs cell shape changes of alveolar type I cells necessary for encasing myofibroblasts that migrate toward the site of secondary septation. All of these cellular events (orange-colored boxes) are due to modulation of the actomyosin cytoskeleton via the Wnt5a–Ror2–Vangl2 axis.
Additional files
-
Supplementary file 1
The Key Resources table lists antibodies, chemicals, peptides, recombinant proteins, commercial assays, cell lines, mouse strains, biological samples, recombinant DNA reagents, and software and algorithms used in this study.
- https://cdn.elifesciences.org/articles/53688/elife-53688-supp1-v1.docx
-
Supplementary file 2
The Sequence-based reagent table lists oligonucleotides used in this study.
- https://cdn.elifesciences.org/articles/53688/elife-53688-supp2-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/53688/elife-53688-transrepform-v1.transparentreporting.pdf





