EphA7 promotes myogenic differentiation via cell-cell contact
Figures
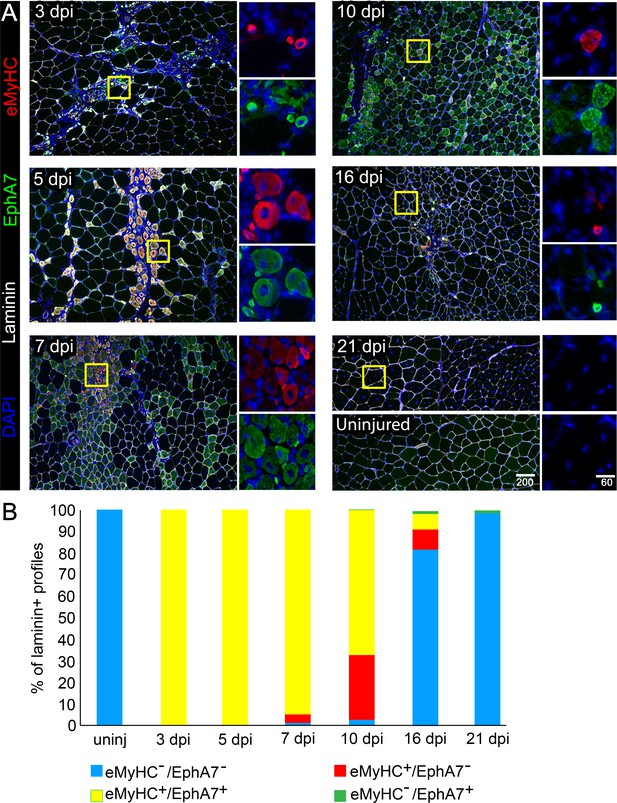
EphA7 expression is restricted to nascent myofibers after injury.
(A) Timecourse of sections of the TA muscle after partial injury via BaCl2 injection. Indirect immunohistochemistry with anti-EphA7 (green), anti-laminin (white) and anti-eMyHC (red) shows that eMyHC expression and EphA7 expression both begin shortly after injury (3 days) and are extinguished when the injury is resolved (by 21 days.) EphA7 staining is limited to laminin+ profiles (nascent or hypertrophying myofibers). T = days post-injury (dpi), boxes indicate regions of color-separated insets. Scale bars = 200 μm in main images, 60 μm in enlarged insets. (B) Coexpression of EphA7 and eMyHC over the timecourse of injury. At early timepoints EphA7 and eMyHC are uniformly coexpressed; as the injury resolves (starting at seven dpi) EphA7 and the eMyHC are downregulated. Infrequent fibers (0.3–1.5%) at later timepoints were scored positive for EphA7 but negative for eMyHC based on staining intensity thresholds.
-
Figure 1—source data 1
EphA7 and embryonic myosin heavy chain coexpression during regeneration.
- https://cdn.elifesciences.org/articles/53689/elife-53689-fig1-data1-v2.xlsx
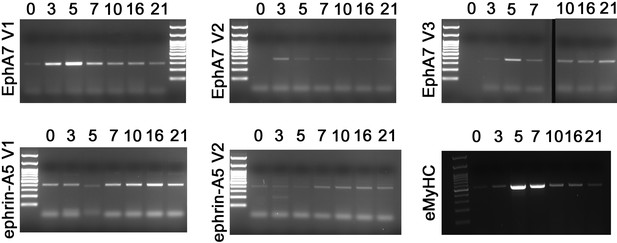
Expression of splice or developmental isoforms during regeneration.
RT-PCR of whole muscle RNA for splice isoforms of EphA7 and ephrin-A5 and a developmental isoform of myosin heavy chain during WT regeneration in vivo. Expected band sizes are 299 bp (EphA7V1/FL), 382 bp (EphA7V2/T1), 386 bp (EphA7V3/T2), 413 bp (ephrin-A5V1/α), 404 bp (ephrin-A5V2/β), and 519 bp (MYH3). Full-length EphA7 as well as both truncated isoforms are present at varying levels, as are both isoforms of ephrin-A5.
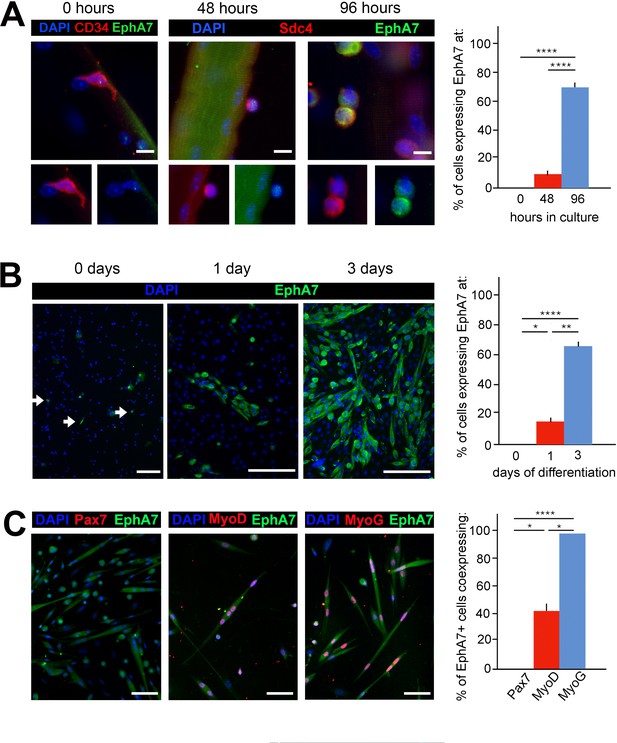
EphA7 is upregulated on satellite cells as they commit to differentiation.
(A) EphA7 expression is absent on satellite cells of freshly-isolated myofibers (marked by expression of CD34 or syndecan-4), but is detectable by 48 hours and prominent by 96 hours. Scale bars = 10 μm. (B) Similarly, primary satellite cells in monoculture do not express EphA7 in significant numbers when initially plated in low serum conditions (0 days); rare EphA7+ cells indicated by arrows. However, within 24 hours (1 day) its expression is upregulated under differentiation-promoting conditions; note that during early differentiation EphA7+ cells are present in clusters rather than evenly dispersed. After 3 days in differentiation medium, the majority of cells are expressing EphA7. Scale bars = 100 μm. (C) Expression of EphA7 is mutually exclusive with the progenitor cell marker Pax7 but always coincident with the differentiation marker myogenin, confirming specific expression in myocytes (image is of cells after 3 days in low serum). A subset of MyoD+ cells, which would include both myoblasts and myocytes, coexpress EphA7. Scale bars = 100 μm. Error bars = SEM, p ≤ 0.05 (*), 0.01 (**), 0.001 (***) or 0.0001 (****).
-
Figure 2—source data 1
EphA7 expression and coexpression in vitro.
- https://cdn.elifesciences.org/articles/53689/elife-53689-fig2-data1-v2.xlsx
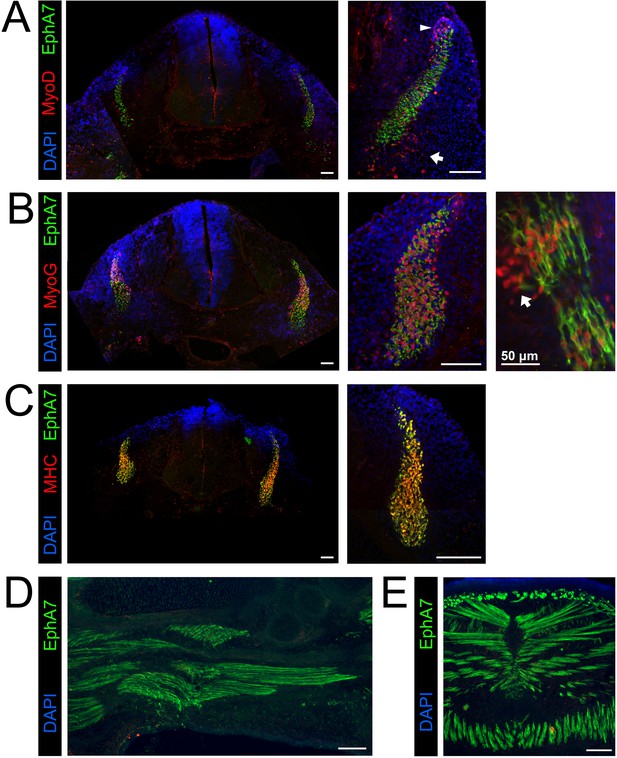
EphA7 is expressed on differentiated myogenic cells during development.
e11.5 embryos sectioned at the level of the forelimb show expression of EphA7 in differentiated myogenic cells in the somites. (A) While some MyoD-expressing cells in the somite coexpress EphA7, undifferentiated MyoD+ migratory myoblasts at the dorsal-medial lip (right panel, arrowhead) and the ventral-lateral lip (right panel, arrow) do not. (B) Myogenin+ myocytes and primary myofibers in the myotome of the somite are almost all positive for EphA7; in oblique sections (far right panel; scale bar = 50 μm) the most recently-differentiated myogenin+ cells (arrow) do not yet express EphA7. (C) Expression of myosin heavy chain in the somite is uniformly coincident with EphA7 expression. (D, E) In e15.5 embryos, EphA7 also identifies myofibers in the forelimb bud (anterior at top) and diaphragm/body wall muscle. Scale bars = 100 μm unless otherwise noted.
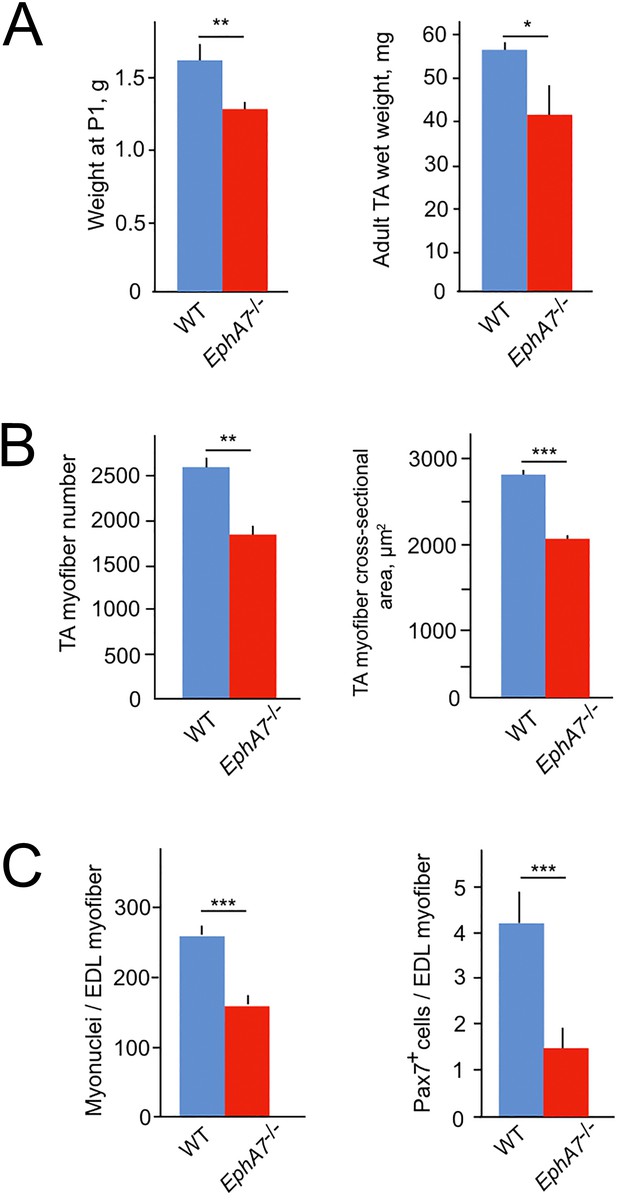
EphA7-/- mice have reduced muscle mass caused by diminished myofiber size and number.
(A) EphA7-/- mice weigh less that WT littermates at birth and continue to have reduced muscle mass as adult animals. (B) This is correlated with a reduction in myofiber number as well as myofiber size. (C) Individual muscle fibers in EphA7-/- mice possess fewer myonuclei and have reduced numbers of progenitor cells during homeostasis. Error bars = SEM, p ≤ 0.05 (*), 0.01 (**), or 0.001 (***).
-
Figure 4—source data 1
Morphometric analysis of EphA7-/- mice.
- https://cdn.elifesciences.org/articles/53689/elife-53689-fig4-data1-v2.xlsx
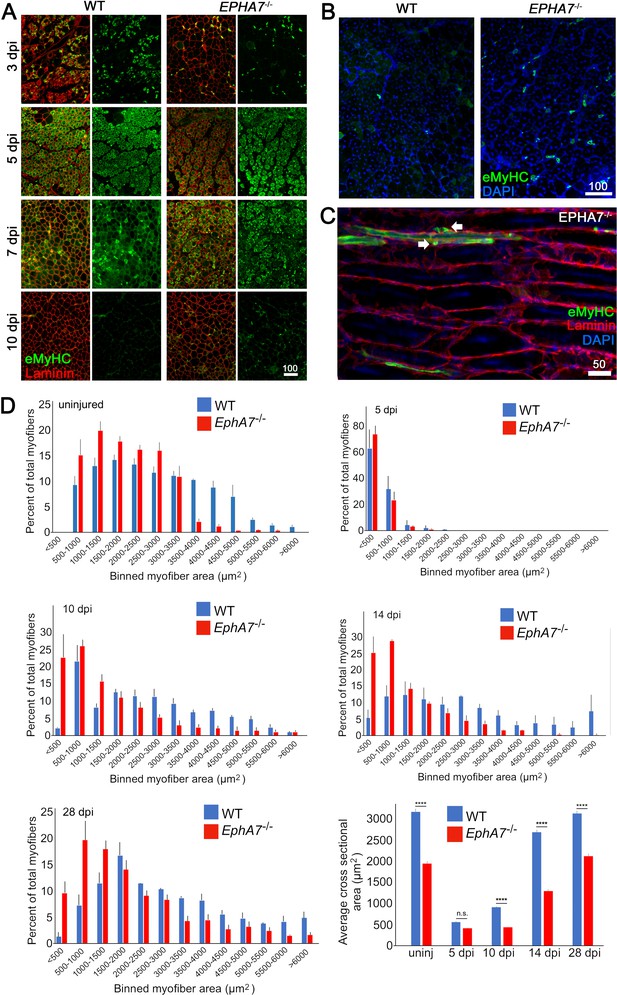
EphA7-/- muscles regenerate after acute injury but maturation and hypertrophy are delayed.
(A) After acute injury, EphA7-/- muscles initially possess fewer nascent (embryonic myosin heavy chain+) myofibers early in regeneration than WT (3 dpi). At later time points eMyHC expression persists in EphA7-/- muscles when it has become extinguished in favor of adult isoforms in WT (7–10 dpi). (B, C) Closer comparison at 10 dpi shows persistent small-caliber eMyHC+ profiles in cross sections of EphA7-/- muscles, which in sagittal section are recognizable as either small nascent myofibers with few myonuclei or differentiated but unfused myocytes (arrows). We also note eMyHC+ cells/processes that do not appear to possess a basal lamina (lower left), another indication of immaturity. Scale bars = 50 or 100 μm as indicated. (D) Quantitation of myofiber caliber in uninjured WT or EphA7-/- muscles confirms the disparities in myofiber size between genotypes, which is eliminated in early stages of regeneration (5 dpi) but is reconstituted at 10, 14 and 28 dpi. Average myofiber area in EphA7-/- muscles was not significantly different between 5 and 10 dpi, or in either genotype between 28 dpi and uninjured muscle. Error bars = SEM, p ≤ 0.0001 (****).
-
Figure 5—source data 1
Comparison of regeneration in WT and EphA7-/- muscle.
- https://cdn.elifesciences.org/articles/53689/elife-53689-fig5-data1-v2.xlsx
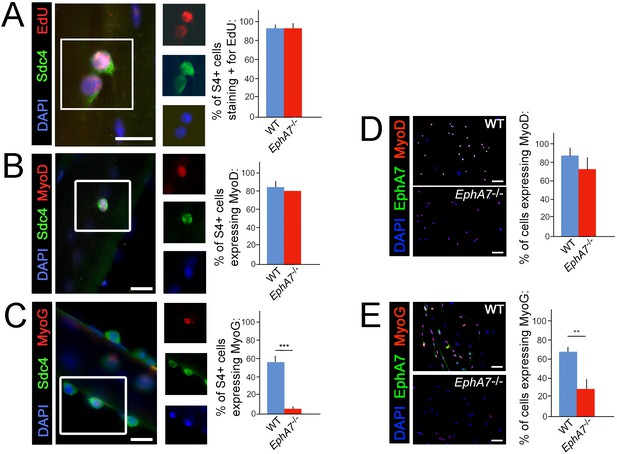
Upregulation of terminal differentiation markers is delayed in EphA7-/- myoblasts.
(A, B) In single fiber culture, the percentage of syndecan-4+ satellite cells incorporating EdU or expressing MyoD at 48 hours is not significantly different between WT and EphA7-/-. (C) However, EphA7-/- satellite cells largely fail to upregulate myogenin (MyoG) at 96 hr. (D, E) Similarly, WT and EphA7-/- satellite cells grown in monoculture express MyoD at statistically similar levels 48 hours after plating, but EphA7-/- cells do not upregulate myogenin to the same extent as WT 96 hours after plating. Scale bars = 10 μm. Error bars = SEM, p ≤0.01 (**) or 0.001 (***).
-
Figure 6—source data 1
Coexpression of EphA7 with markers of proliferation, specification, and differentiaion.
- https://cdn.elifesciences.org/articles/53689/elife-53689-fig6-data1-v2.xlsx
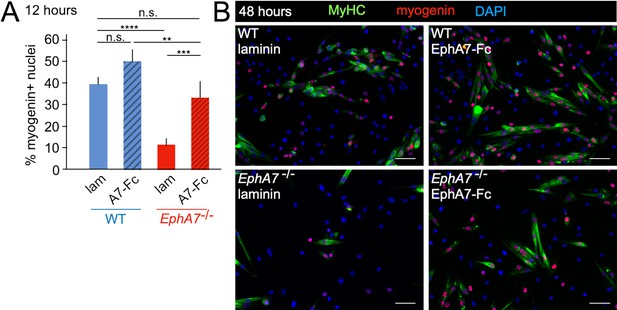
Exogenous EphA7 extracellular domain rescues differentiation of EphA7-/- satellite cells.
WT and EphA7-/- cells were cultured on coverslips coated with either laminin alone (lam) or laminin functionalized with EphA7-Fc chimera (A7–Fc) in growth medium (F-12 + 15% horse serum). (A) 12 hours of exposure to EphA7-Fc rescued differentiation of EphA7-/- cells (scored by expression of myogenin) to WT levels, while more WT cells on EphA7-Fc were differentiated than on laminin. Error bars = SEM, p ≤ 0.01 (**), 0.001 (***) or 0.0001 (****). (B) EphA7-/- myoblasts acquire morphological and molecular characteristics of differentiated myocytes similar to WT cells when exposed to EphA7-Fc. Scale bars = 50 μm.
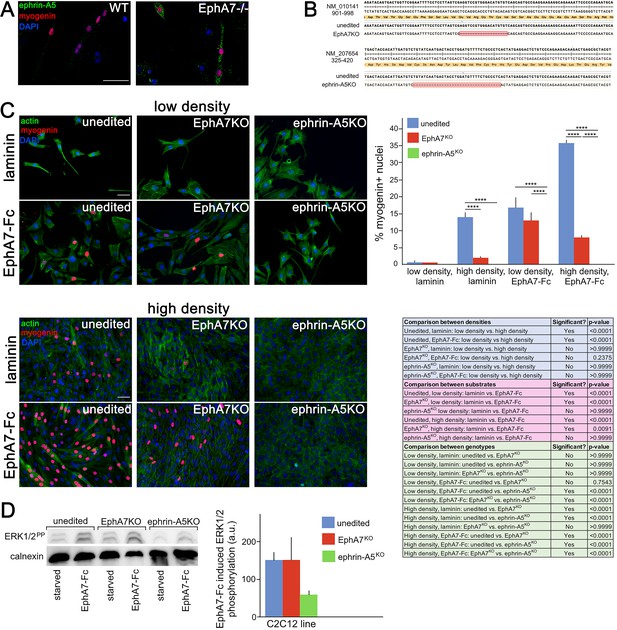
Ephrin-A5 meets the criteria for the EphA7 receptor on myoblasts.
(A) Ephrin-A5 is expressed at higher levels on EphA7-/- adult primary myogenic cells than it is on WT. (B) Mutations were made in the EphA7 and ephrin-A5 coding sequences of C2C12 myoblasts using double-nickase targeting, and single clones were expanded and analyzed by RT-PCR for inactivating mutations. Note: The red box in B indicates provisional sequencing of the targeted mutation in EphA7. (C) In high serum conditions, differentiation (indicated by expression of myogenin) is minimal in low density cultures of unedited, EphA7KO, and ephrin-A5KO C2C12 cells. Unedited C2C12 cells respond to high density by differentiating even in the presence of high serum, but EphA7KO and ephrin-A5KO C2C12 cells do not. At either low or high densities, unedited and EphA7KO C2C12 cells differentiate in high serum in response to exposure to EphA7-Fc, but ephrin-A5KO C2C12 cells do not. Table shows all significance comparisons by density, substrate, and genotype. (D) Unedited and EphA7KO C2C12 cells phosphorylate ERK1/2 following 10' exposure to soluble EphA7-Fc to a greater degree than do ephrin-A5KO C2C12 cells; all values were normalized to loading control. Error bars = SEM, p ≤ 0.01 (**), 0.001 (***) or 0.0001 (****). Scale bars = 50 μm.
-
Figure 8—source data 1
Expression of myogenin in unedited, EphA7KO, and ephrin-A5KO cells at high and low density, with and without exogenous EphA7-Fc.
- https://cdn.elifesciences.org/articles/53689/elife-53689-fig8-data1-v2.xlsx
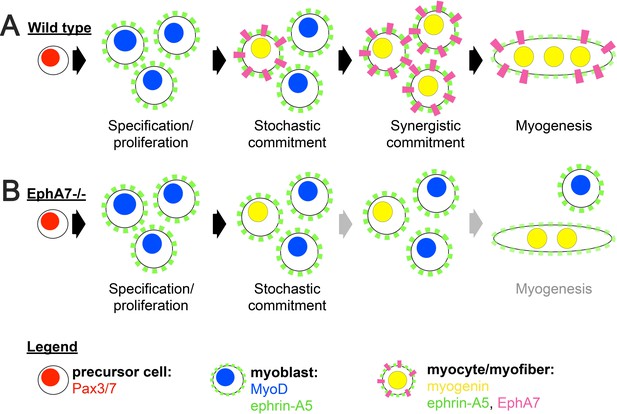
Proposed model.
(A) We propose a model in which initial, stochastic commitment of individual myoblasts results in their expression of EphA7, which then induces rapid, synergistic commitment of adjacent ephrin-A5-expressing myoblasts via the community effect. (B) In the absence of EphA7, stochastic commitment still occurs but synergistic commitment does not, resulting in a decreased number of myocytes and later in fewer, smaller syncytial myofibers.





