Sox8 and Sox9 act redundantly for ovarian-to-testicular fate reprogramming in the absence of R-spondin1 in mouse sex reversals
Figures
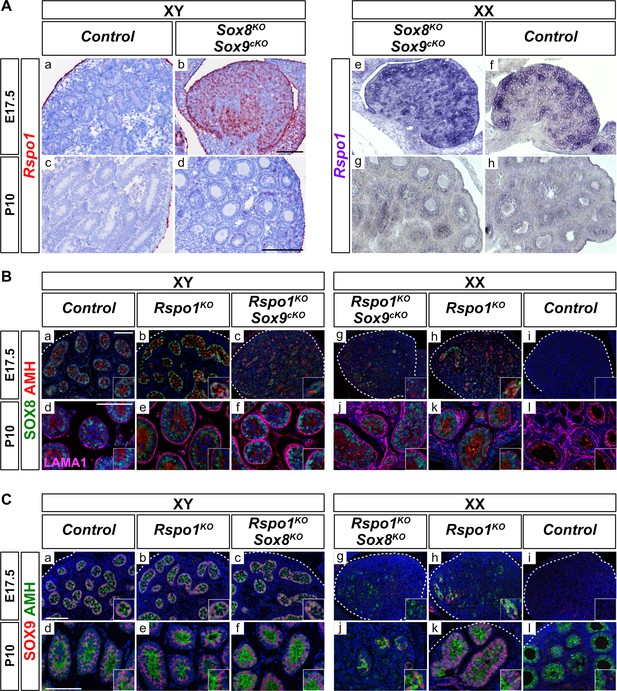
Expression of Rspo1, Sox8, and Sox9 in E17.5 and P10 gonads.
Expression of Rspo1, as revealed by in situ hybridizations (A), and of SOX8 and SOX9, as revealed by immunostaining (B, C) on gonadal sections from embryonic day 17.5 (E17.5) and 10 days post-natal (P10) mice. In XY wild-type testes, Rspo1 is mainly expressed in the coelomic epithelium (Aa) and tunica albuginea (Ac). In XX wildtype ovaries, Rspo1 is expressed throughout the gonad at E17.5 (Af), and down-regulated in post-natal animals, as shown at P10 (Ah). In XY and XX Sox8-/-; Sox9flox/flox; Sf1:creTg/+ (Sox8KO Sox9cKO) mutant mice, the Rspo1 expression profile (Ab,d,e,g) is similar to wildtype ovaries (Af,h), indicating an ovarian fate. For SOX8 expression, in XY control testes (Ba,d) and in XY Rspo1KO gonads developing as testes (Bb,e), SOX8 is expressed in testis cords at E17.5 and P10. Co-immunolabeling with AMH confirmed the identity of Sertoli cells. In XX mice, though AMH is expressed in post-natal control ovaries, these cells were SOX8-negative, indicating that they are granulosa cells (Bj). However, SOX8 and AMH positive testis cords were found in XX Rspo1KO female-to-male sex reversal gonads (Bh,k). SOX8 is also expressed in the absence of Sox9 in XY and XX Rspo1-/-; Sox9flox/flox; Sf1:creTg/+ (Rspo1KO Sox9cKO) gonads at E17.5 and P10 (Bc,g,f,j). At P10, LAMA1 staining demarcates testis cords (Bd-f, j–k) and follicles (Bl). SOX9 expression was found in XY control testes (Ca,d), and in XY Rspo1KO gonads developing as testes (Cb,e). Co-immunolabeling with AMH confirmed the identity of Sertoli cells. As shown, SOX9 and AMH positive testis cords are found in XX Rspo1KO sex reversal gonads at E17.5 and P10 (Ch,k). In addition, SOX9 is also expressed in absence of Sox8 in XY Rspo1-/-; Sox8-/- (Rspo1KO Sox8KO) gonads developing as testes at E17.5 and P10 (Cc,f), and in XX Rspo1KO Sox8KO gonads developing as ovotestes at P10 (Cd). In XX control mice, SOX9 and AMH expression is absent in fetal ovaries (Ci). In post-natal female animals, SOX9 is expressed by theca cells, which are AMH-negative (Cj). All scale bars 100 μm.
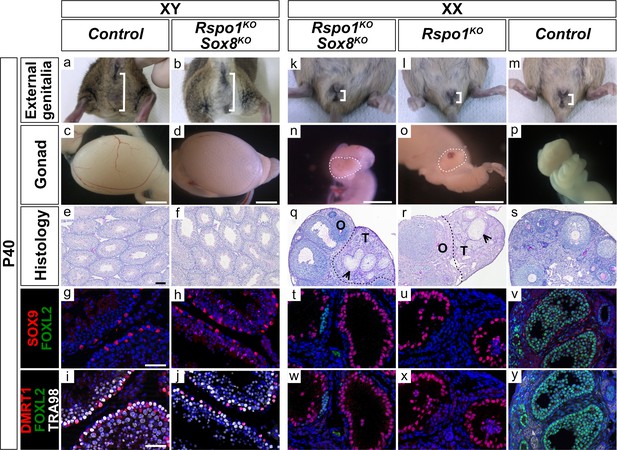
External genitalia and gonad development in adult XY and XX Rspo1KO Sox8KO double mutant mice.
External genitalia from adult P40 mice (a–b, k–m), macroscopic view of gonads (c–d, n–p) (Scale bars 1.5 mm), histology as revealed by PAS staining on gonadal sections (e–f, q–s) (Scale bars 100 µm), and immunostaining of SOX9 (Sertoli cell marker, in red) (g–h, t–v), FOXL2 (granulosa cell marker, in green) (g–j, t–y), DMRT1 (Sertoli and germ cell marker, in red) (i–j, w–y), TRA98 (germ cell marker, in white) (i–j, w–y) and DAPI (nuclear marker, in blue) (g–j, t–y) on gonadal sections (Scale bars 50 µm). Inactivation of both Rspo1 and Sox8 in XY Rspo1KO Sox8KO double mutant mice did not cause a sex reversal (a–j). XY Rspo1KO Sox8KO gonads developed as testes with seminiferous tubules (f) containing SOX9 and DMRT1 positive Sertoli cells (h–j), as in control testes (e, g, i). As shown, XX control ovaries developed follicles (s) containing FOXL2-positive granulosa cells (v, y). Adult ovotestes in XX Rspo1KO Sox8KO mice (n, q) were indistinguishable from XX Rspo1KO mice (o, r). These gonads contained an ovarian ‘O’ compartment with follicles and a testicular ‘T’ compartment with seminiferous tubule-like structures, as indicated by arrowheads (q, r). The seminiferous tubule-like structures in XX Rspo1KO Sox8KO and XX Rspo1KO ovotestes contained SOX9 and DMRT1 positive Sertoli cells (t–u, w–x), as in control testes (g, i), but lacked TRA98-positive germ cells (w, x).
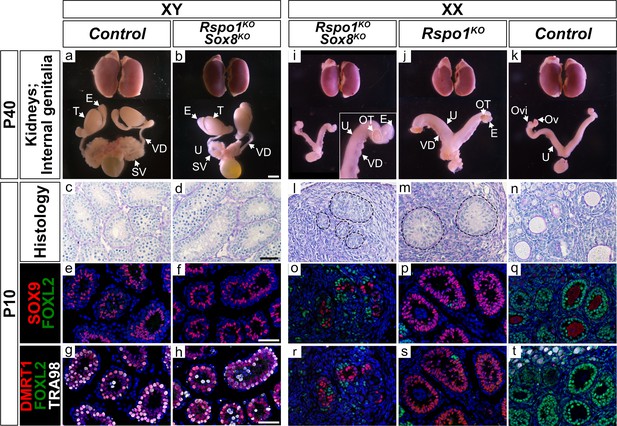
Reproductive tract of XY and XX Rspo1KO Sox8KO adult P40 mice and analyses in juvenile P10 mice.
Internally, at P40, XY control mice and XY Rspo1KO Sox8KO mice developed as male with testes ‘T’, epididymides ‘E’, vasa deferentia ‘VD’, and seminal vesicles ‘SV’ (a, b). XX control mice developed ovaries ‘Ov’, oviducts ‘Ovi’, and uteri ‘U’ (k). Both XX Rspo1KO mice and XX Rspo1KO Sox8KO mice exhibited hermaphroditism of the reproductive tracts (i, j). Kidneys are shown for comparison (a–b, i–k). In P10 mice, XY control and XY Rspo1KO Sox8KO gonads exhibited seminiferous tubules (c, d) containing SOX9 and DMRT1 positive Sertoli cells (g–h). XX control ovaries exhibited follicles (n) containing FOXL2-postive granulosa cells (q, t). Gonads in XX Rspo1KO and XX Rspo1KO Sox8KO mice also contained SOX9 and DMRT1 positive Sertoli cells (o–p, r–s), indicating XX sex reversal. As shown in Figure 2, ovotestis development is indistinguishable in XX Rspo1KO and XX Rspo1KO Sox8KO mice by P40. Internal organs scale bar 3 mm (a–b, i–k), histology and immunostainings scale bar 50 µm (c–h, l–t).
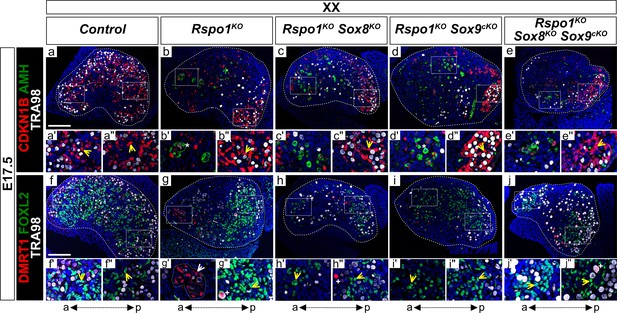
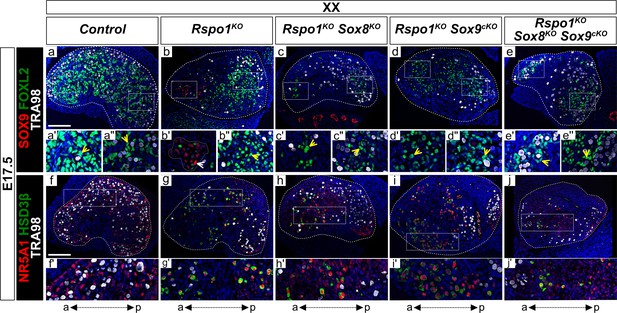
Precocious granulosa cell differentiation in XX Rspo1KO Sox8KO Sox9cKO triple mutant fetuses at E17.5.
Immunofluorescence of CDKN1B (P27) (mitotic arrest marker, in red) (a–e''), AMH (Sertoli marker and mature granulosa cell marker, in green) (a–e''), DMRT1 (Sertoli and germ cell marker, in red) (f–j''), FOXL2 (granulosa cell marker, in green) (f–j''), TRA98 (germ cell marker, in white) (a–j''), and DAPI (nuclear marker, in blue) (a–j'') on gonadal sections from E17.5 fetuses (main panels scale bar 100 µm). The anterior ‘a’ and posterior ‘p’ axis is shown below each column. For main panels (a–j), highlighted anterior and posterior areas are shown in the respective single and double primed letter panels. Yellow arrowheads indicate granulosa cells expressing CDKN1B or FOXL2, asterisks indicate cells expressing AMH, white arrowheads indicate Sertoli cells expressing DMRT1, and plus symbols indicate germ cells expressing DMRT1 and TRA98. Gonads in XX control fetuses developed as ovaries, as shown by FOXL2 and CDKN1B expression in pre-granulosa cells throughout the gonad (a', a'', f', f'', yellow arrowheads). These fetal ovaries lacked mature granulosa cells expressing AMH (a). In contrast, XX Rspo1-/- (Rspo1KO), XX Rspo1-/-; Sox8-/- (Rspo1KO Sox8KO), XX Rspo1-/-; Sox9flox/flox; Sf1:creTg/+ (Rspo1KO Sox9cKO), and XX Rspo1KO Sox8KO Sox9cKO gonads exhibited down-regulation of CDKN1B (b–e) and ectopic AMH expression in the anterior area (b’-e’, asterisks), indicating Sertoli cells or mature granulosa cells. However, while XX Rspo1KO gonads contained Sertoli cells expressing DMRT1 (g', white arrowhead), these cells were rare in XX double and triple mutants (h–j) (for XX triple mutants, 1 out of 8 gonads studied from n = 4 fetuses). Note that some DMRT1-positive cells are germ cells expressing TRA98 (g'', h'', j'', plus symbols). Thus, while granulosa cells differentiate precociously in XX Rspo1KO gonads lacking Sox8 and/or Sox9 at E17.5, these cells have not yet reprogrammed as Sertoli cells in XX Rspo1KO Sox8KO and XX Rspo1KO Sox9cKO mice. In XX triple mutant fetuses, granulosa cell reprogramming as Sertoli cells may be delayed, or blocked.
Absence of SOX9 expression in gonads from XX Rspo1KO Sox8KO Sox9cKO fetuses and presence of steroidogenic cells at E17.5.
Immunofluorescence of SOX9 (Sertoli cell marker, in red) (a–e''), FOXL2 (granulosa cell marker, in green) (a–e''), NR5A1 (SF1) (supporting and steroidogenic cell marker, in red) (f–j'), HSD3B1/2 (HSD3β) (steroidogenic cell marker, in green) (f–j'), TRA98 (germ cell marker, in white) (a–j'), and DAPI (nuclear marker, in blue) (a–j') on gonadal sections from E17.5 fetuses (main panel scale bar 100 µm). The anterior ‘a’ and posterior ‘p’ axis is shown below each column. For main panels (a–j), highlighted anterior and posterior areas are shown in the respective single and double primed letter panels. Yellow arrowheads indicate granulosa cells expressing FOXL2 and white arrowheads indicate Sertoli cells expressing SOX9. Gonads in XX control fetuses developed as ovaries, as shown by FOXL2 expression in pre-granulosa cells (a’, yellow arrowhead). In contrast, XX Rspo1-/- (Rspo1KO) gonads exhibited Sertoli cells expressing SOX9 forming testis cords (b’, white arrowhead). At this stage, XX Rspo1-/-; Sox8-/- (Rspo1KO Sox8KO), XX Rspo1-/-; Sox9flox/flox; Sf1:creTg/+ (Rspo1KO Sox9cKO), and XX Rspo1KO Sox8KO Sox9cKO gonads lacked SOX9-positive Sertoli cells because sex reversal is delayed (c), or because of genetic inactivation of Sox9flox/flox with Sf1:creTg/+ (d, e). The XX single, double, and triple mutant gonads contained steroidogenic cells expressing NR5A1 and HSD3β (g'–j'), which were absent in control fetal ovaries (f').
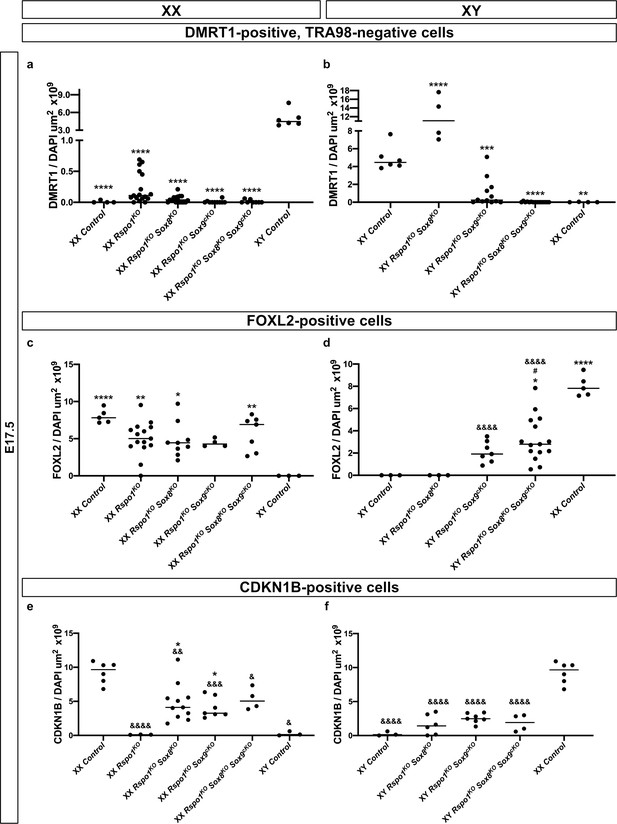
Quantification of immunostained cells expressing DMRT1, FOXL2, and CDKN1B at E17.5.
Histogram showing the distribution of immunostained Sertoli expressing DMRT1 and negative for TRA98 (a–b), granulosa cells expressing FOXL2 (c–d), and quiescent cells expressing CDKN1B (e–f) per gonad section area (μm2), as demarcated by DAPI shown in Figures 3 and 4. As represented in (a–b), XX control, XX Rspo1-/- (Rspo1KO), XX Rspo1KO gonads lacking Sox8 and/or Sox9, XY Rspo1KO gonads lacking Sox9 or both Sox8 and Sox9 (thus XX fetal ovaries and all mutants shown, with exception to XY Rspo1-/-; Sox8-/- (Rspo1KO Sox8KO) gonads), showed an absence or reduced number of Sertoli cells expressing DMRT1, as compared with XY control fetal testes at this stage. Sertoli cells are TRA98-negative. In contrast with these trends, XY Rspo1KO Sox8KO gonads retained Sertoli cells (b). Next, in contrast with XY control and XY Rspo1KO Sox8KO fetuses developing testes, gonads from XX control, XX Rspo1KO, XX Rspo1KO Sox8KO, XY and XX Rspo1-/-; Sox9flox/flox; Sf1:creTg/+ (Rspo1KO Sox9cKO), and XY and XX triple mutant fetuses in general contained granulosa cells expressing FOXL2 (c, d). In XX control fetal ovaries, the granulosa cells remained quiescent, as indicated by high CDKN1B levels (e, f). In mutants with granulosa cells however, CDKN1B is down-regulated (e, f), indicating reentry into the cell cycle. This has been associated with precocious granulosa cell differentiation, a cell type that could reprogram as a Sertoli cell. Thus, in fetuses, XY and XX Rspo1KO Sox8KO Sox9cKO triple mutant gonads contain mature granulosa cells that were poised for sex reversal. One-way ANOVA and Tukey-Kramer post-tests revealed statistically significant differences. For p-values<0.05,<0.01,<0.001, and <0.0001, asterisks (*, **, ***, ****) represent significant differences compared with XY control cell numbers, respectively and ampersands (&, &&, &&&, &&&&) represent significant differences compared with XX control cell numbers, respectively. For convenience, significant differences between non-control groups are shown. Non-significant differences are not shown.
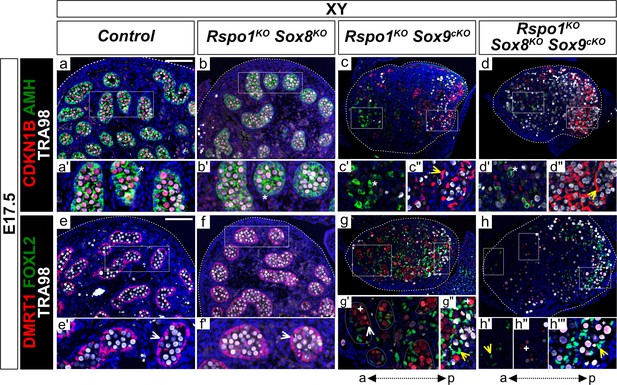
Lack of testis cords in XY Rspo1KO Sox8KO Sox9cKO triple mutant fetuses at E17.5.
Immunofluorescence of CDKN1B (P27) (mitotic arrest marker, in red) (a–d''), AMH (Sertoli marker and mature granulosa cell marker, in green) (a–d''), DMRT1 (Sertoli and germ cell marker, in red) (e–h'''), FOXL2 (granulosa cell marker, in green) (e–h'''), TRA98 (germ cell marker, in white) (a–h'''), and DAPI (nuclear marker, in blue) (a–h) on gonadal sections from E17.5 fetuses (main panel scale bar 100 µm). For gonads in panels (c–d), the anterior ‘a’ and posterior ‘p’ axis is shown below each column. Below each main panels (a–h), highlighted areas are shown in respective primed letter panels. Yellow arrowheads indicate granulosa cells expressing CDKN1B or FOXL2, asterisks indicate cells expressing AMH, white arrowheads indicate Sertoli cells expressing DMRT1, and plus symbols indicate germ cells expressing DMRT1 and TRA98. Gonads in XY Rspo1-/-; Sox8-/- (Rspo1KO Sox8KO) fetuses exhibited AMH and DMRT1 positive Sertoli cells organized as testis cords (b, f) and lacked FOXL2-positive granulosa cells (f), as in control testes (a, e). Cells expressing AMH were found in XY Rspo1-/-; Sox9flox/flox; Sf1:creTg/+ (Rspo1KO Sox9cKO) and XY Rspo1KO Sox8KO Sox9cKO gonads (c’ and d’, asterisks), indicating Sertoli cells or mature granulosa cells. Indeed, both exhibited granulosa cells expressing CDKN1B and FOXL2 (c', d', g', h', h''', yellow arrowheads). However, while XY Rspo1KO Sox9cKO gonads exhibited DRMT1-positive, TRA98-negative Sertoli cells (g', white arrowhead), these cells were scarce in XY triple mutant gonads (h) (6 of 6 XY triple mutant gonads studied from n = 3 fetuses). Note that some DMRT1 expressing cells in XY Rspo1KO Sox9cKO and XY triple mutant gonads are germ cells expressing TRA98 (g', g'', h'', h''', plus symbols). Thus, although XY Rspo1KO Sox9cKO and XY triple mutant gonads contain mature granulosa cells at E17.5, these cells do not reprogram as Sertoli cells in XY triple mutant fetuses.
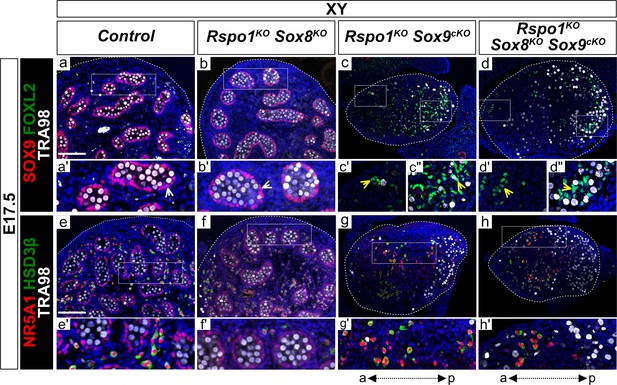
Absence of SOX9 expression in gonads from XY Rspo1KO Sox8KO Sox9cKO fetuses and presence of steroidogenic cells at E17.5.
Immunofluorescence of SOX9 (Sertoli cell marker, in red) (a–d''), FOXL2 (granulosa cell marker, in green) (a–d''), NR5A1 (SF1) (supporting and steroidogenic cell marker, in red) (e–h'), HSD3B1/2 (HSD3β) (steroidogenic cell marker, in green) (e–h'), TRA98 (germ cell marker, in white) (a–h'), and DAPI (nuclear marker, in blue) (a–h') on gonadal sections from E17.5 fetuses (main panel scale bar 100 µm). For gonads in (c–d, g–h), the anterior ‘a’ and posterior ‘p’ axis is shown below each column. Also, for main panels in (a–h), highlighted areas are shown in the respective single and double primed letter panels. Yellow arrowheads indicate granulosa cells expressing FOXL2 and white arrowheads indicate Sertoli cells expressing SOX9. Gonads in XY Rspo1-/-; Sox8-/- (Rspo1KO Sox8KO) fetuses exhibited SOX9-positive Sertoli cells organized as testis cords and lacked FOXL2-positive granulosa cells (b, b'), as in control testes (a, a'). As in XY Rspo1KO Sox9cKO gonads (c), genetic inactivation of Sox9 in XY Rspo1KO Sox8KO Sox9cKO gonads resulted in the absence of SOX9-positive Sertoli cells (d). Instead, these gonads exhibited granulosa cells expressing FOXL2 (c’-d’, asterisks). As in fetal testes (e, f), XY Rspo1KO Sox9cKO and XY triple mutant gonads exhibited steroidogenic cells expressing NR5A1 and HSD3β (g, h).
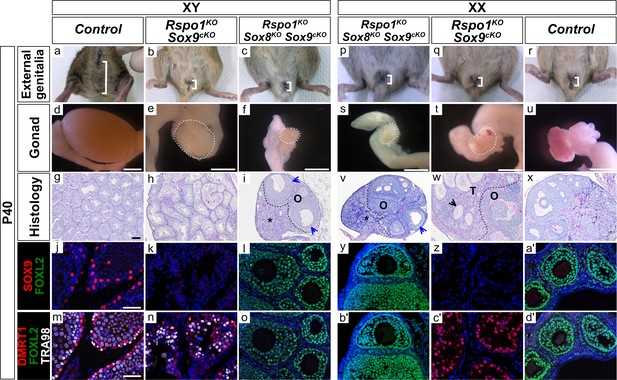
Absence of seminiferous tubules in XY and XX Rspo1KO Sox8KO Sox9cKO triple mutant adult mice.
External genitalia from adult P40 mice (a–c, p–r), macroscopic view of gonads (d–f, s–u) (Scale bars 1.5 mm), histology as revealed by PAS staining on gonadal sections (g–i, v–x) (Scale bars 100 µm), and immunostaining of SOX9 (Sertoli cell marker, in red) (j–l, y–a'), FOXL2 (granulosa cell marker, in green) (j–o, y–d'), DMRT1 (Sertoli and germ cell marker, in red) (m–o, b'–d'), TRA98 (germ cell marker, in white) (m–o, b'–d'), and DAPI (nuclear marker, in blue) (j–o, y–d') on gonadal sections (Scale bars 50 µm). As shown, though adult XY Rspo1-/-; Sox9fl/fl; Sf1:creTg/+ (Rspo1KO Sox9cKO) double mutant gonads developed a short anogenital distance (b), internally these mice developed hypoplastic testes (compare e with d). XX Rspo1KO Sox9cKO gonads developed as ovotestes (t), as in XX Rspo1KO single mutants and XX Rspo1KO Sox8KO double mutants (see Figure 2r, q). Although Sox9fl/fl is inactivated by Sf1:creTg/+ in Rspo1KO Sox9cKO mice (k, z), XY double mutant gonads exhibited seminiferous tubules (h) containing DMRT1-positive Sertoli cells which are TRA98-negative (n), as in control testes (g, m). XX double mutant gonads contained an ovarian compartment ‘O’ with follicles and a testicular ‘T’ compartment with seminiferous tubule-like structures, as indicated by black arrowheads (w). The seminiferous tubule-like structures contained DMRT1-positive Sertoli cells (c'), as in control testes (m), but lacked TRA98-positive germ cells (c'). Both the XY and XX Rspo1KO Sox8KO Sox9cKO triple mutant mice develop externally as female with a short anogenital distance (c, p) as in the double mutants and control female (b, q–r). Despite this, the triple mutants gonads (f, s) developed as atrophied ovaries (i, v), which were smaller than control ovaries (u, x). XY and XX triple mutant gonads exhibited an ovarian ‘O’ compartment and a distinct interstitial compartment, as indicated by asterisks (i, v). The gonads contained follicles up to the antral stage, though some exhibited irregular granulosa cell organization, as indicated by blue arrowheads (i, v). Notably, XY and XX triple mutants lacked testicular sex cords (i, v) that were present in XY and XX Rspo1KO Sox9cKO gonads (h, w). Immunostaining on XY and XX Rspo1KO Sox8KO Sox9cKO gonads confirmed the absence of SOX9 and DMRT1 positive Sertoli cells and the presence of ovarian follicles with granulosa cells expressing FOXL2 (l, o, y, b'), as in control ovaries (a', d'). For these analyses, n = 3 XY and n = 3 XX triple mutant mice were examined.
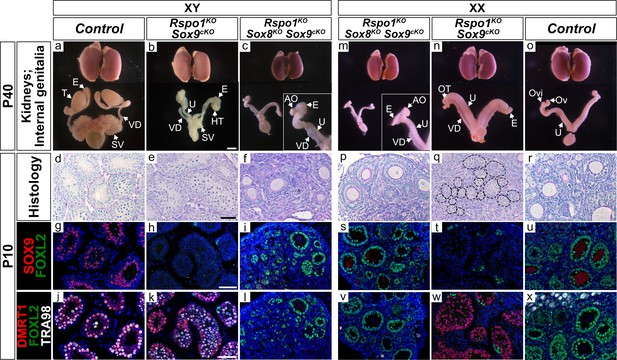
Reproductive tract of XY and XX Rspo1KO Sox8KO Sox9cKO adult P40 mice and analyses in juvenile P10 mice.
Internally, XX control females developed ovaries ‘Ov’, oviducts ‘Ovi’, and uteri ‘U’ (o). In contrast, XY control mice internally developed testes ‘T’, epididymides ‘E’, vasa deferentia ‘VD’, and seminal vesicles ‘SV’ (a). Both XY and XX triple mutants exhibited hermaphroditism of the reproductive tracts (c, m), like XY and XX Rspo1KO Sox9cKO double mutant mice (b, n). However, while XY Rspo1KO Sox9cKO mice developed testes ‘HT’ that were hypoplastic (b) and XX Rspo1KO Sox9cKO mice developed ovotestes ‘OT’ (n), XY and XX triple mutants developed atrophied ovaries ‘AO’ (c, m). Kidneys are shown for comparison (a–c, m–o). In P10 mice, XY and XX triple mutants exhibited ovarian follicles (f, p) containing FOXL2-positive granulosa cells (i, l, s, v) like XX control ovaries (u, x). This contrasted gonad development in XY and XX Rspo1KO Sox9cKO mice, exhibiting seminiferous tubules (e) or seminiferous tubule-like structures (q, circled) with DMRT1-positive Sertoli cells (k, w), similar to XY control testes (d, g, j). Internal organs scale bar 3 mm (a–c, m–o), histology and immunostaining scale bar 50 µm (d–l, p–x). For analyses at P40, n = 3 XY and n = 3 XX triple mutant mice were examined. For P10, n = 2 XY and n = 5 XX triple mutant mice were analyzed.
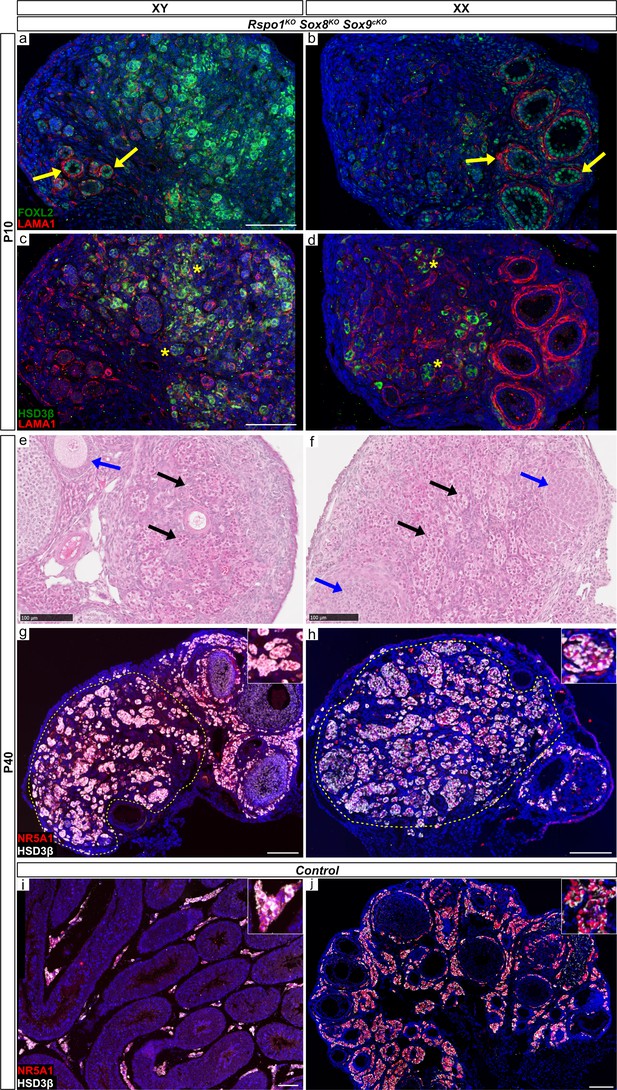
Organization of adult XY and XX Rspo1KO Sox8KO Sox9cKO triple mutant gonads.
Immunofluorescence of LAMA1 (basal lamina marker, in red), FOXL2 (granulosa cell marker, green), and HSD3B1/2 (HSD3β) (interstitial/steroidogenic cell marker, in green) of juvenile P10 XY and XX Rspo1KO Sox8KO Sox9cKO triple mutant gonads reveals follicles (a, b, yellow arrows) and steroidogenic cell clusters (c, d, asterisks). Histology (H and E) staining of adult P40 triple mutant gonads show collapsed/atrophied interstitial cells (e, f, black arrows) and immature/atrophied follicles (e, f, blue arrows). Immunofluorescence of NR5A1 (somatic cell marker, in red) and HSD3B1/2 (HSD3β) (interstitial/steroidogenic cell marker, in white) reveals a compartment of interstitial cells (g, h, circled). In control testes or ovaries, interstitial cells are found between seminiferous tubules or follicles (i, j). All scale bars 100 µm. For these analyses, n = 3 XY and n = 3 XX triple mutant mice were examined.
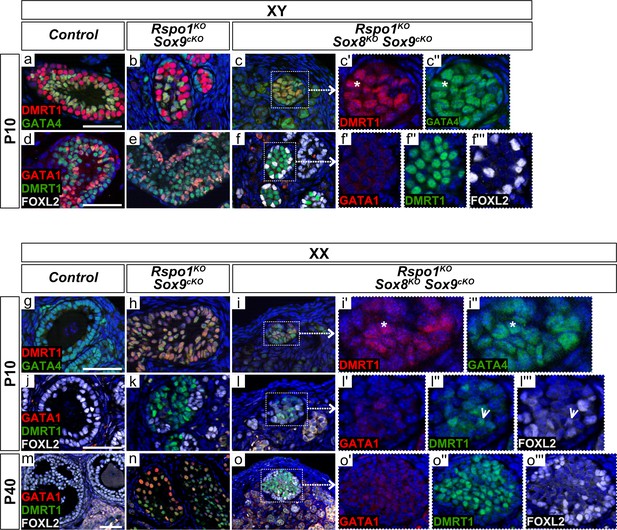
Rare immature Sertoli cells in XY and XX Rspo1KO Sox8KO Sox9cKO triple mutant gonads.
Both XY or XX triple mutants (3 of 10 XY and 6 of 16 XX post-natal gonads studied) exhibited rare cells expressing DMRT1, a Sertoli cell marker, and GATA4, a somatic cell marker (asterisks in c' and c'', and in i' and i''), but lacked DMRT1-positive Sertoli cells expressing the mature Sertoli cell marker GATA1 (f', l', o'). Cells expressing DMRT1 (f, l, o) were sometimes found in close proximity with cells expressing the granulosa cell marker FOXL2 (f'', f''', l'', l''', o'', o'''), and some cells co-expressed DMRT1 and FOXL2 (l'', l''', arrowheads). Though GATA1-positive cells are absent in XX Rspo1KO Sox9cKO mice at P10 (k), they were present in P40 gonads (n). Scale bars 50 µm.
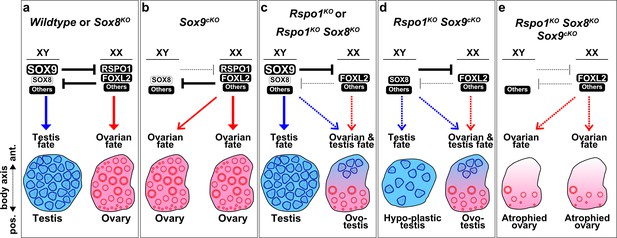
Gonad fate in wildtype, Rspo1, and Sox mutant mice.
In wildtype mice, SOX9, SOX8, and other factors promote testicular differentiation in XY mice, and RSPO1, FOXL2, and other factors promote ovarian differentiation in XX mice, as indicated by arrows (a). Antagonism exists between the testis and ovarian pathway, as indicated by ‘T’ bars (a). SOX9 and RSPO1 are essential for testicular and ovarian differentiation respectively, since XY Sox9cKO mice develop ovaries (b) and XX Rspo1KO mice develop partial sex-reversal ovotestes (c). However, we previously demonstrated that SOX9 is dispensable for testicular development in XX Rspo1KO mice, by studying XX Rspo1KO Sox9cKO mice (d). Also, gonads in XY Rspo1KO Sox9cKO mice develop as hypoplastic testes (d), indicating that RSPO1 is required for ovarian differentiation in XY Sox9cKO mice. In studying a Sox8KO mutation in XY and XX mice or mice lacking Rspo1, it was evident that SOX8 is dispensable for testicular, ovarian, or ovotesticular development (a, c). In this study however, we demonstrated that SOX8 is required for hypoplastic testicular or ovotesticular differentiation in XY and XX Rspo1KO Sox9cKO mice (d) by studying triple mutants (e). Gonads in both XY and XX Rspo1KO Sox8KO Sox9cKO mice lacked testis cords and developed as atrophied ovaries (e). Thus, SOX8 or SOX9 is sufficient and both SOX are required for testicular differentiation in gonads lacking RSPO1.
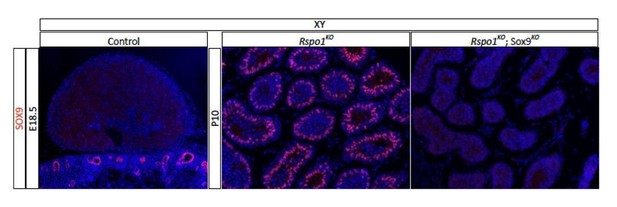
Left panel – Immunostaining analysis using anti-SOX9 antibody on adrenal cross-sections at E18.5.
Nuclear SOX9 is readily detectable in the ureter tips of the kidney, but not in the medulla of the adrenal gland. This confirmed that this antibody does not cross-react with SOX10. Middle and right panel – Immunostaining analyses using anti-SOX9 antibody on testes at P10. Anti-SOX9 antibodies reveal expression of SOX9 in XY Rspo1KO gonads, but not in XY Rspo1KOSox9cKO gonads, which we have demonstrated express SOX8. Thus, this anti-SOX9 antibody does not cross react with SOX8 antigen.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain (Mus musculus) | Rspo1-/- | (Chassot et al., 2008) | henceforth Rspo1KO | |
| Strain, strain (Mus musculus) | Sox8-/- | (Sock et al., 2001) | henceforth Sox8KO | |
| Strain, strain (Mus musculus) | Sox9fl/fl; Sf1:creTg/+ | (Lavery et al., 2011) | henceforth Sox9cKO | |
| Strain, strain (Mus musculus) | Rspo1-/-; Sox8-/- | This paper | henceforth Rspo1KO Sox8KO | |
| Strain, strain (Mus musculus) | Rspo1-/-; Sox9fl/fl; Sf1:creTg/+ | (Lavery et al., 2012) | henceforth Rspo1KO Sox9cKO | |
| Strain, strain (Mus musculus) | Rspo1-/-; Sox8-/-; Sox9fl/fl; Sf1:creTg/+ | This paper | henceforth Rspo1KOSox8KO Sox9cKO | |
| Antibody | anti-AMH/MIS (C-20) (Goat polyclonal) | Santa Cruz RRID:AB-649207 | Cat# sc-6886 | IF(1:100) |
| Antibody | anti-DMRT1 (Rabbit polyclonal) | Sigma RRID:AB_10600868 | Cat# HPA027850 | IF(1:100) |
| Antibody | anti-FOXL2 (Goat polyclonal) | Novus RRID:AB_2106188 | Cat# NB100-1277 | IF(1:200) |
| Antibody | anti-GATA1 (N6) (Rat monoclonal) | Santa Cruz RRID:AB_627663 | Cat# sc-265 | IF(1:200) |
| Antibody | anti-GATA4 (C20) (Goat polyclonal) | Santa Cruz RRID:AB_2108747 | Cat# sc-1237 | IF(1:200) |
| Antibody | anti-3β-HSD (P18) (Goat polyclonal) | Santa Cruz RRID:AB_2279878 | Cat# sc-30820 | IF(1:200) |
| Antibody | anti-P27/CDKN1B (Kip1) (Rabbit polyclonal) | Santa Cruz RRID:AB_632129 | Cat# sc-528 | IF(1:200) |
| Antibody | anti-Laminin LAMA1 (Rabbit polyclonal) | Sigma RRID:AB_477163 | Cat# L9393 | IF(1:150) |
| Antibody | anti-NR5A1/SF-1 (Rabbit polyclonal) | Gift from Ken Morohashi | IF(1:1000) | |
| Antibody | anti-SOX8 (Guineapig, polyclonal) | Gift from Elisabeth Sock (Stolt et al., 2005) | IF(1:1000) | |
| Antibody | anti-SOX9 (Rabbit polyclonal) | Sigma RRID:AB_1080067 | Cat# HPA001758 | IF(1:200) |
| Antibody | anti-TRA98 (Rat monoclonal) | Abcam RRID:AB_1659152 | Cat# ab82527 | IF(1:200) |
| Recombinant DNA reagent | Rspo1 riboprobe | (Parma et al., 2006) | ||
| Recombinant RNA reagent | Rspo1 (RNAscope riboprobe) | Advanced Cell Diagnostics | ||
| Software, algorithm | Affinity Photo | Serif Europe Ltd., Nottingham United Kingdom | https://affinity.serif.com/en-us/photo/ | |
| Software, algorithm | Affinity Designer | Serif Europe Ltd., Nottingham United Kingdom | https://affinity.serif.com/en-us/ | |
| Software, algorithm | Graphpad Prism | Graphpad Software, Inc, La Jolla, CA | http://www.graphpad.com/ | |
| Other | DAPI stain | Vector Laboratory | H-1500 | (1µg/ml) |





