Synergistic and antagonistic drug interactions in the treatment of systemic fungal infections
Figures
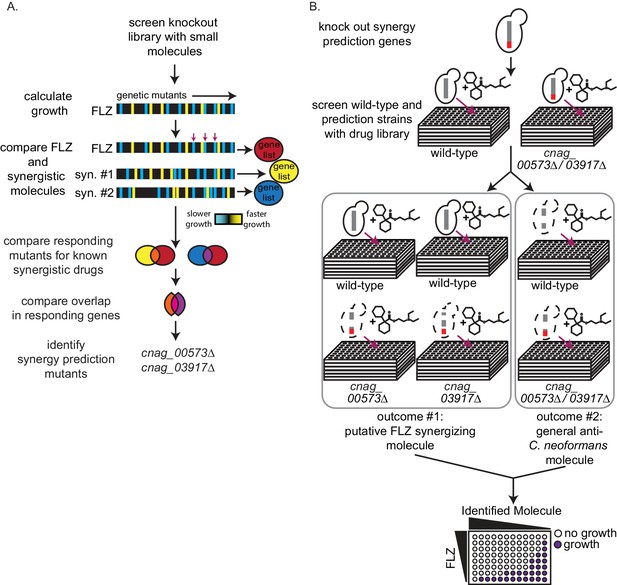
High-throughput screening for fluconazole interacting molecules using synergy prediction mutants.
(A) Outline of overlap2 method (O2M), which is also presented in Brown et al., Wambaugh et al., and Wambaugh and Brown. O2M requires a chemical-genetic dataset which can be generated by growing a collection of mutants in the presence of >100 small molecules individually. Growth scores are then calculated for each small molecule + mutant combination. In the heatmaps, the vertical line represents a different mutant. Blue represents slower growth compared to wild-type cells and yellow represents faster growth compared to wild-type cells. Comparing our starting drug (FLZ) and known synergistic molecules, we can identify genes whose knockout mutants show similar growth scores to the starting drug and all known synergistic partners (red arrows). These are the synergy prediction mutants. (B) Screening method to identify molecules that synergize with FLZ as well as anti-C. neoformans molecules. Synergy prediction mutants are created (red chromosome indicates gene knockout) and both synergy prediction mutant and wild type are grown in the library of small molecules (top section). Growth of wild type and synergy prediction mutants are accessed. Greyed out dotted yeast cell indicates differential growth compared to wild type (middle section). These molecules are then validated in a checkerboard assay (bottom section).
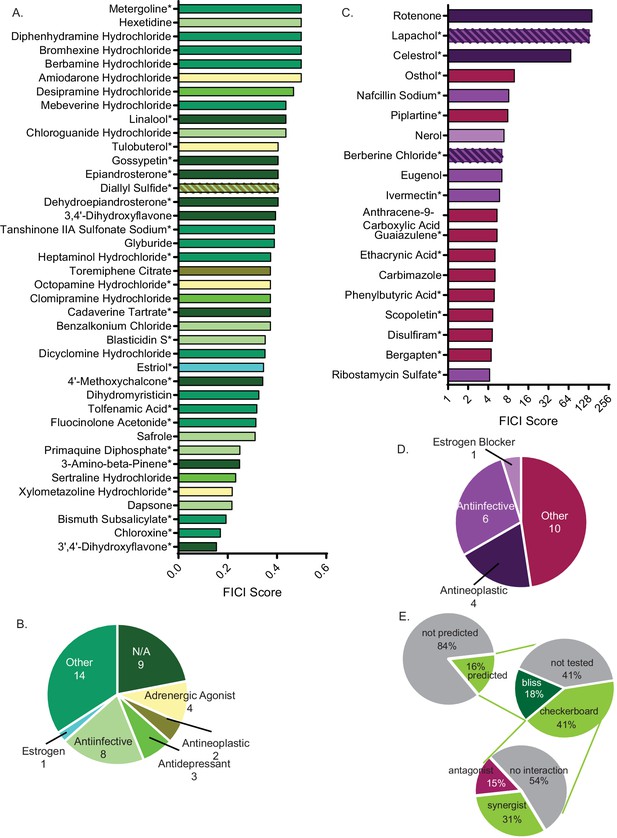
Synergistic and antagonistic molecules identified from high-throughput screen.
(A) Average fractional inhibitory concentration index (FICI) score of synergistic molecules identified from our high-throughput screen. Color of bar corresponds with bioactivities listed in B. FICIs are listed in linear scale. Source data are in Figure 2—source data 1. (B) Categories of bioactivities of synergistic molecules with the corresponding number of molecules in each category. (C) FICI scores in log2 scale of antagonistic molecules from screen. Colors correspond with bioactivities listed in D. Source data are in Figure 2—source data 2 (D) Categories of bioactivities of antagonistic molecules with corresponding number of molecules. All bioactivities came from Microsource Spectrum molecule list which is also seen in Table 1. (E) Representation of percentage of molecules from the entire Microsource Spectrum Library predicted to synergize with fluconazole based on screening results (top), molecules tested in various assays (middle), and molecules yielding an interaction from checkerboards (bottom). * represents FICI for 50% inhibition of C. neoformans (when 90% inhibition was not found). All other scores listed are the FICI for 90% inhibition (FICI90) unless otherwise stated. Molecules not tested were not available commercially. All average FICI scores represent an average of at least two independent tests, performed in our prior works (Brown et al., 2014; Wambaugh and Brown, 2018; Wambaugh et al., 2017). All data are against C. neoformans strain CM18.
-
Figure 2—source data 1
FICI scores of synergistic small molecule combinations.
FICI scores for small molecules listed in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90% inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig2-data1-v1.xlsx
-
Figure 2—source data 2
FICI scores of antagonistic small molecule combinations.
FICI scores for small molecules listed in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90% inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig2-data2-v1.xlsx
-
Figure 2—source data 3
Bliss Independence scores of small molecule combinations.
FICI scores for small molecules listed in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90%) inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig2-data3-v1.xlsx
-
Figure 2—source data 4
FICI scores of non-synergistic small molecule combinations.
FICI scores for small molecules listed in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90% inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig2-data4-v1.xlsx
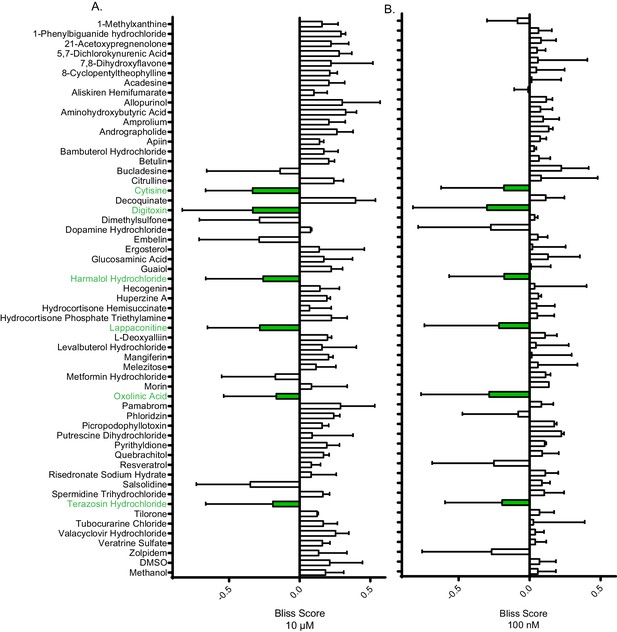
Bliss independence scores of non-single agent molecules.
Average bliss independence score of molecules at (A) 10 µM and (B) 100 nM. Molecules were considered synergistic if they exhibited a negative score in both concentrations the majority of times tested (green labels). Average scores represent a minimum of two independent replicates. Error bars represent the standard deviation. Source data are in Figure 2—source data 3.
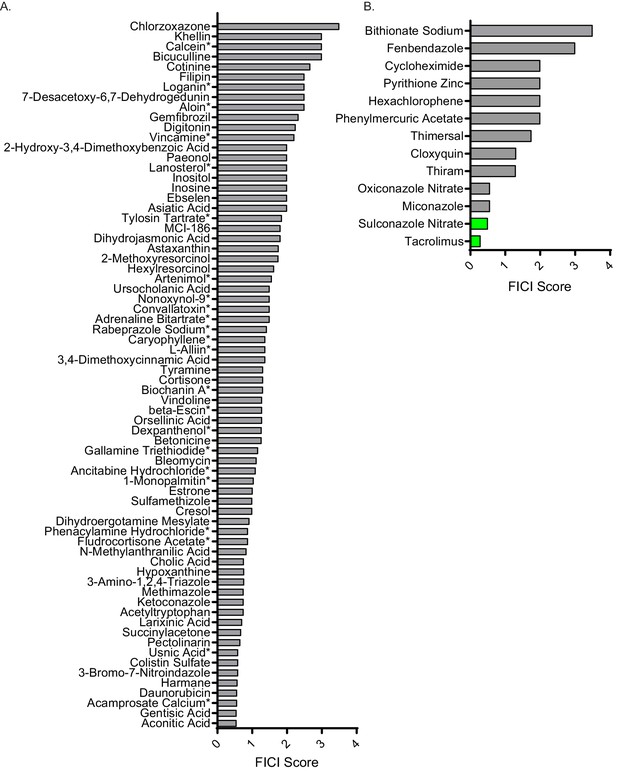
FICI scores of non-interacting molecules and general antifungals.
(A) Fractional inhibitory concentration index (FICI) of molecules identified from our high-throughput screen that did not interact with FLZ. (B) FICI scores of molecules identified as general anti-C. neoformans molecules from our high-throughput screen. Synergistic interactions with FLZ labeled in green and non-interacting molecules labeled in grey. * represents FICI for 50% inhibition of C. neoformans all others are FICI for 90% inhibition. Average FICI scores represent a minimum of two independent replicates. Source data are in Figure 2—source data 4. All data are against C. neoformans strain CM18.
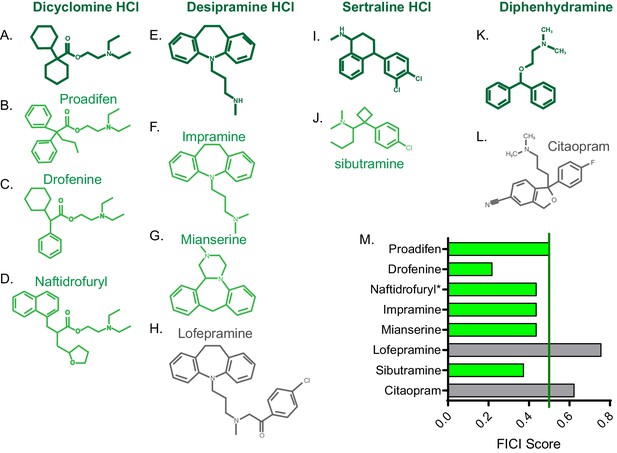
FICI scores of structurally similar molecules.
Chemical structure of (A) dicyclomine HCl and structurally similar molecules (B) proadifen, (C) drofenine, and (D) naftidrofuryl. Chemical structure of (E) desipramine HCl and structurally similar molecules (F) impramine, (G) mianserine, and H) lofepramine. Chemical structure of (I) sertraline HCl and structurally similar (J) sibutramine. Chemical structure of (K) diphenhydramine HCl and structurally similar (L) citalopram. (M) Fractional inhibitory concentration index (FICI) score of structurally similar molecules. Synergistic interactions with FLZ labeled in green and non-interacting molecules labeled in grey. * represents FICI for 50% inhibition of C. neoformans strain CM18 all others listed are FICI for 90% inhibition. Average FICI scores represent a minimum of two independent replicates. All average FICI scores represent an average of at least two independent tests. Source data are Figure 3—source data 1.
-
Figure 3—source data 1
FICI scores of small molecules with structures similar to newly identified fluconazole-synergizers.
FICI scores for small molecules listed in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90% inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig3-data1-v1.xlsx
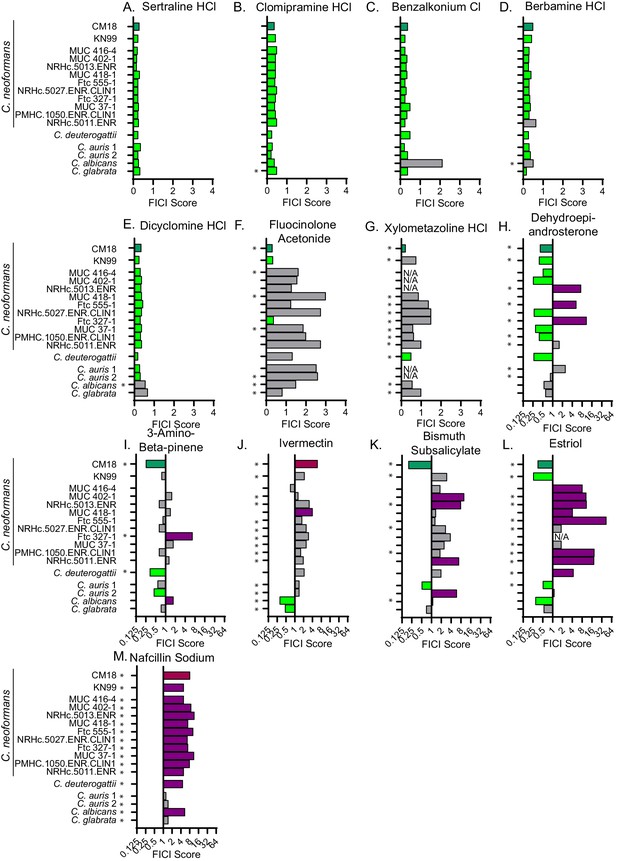
Synergistic and antagonistic combinations affect other fungal strains and species.
Fractional inhibitory concentration index (FICI) scores of synergistic and antagonistic combinations with FLZ in other fungal strains/species for (A) Sertraline (B) Clomipramine HCl (C) Benzalkonium Cl (D) Berbamine HCl (E) Dicyclomine HCl (F) Fluocinolone Acetonide (G) Xylometazoline HCl (H) Dehydroepiandrosterone (I) 3-Amino-beta-pinene (J) Ivermectin (K) Bismuth Subsalicylate (L) Estriol (M) Nafcillin Sodium. * represents FICI for 50% inhibition all other scores listed are the FICI90. Strains/species listed on left and Supplementary file 4. CM18 (top) represents original result. Green bars represent FICI scores ≤ 0.5 yielding a synergistic result. Violet bars represent FICI scores ≥ 4 yielding an antagonistic result. No interactions are in grey bars. FICI Scores presented in either linear or log2 scale. HCl = hydrochloride, Cl = chloride. All average FICI scores represent an average of at least two independent tests. Source data are Figure 4—source data 1.
-
Figure 4—source data 1
FICI scores of synergistic small molecule combinations against a variety of fungal species and strains.
FICI scores for small molecules listed in combination with fluconazole. Whether FICI scores represent 90% inhibition of 50% inhibition is marked in Figure 4.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig4-data1-v1.xlsx
-
Figure 4—source data 2
FICI scores of nafcillin in combination with ketoconazole.
FICI scores 50% inhibition for nafcillin in combination with ketoconazole for KN99 C. neoformans.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig4-data2-v1.xlsx
-
Figure 4—source data 3
FICI scores of dicyclomine in FLZ resistant strains and species.
FICI scores for dicyclomine in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90% inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig4-data3-v1.xlsx
-
Figure 4—source data 4
FICI scores of dicyclomine in combination with ketoconazole.
FICI scores for 90% inhibition for dicyclomine in combination with ketoconazole for KN99 C. neoformans.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig4-data4-v1.xlsx
-
Figure 4—source data 5
FICI scores of nafcillin in FLZ resistant strains and species.
FICI scores for nafcillin in combination with fluconazole. All scores are FICI 50.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig4-data5-v1.xlsx
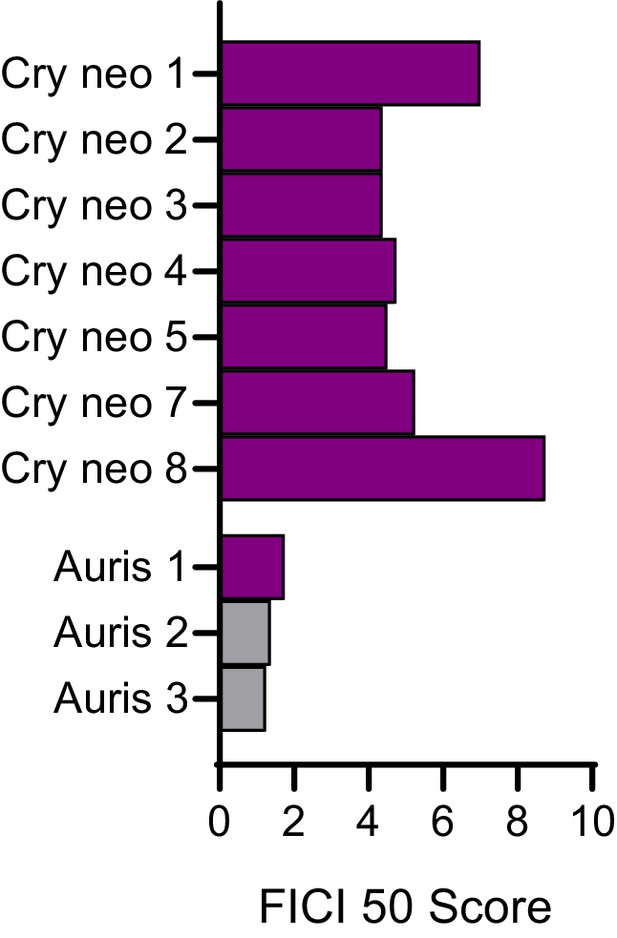
Nafcillin is antagonistic in most FLZ resistant strains and species.
Fractional inhibitory concentration index (FICI) scores of nafcillin in combination with FLZ in clinical isolates of C. neoformans and Candida auris that are considered FLZ resistant. All scores are FICI 50. Violet bars represent FICI scores ≥ 4 yielding an antagonistic result. No interactions are in grey bars. Source data are available in Figure 4—source data 5.
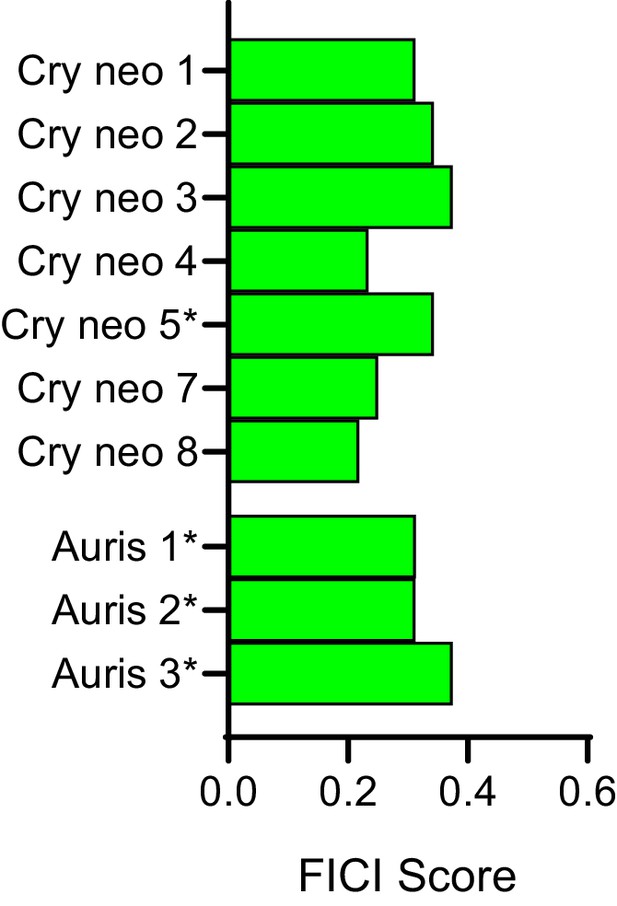
Dicyclomine is synergistic in most FLZ resistant strains and species.
Fractional inhibitory concentration index (FICI) scores of dicyclomine in combination with FLZ in clinical isolates of C. neoformans and Candida auris that are considered FLZ resistant. * represent FICI 50 all others are FICI 90. Green bars represent FICI scores ≤ 0.5 yielding a synergistic result. Source data are available in Figure 4—source data 3.
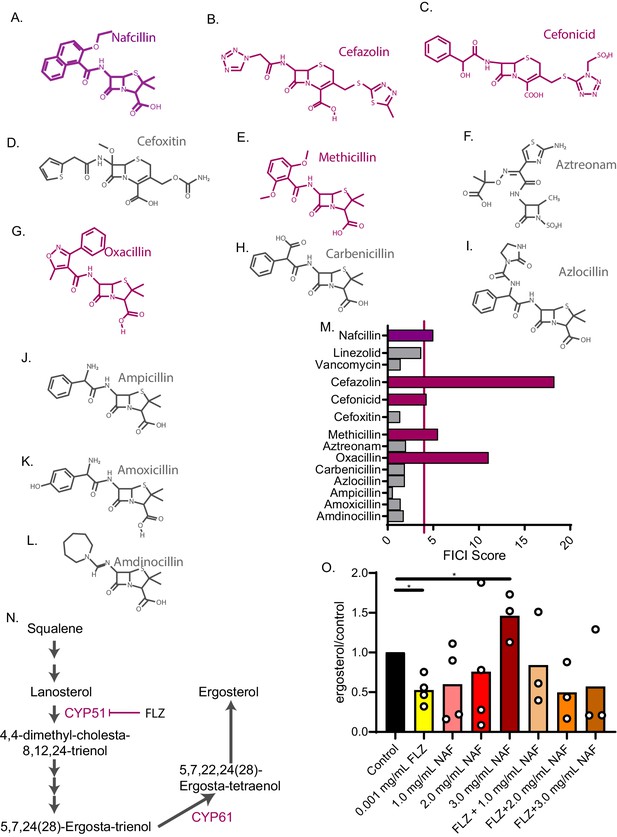
Nafcillin Sodium affects ergosterol levels.
Molecular structures of beta-lactam antibiotics shown for (A) Nafcillin Sodium (B) Cefazolin (C) Cefonicid (D) Cefoxitin (E) Methicillin (F) Aztreonam (G) Oxacillin (H) Carbenicillin (I) Azlocillin (J) Ampicillin (K) Amoxicillin (L) Amdinocillin. (M) FICI scores for 50% inhibition of C. neoformans of various antibiotics related to nafcillin sodium tested with fluconazole. Violet bars over the red line illustrate a FICI score of ≥4 indicating antagonism. (N) Ergosterol biosynthesis pathway illustrating cytochrome P450 enzymes. (O) Ergosterol quantification from cell treated with Nafcillin (NAF), FLZ, or NAF+FLZ. Data normalized to control treated. *=p value is 0.03 (Mann-Whitney test). All average FICI scores represent an average of at least two independent tests (technical and biological replicates). Source data are in Figure 5—source data 1.
-
Figure 5—source data 1
FICI scores of small molecules with structures similar to nafcillin.
FICI scores for small molecules listed in combination with fluconazole. All scores are for FICI 90% inhibition unless listed. *=FICI scores for 50% inhibition (when 90% inhibition of fungal growth could not be obtained.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig5-data1-v1.xlsx
-
Figure 5—source data 2
FICI scores of nafcillin in combination with amphotericin B.
FICI scores for 50% inhibition for nafcillin in combination with amphotericin B for KN99 C. neoformans.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig5-data2-v1.xlsx
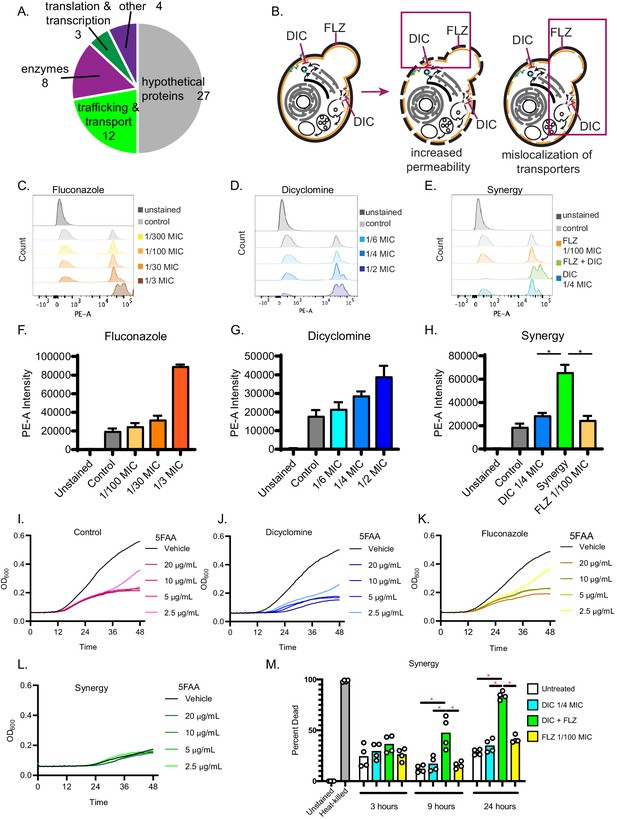
Dicyclomine affects permeability and nutrient transporters.
(A) Pie chart with processes of deletion mutants that were resistant to dicyclomine. Numbers represent number of mutants. (B) Prediction for dicyclomine (DIC) + FLZ synergy mechanism. (C–E) Representative flow plots of propidium iodide staining. (F–H) Quantification of propidium iodide staining. Data are averages of three independent replicates. (I–L) Growth curves of C. neoformans with and without various concentrations of 5-FAA in addition to control (1x YNB + 2% glucose), dicyclomine (0.3 mg/mL or ¼ MIC), fluconazole (0.1 µg/mL or 1/100 MIC), or synergy (0.3 mg/mL dicyclomine + 0.1 µg/mL FLZ) treatment. Each experiment contained four technical replicates that were inoculated from the same culture. The lines represent the average of two experiments are presented in the figure. Source data are in Figure 6—source data 1. (M) Quantification of percent of dead cells after treatment with dicyclomine, FLZ, or synergy after 3, 9, and 24 hr. *=p value is 0.0286 (Mann-Whitney).
-
Figure 6—source data 1
Growth rate of cells grown in the presence of toxic amino acid analog 5-FAA.
OD600 from each of two different biological replicates. These data were averaged to produce the graphs in Figure 6I–L.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig6-data1-v1.xlsx
-
Figure 6—source data 2
CFUs after heat-killing C. neoformans.
Colony forming units (CFUs) of C. neoformans after heat-killing cells in a water bath >65° C for about an hour. The starting concentration was 3 × 106 cells.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig6-data2-v1.xlsx
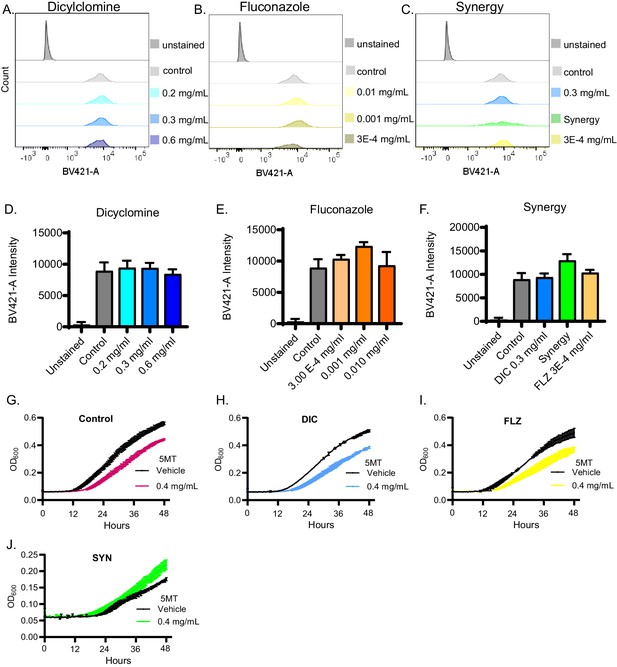
Dicyclomine additional effects on fungal chitin staining and nutrient intake.
(A–C) Representative flow cytometry of Calcofluor white staining with various concentrations of Dicyclomine (DIC) (blue), FLZ (yellow), or synergy (green). (D–F) Quantification of average fluorescence intensity of calcofluor white staining flow cytometry. Average of three replicates shown. (G–J) Growth curves of C. neoformans with or without 0.4 mg/mL of 5-MT after treatment with (G) control, (H) dicyclomine, (I) FLZ, or (J) Synergy (SYN). Source data are in Figure 6—figure supplement 1—source data 1. 100,00 cells were counted for each flow cytometry sample.
-
Figure 6—figure supplement 1—source data 1
Growth rate of cells grown in the presence of toxic amino acid analog 5-MT.
OD600 from each of two different biological replicates. Each biological replicate represents the average of four technical replicates. These data were averaged to produce the graphs in Figure 6—figure supplement 1G–J.
- https://cdn.elifesciences.org/articles/54160/elife-54160-fig6-figsupp1-data1-v1.xlsx
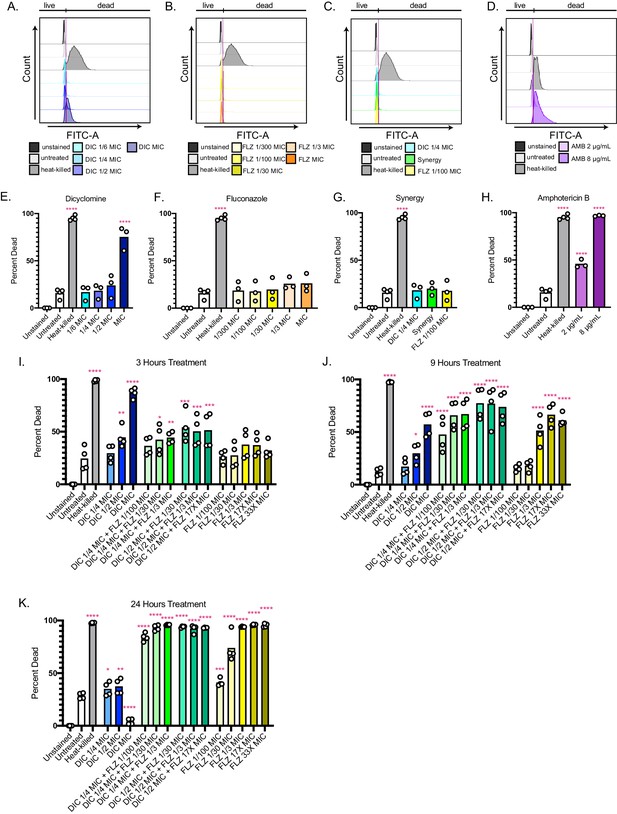
Fungicidal effects of dicyclomine and combination treatment.
Representative flow plots for dicyclomine (A) fluconazole (B) synergistic combination (C) and amphotericin B (AMB) (D) showing the gates for live and dead cells. Quantification of percent of dead cells for dicyclomine (E) fluconazole (F) synergistic combination (G) and amphotericin B (H). **** represent p<0.0001 compared to untreated (uncorrected Fisher’s LSD). Quantification of percent of dead cells after treatment for 3 hr (I) 9 hr (J) and 24 hr (K). * represents p=0.02, ** represents p=0.008, *** represent p>0.001 and<0.001 , **** represents p<0.0001 compared to untreated (uncorrected Fisher’s LSD). Heat-killed cells were plated out for survival (Figure 6—source data 2).
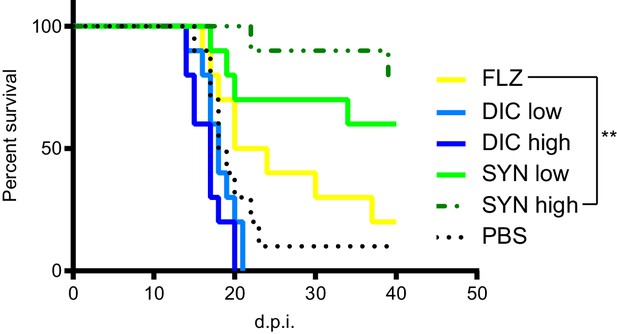
Dicyclomine + FLZ synergy increases survival of mice with cryptococcosis Survival of CD-1 outbred mice given FLZ (8 mg/kg), DIC low (1.15 mg/kg), DIC high (2.30 mg/kg), Synergy (SYN) low (FLZ + 1.15 mg/kg DIC), SYN high (FLZ + 2.30 mg/kg DIC) or PBS treatments.
N = 10. ; **=p value is 0.0036 (Mantel-Cox test).
Tables
Minimum inhibitory concentrations for fluconazole interacting molecules.
All values are against C. neoformans strain CM18. MIC for 90% inhibition (MIC90) listed when possible. MIC50 = MIC for 50% inhibition. Molecules were dissolved in to their highest soluble concentration in DMSO. MICs were determined below the inhibitory concentrations of DMSO (~5%) in YNB minimal media.
| Small molecule | Bioactivity | MIC 50 (mM) | MIC 90 (mM) | Result |
|---|---|---|---|---|
| Anthracene-9-Carboxylic Acid | Cl transport inhibitor | 0.2 | 1.7 | antagonistic |
| Berberine Chloride | antiarrhythmic, alpha2 agonist, cholinesterase, anticonvulsant, antiinflammatory, antibacterial, antifungal, antitrypanosomal, antineoplastic, immunostimulant | 1.4 | 2.6 | antagonistic |
| Bergapten | antipsoriatic, antiinflammatory | 1.2 | 2.4 | antagonistic |
| Carbimazole | antithyroid | 0.2 | 0.9 | antagonistic |
| Celastrol | antineoplastic, antiinflamatory, NO synthesis inhibitor, chaperone stimulant | 0.07 | 0.2 | antagonistic |
| Disulfram | alcohol antagonist | 0.007 | 0.007 | antagonistic |
| Ethacrynic Acid | diuretic | 0.4 | 1.1 | antagonistic |
| Eugenol | analgesic (topical), antiseptic, antifungal | 2.4 | 14 | antagonistic |
| Guaiazulene | antioxidant, inhibits lipid peroxidation inhibitor, antiinflammatory, hepatoprotectant; LD50(rat) 1550 mg/kg po | 3.5 | 11 | antagonistic |
| Ivermectin | antiparasitic | 1.9 | 1.9 | antagonistic |
| Lapachol | antineoplastic, antifungal | 36 | N/A | antagonistic |
| Nafcillin Sodium | antibacterial | 2.4 | N/A | antagonistic |
| Nerol | weak estrogen receptor blocker | 0.2 | 0.7 | antagonistic |
| Osthol | N/A | 0.5 | 1.8 | antagonistic |
| Phenylbutyric Acid | antiinflammatory, antihyperammonemic (Na salt) | 0.7 | 2.9 | antagonistic |
| Piplartine | anti-asthma, antibronchitis | 3.3 | 190 | antagonistic |
| Ribostamycin Sulfate | antibacterial | 9.4 | 19 | antagonistic |
| Rotenone | acaricide, ectoparasiticide, antineoplastic, mitochondrial poison | 0.002 | 0.002 | antagonistic |
| Scopoletin | NO synthesis (inducible) inhibitor, anticoagulant | 2.9 | 11 | antagonistic |
| 3,4'-Dihydroxyflavone | N/A | 1.4 | 8.3 | synergistic |
| 3',4'-Dihydroxyflavone | N/A | 8.9 | N/A | synergistic |
| 3-Amino-beta-Pinene | N/A | 12 | 50 | synergistic |
| 4'-Methoxychalcone | N/A | 0.1 | N/A | synergistic |
| Amiodarone Hydrochloride | adrenergic agonist, coronary vasodilator, Ca channel blocker | 0.003 | 0.007 | synergistic |
| Benzalkonium Chloride | antiinfective (topical) | 0.015 | 0.015 | synergistic |
| Berbamine Hydrochloride | antihypertensive, skeletal muscle relaxant | 0.03 | 0.06 | synergistic |
| Bismuth Subsalicylate | antidiarrheal, antacid, antiulcer | 1.5 | N/A | synergistic |
| Blasticidin S | antibiotic, antifungal; LD50 (rat po) 16 mg/kg | 0.23 | 0.72 | synergistic |
| Bromhexine Hydrochloride | expectorant | 15 | 15 | synergistic |
| Cadaverine Tartrate | N/A | 2.3 | 5.8 | synergistic |
| Chloroguanide Hydrochloride | antimalarial | 1 | 1 | synergistic |
| Chloroxine | chelating agent | 0.002 | 0.007 | synergistic |
| Clomipramine Hydrochloride | antidepressant | 0.65 | 0.78 | synergistic |
| Dapsone | antibacterial, leprostatic, dermatitis herpetiformis suppressant | 29 | N/A | synergistic |
| Dehydroepiandrosterone | N/A | 1.5 | 12 | synergistic |
| Desipramine Hydrochloride | antidepressant | 1.9 | 1.9 | synergistic |
| Diallyl Sulfide | antibacterial, antifungal, antineoplastic, antihypercholesterolaemic, hepatoprotectant | 7.2 | 33 | synergistic |
| Dicyclomine Hydrochloride | anticholinergic | 4.9 | 4.9 | synergistic |
| Dihydromyristicin | GSH transferase inducer | 280 | N/A | synergistic |
| Diphenhydramine Hydrochloride | antihistaminic | 18 | N/A | synergistic |
| Epiandrosterone | N/A | 1.2 | 3.2 | synergistic |
| Estriol | estrogen | 25 | 25 | synergistic |
| Fluocinolone Acetonide | glucocorticoid, antiinflammatory | 1.8 | 3.7 | synergistic |
| Glyburide | antihyperglycemic | 0.3 | 2 | synergistic |
| Gossypetin | N/A | 1.6 | N/A | synergistic |
| Heptaminol Hydrochloride | vasodilator | 6.2 | N/A | synergistic |
| Hexetidine | antifungal | 0.05 | 0.3 | synergistic |
| Linalool (+) | N/A | 0.8 | 3.1 | synergistic |
| Mebeverine Hydrochloride | muscle relaxant (smooth) | 4.4 | 8.9 | synergistic |
| Metergoline | analgesic, antipyretic | 0.2 | 0.2 | synergistic |
| Octopamine Hydrochloride | adrenergic agonist | 25 | N/A | synergistic |
| Primaquine Diphosphate | antimalarial | 3.7 | N/A | synergistic |
| Safrole | anesthetic (topical) and antiseptic, pediculicide | 20 | 41 | synergistic |
| Sertraline Hydrochloride | antidepressant, 5HT uptake inhibitor | 0.2 | 0.3 | synergistic |
| Tanshinone IIA Sulfonate Sodium | free radical scavenger | 0.9 | 1.2 | synergistic |
| Tolfenamic Acid | antiinflammatory, analgesia | 0.8 | 7.7 | synergistic |
| Toremiphene Citrate | antineoplastic, anti-estrogen | 28 | 28 | synergistic |
| Tulobuterol | bronchodilator, beta adrenergic agonist | 23 | N/A | synergistic |
| Xylomethazoline Hydrochloride | adrenergic agonist, nasal decongestant | 2.4 | N/A | synergistic |
Additional files
-
Supplementary file 1
Small molecules predicted to synergize with fluconazole by O2M.
Small molecules predicted to interact with FLZ. Bioactivity and Status determined by Microsource Spectrum Library. The specific manufacturers we purchased molecules from are listed in last column. Molecules we did not purchase (for various reasons), have the manufacturer listed as N/A. INN, International Nonproprietary Names; USAN, United States Accepted Name; BAN, British Approved Names; JAN, Japanese Adopted Name; USP, United States Pharmacopeia; NF, National Formulary.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp1-v1.xlsx
-
Supplementary file 2
Minimum inhibitory concentrations of non-interacting molecules.
Small molecules predicted to interact with FLZ but had no interaction. Minimum inhibitory concentration for 50% inhibition (MIC 50) and 90% inhibition (MIC 90) of small molecules predicted to interact with FLZ but resulted in no interaction. All values are against C. neoformans strain CM18. Bioactivity determined by Microsource Spectrum Library.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp2-v1.xlsx
-
Supplementary file 3
Minimum inhibitory concentrations of general anti-C. neoformans molecules.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp3-v1.xlsx
-
Supplementary file 4
Additional fungal species and strains used.
Strains and strain sources used in this study.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp4-v1.xlsx
-
Supplementary file 5
Minimum inhibitory concentrations for various fungal strains/species.
Minimum inhibitory concentration for 50% inhibition (MIC 50) and 90% inhibition (MIC 90) of small molecules that interacted with FLZ in various fungal species. N/A represents molecules that did not have an MIC.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp5-v1.xlsx
-
Supplementary file 6
Minimum inhibitory concentrations for FLZ resistant strains and species.
Minimum inhibitory concentrations for 50% inhibition (MIC 50) and 90% inhibition (MIC 90) of FLZ resistant species. N/A represents molecules that did not have an MIC.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp6-v1.xlsx
-
Supplementary file 7
Gene deletion mutants resistant to Dicyclomine.
Gene knockouts in KN99 resistant to dicyclomine at 1.65 mg/mL.
- https://cdn.elifesciences.org/articles/54160/elife-54160-supp7-v1.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/54160/elife-54160-transrepform-v1.docx