A neuropeptide regulates fighting behavior in Drosophila melanogaster
Figures
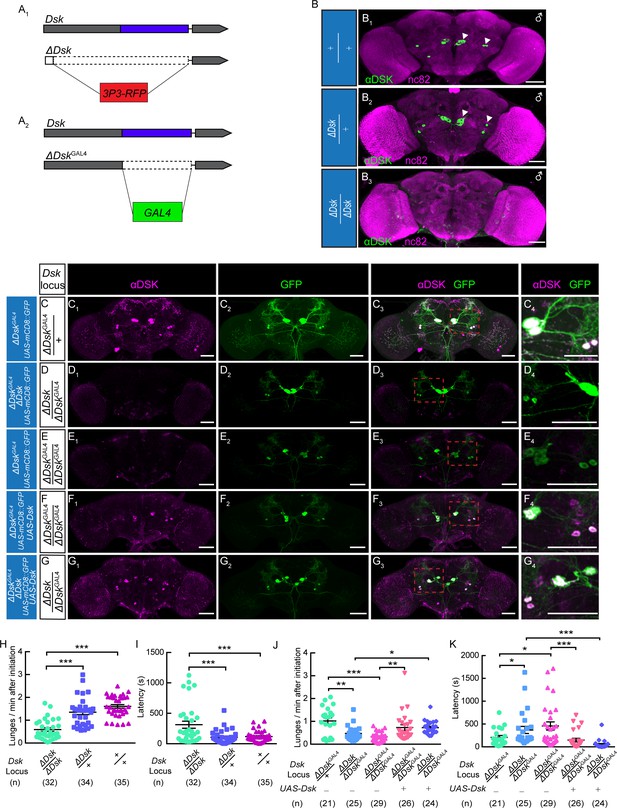
The Dsk Gene Is Essential for Modulating Male-Male Aggression.
(A) Generation of ΔDsk (A1) and ΔDskGAL4 (A2). Dashed boxes indicate the region replaced by 3P3-RFP (A1) or GAL4 (A2) cassette. (B) Male adult brains of the indicated genotypes were stained with anti-DSK antibody (green) and counter-stained with nc82 antibody (magenta) to label neuropil. Arrowheads: Dsk-expressing neurons. (C–G) ΔDskGAL4 driven UAS-mCD8:GFP expression in +/ΔDskGAL4 (C), ΔDsk/ΔDskGAL4 (D), ΔDskGAL4/ΔDskGAL4 (E), ΔDskGAL4/ΔDskGAL4 rescued by UAS-Dsk (F), ΔDsk/ΔDskGAL4 rescued by UAS-Dsk (G). Male brains were stained with anti-DSK antibody (magenta; C1–G1) and anti-GFP antibody (green; C2–G2). the anti-DSK antibody signal was undetected in Dsk mutant background (D1 and E1), but recovered when the Dsk mutant was rescued by ΔDskGAL4 driven UAS-Dsk expression (F1 and G1). C4-G4: regions in the red dashed boxes of C3-G3. Scale bars represent 50 μm. (H–I) Dsk mutants show reduced number of lunges (H) and prolonged fighting latency (I) compared with wildtype and heterozygous controls. (J–K) Number of lunges per minute after initiation (J) and fighting latency (K) for indicated genotype. Reduced aggression phenotypes of Dsk mutants were rescued by ΔDskGAL4 driven UAS-Dsk expression. *p<0.05, **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
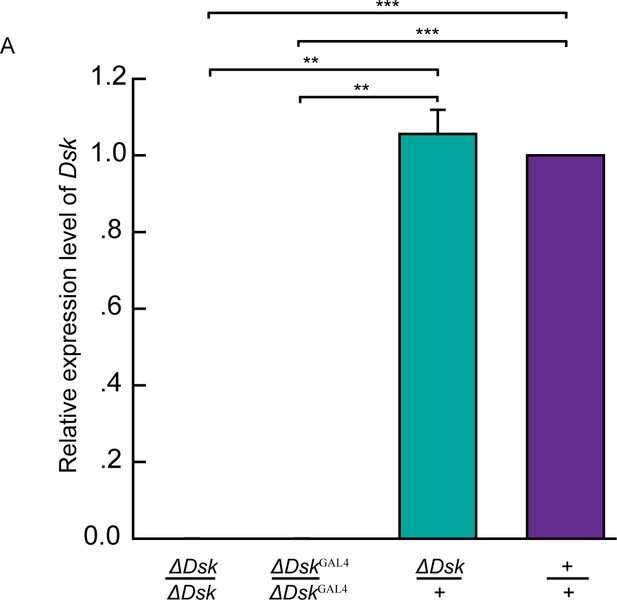
Molecular validation of mutants for Dsk.
(A) Quantification of Dsk mRNA by qRT-PCR in the homozygotes, heterozygotes, and wildtypes. The Dsk transcripts were not detectable in ΔDsk /ΔDsk and ΔDskGAL4/ΔDskGAL4 mutants. Triplicate assays per cDNA sample were carried out independently, the results were obtained from three independent RNA samples. **p<0.01, ***p<0.001 (one-way ANOVA and post-hoc Student’s T-test). Error bars indicate SEM.
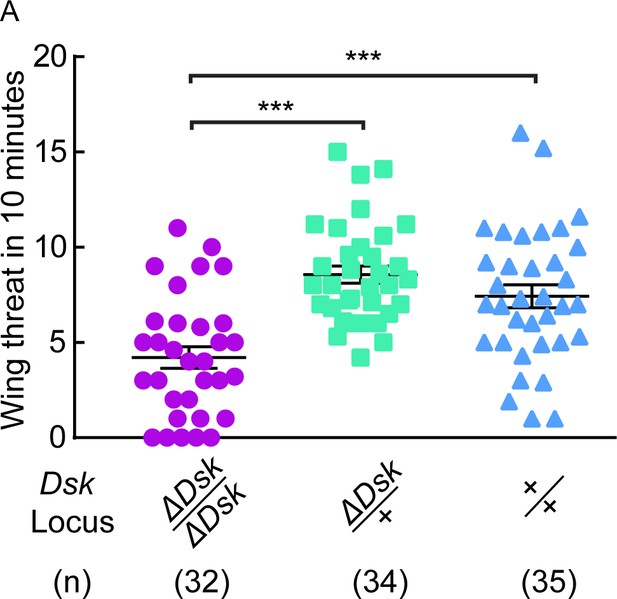
ΔDsk suppresses wing threat frequency in males.
(A) ΔDsk mutants show reduced number of wing threats compared to wildtype and heterozygous controls. ***p<0.001 (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
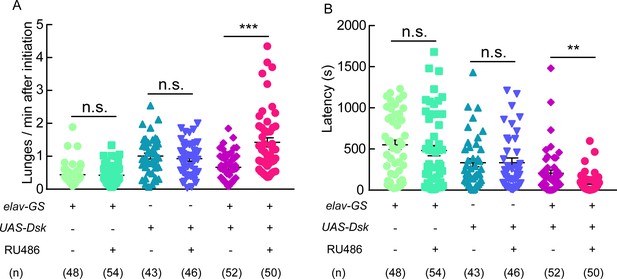
Aggressive behavior was promoted by Dsk overexpression.
(A–B) RU486 induced overexpression of Dsk increased number of lunges per minute after initiation (A) and shortened fighting latency (B) in males. **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
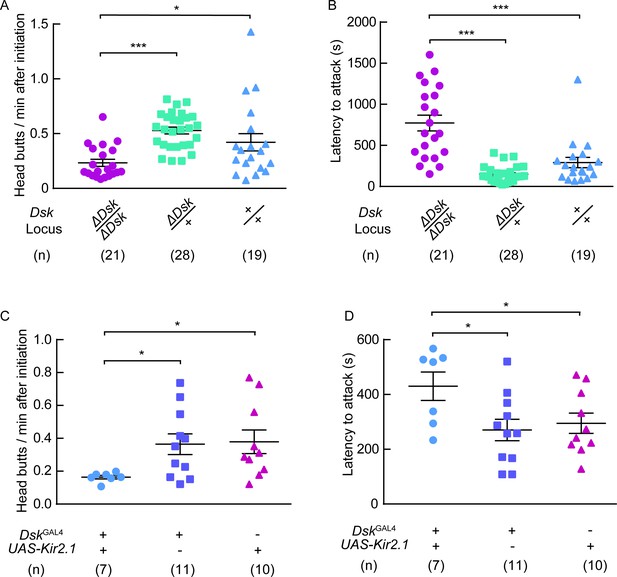
DSK signal modulates female-female aggression.
(A) Head butts frequency of female-female aggression. Homozygotes of ΔDsk showed a significant decrease in head butts compared to heterozygotes or wildtypes. (B) Fighting latency of female-female aggression. Fighting latencies were significantly longer in Dsk mutants than in heterozygotes or wildtypes. (C–D) Kir2.1 inactivation of DskGAL4 neurons in females decreased head butts frequency (C) and prolonged fighting latency (D). *p<0.05, ***p<0.001 (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
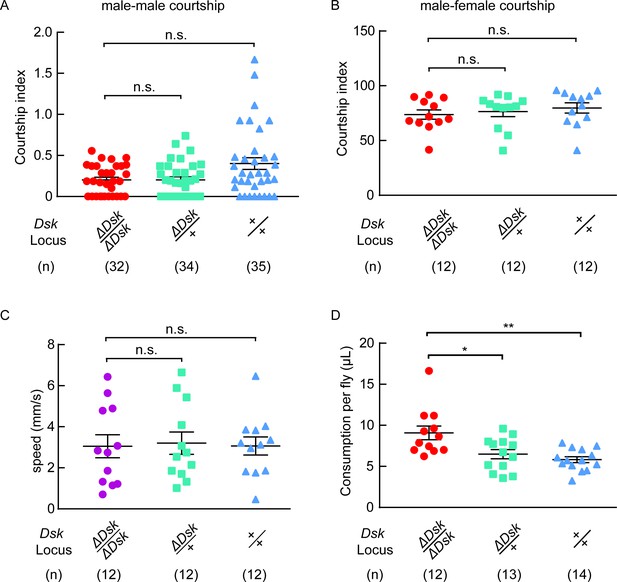
Courtship, locomotion and feeding behavior of ΔDsk mutants.
(A) Courtship indices for male-male courtship. (B) Courtship indices for male-female courtship. (C) The locomotive speed of ΔDsk mutants. (D) The feeding behavior of ΔDsk mutants. ΔDsk mutants show increased food consumption compared to wildtype and heterozygous controls in the CAFE essay. *p<0.05, **p<0.01, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
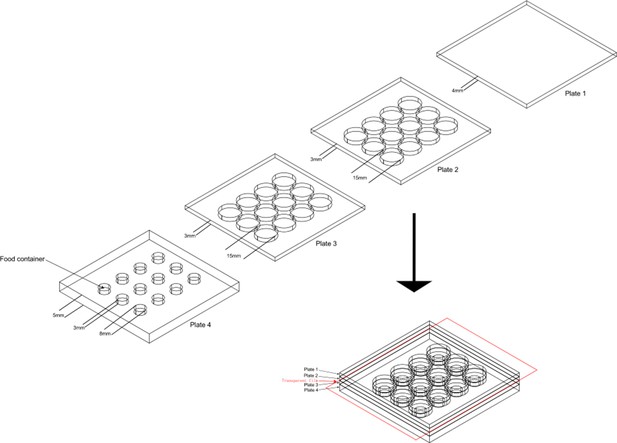
The design of aggression chamber.
The aggression chamber consists of four acrylic plates. The bottom plate (Plate 4) contains 12 wells for food patches (diameter: 8 mm; depth: 3 mm). The lower (Plate 3) and upper plates (Plate 2) have 12 cylindrical arenas (diameter: 15 mm; height of each plates: 3 mm). A transparent film is sandwiched by Plate two and Plate three to separate the two flies and removal of the transparent film allows encounters of the two flies.
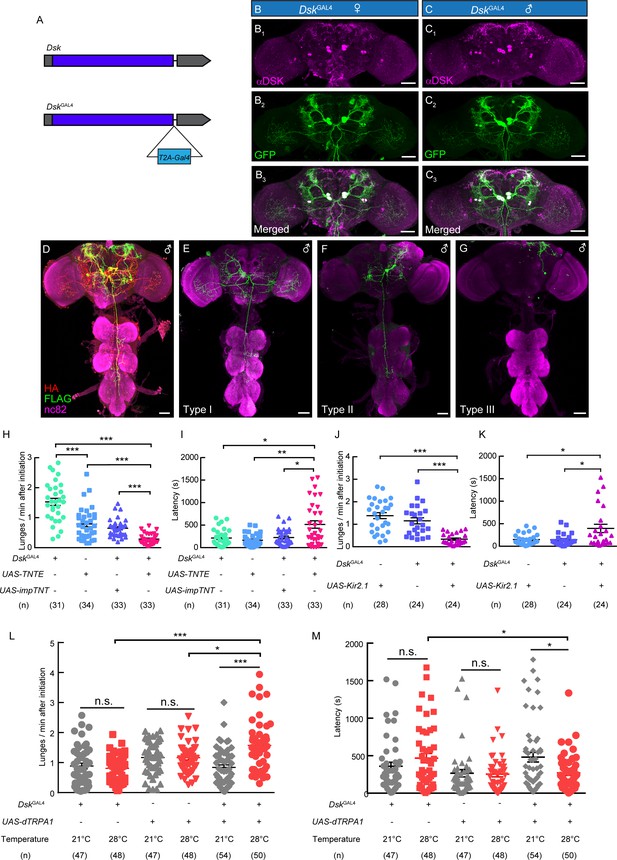
DskGAL4 Neurons Modulate Male-Male Aggressive Behavior.
(A) Generation of DskGAL4 Knock-in line. GAL4 was fused to the end of open reading frame (ORF) of Dsk with a T2A peptide linker. (B–C) Anatomical features of DSK neurons revealed by DskGAL4 driven UAS-mCD8:GFP expression. Adult female brains stained with anti-DSK antibody (magenta; B1) and anti-GFP antibody (green; B2); adult male brains stained with anti-DSK antibody (magenta; C1) and anti-GFP antibody (green; C2). Scale bars represent 50 μm. (D–G) Characterization of individual DskGAL4 neurons using MultiColor FlpOut (MCFO) method in males. Eight DSK-expressing neurons were classified into three neuronal cell types (D–G). Scale bars represent 50 μm. (H–I) TNTE inactivation of DskGAL4 neurons in males reduced lunge frequency (H) and prolonged fighting latency (I). (J–K) Kir2.1 inactivation of DskGAL4 neurons in males decreased lunge frequency (J) and prolonged fighting latency (K). (L–M) Number of lunges per minute after initiation (L) and fighting latency (M) for males during thermogenetic activation of DskGAL4 neurons. *p<0.05, **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
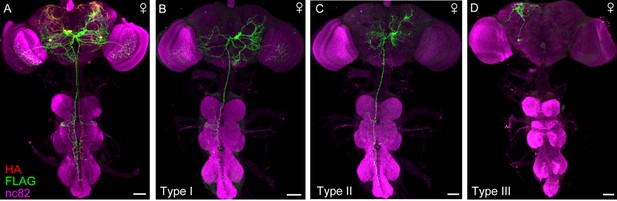
Projection patterns of DskGAL4 neurons in female.
(A–D) Characterization of individual DskGAL4 neurons using MultiColor FlpOut (MCFO) method in females (A). Eight DSK-expressing neurons were classified into three neuronal cell types (B–D). Scale bars represent 50 μm.
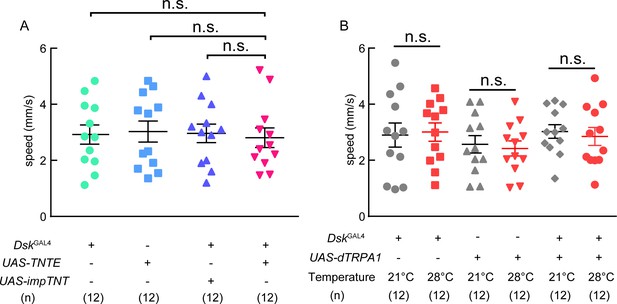
Locomotion behavior of TNT and trpA1 experiments.
(A) The locomotive speed of TNT experiment. (B) The locomotive speed of trpA1 experiment. Inactivation or activation of DskGAL4 neurons could not affect locomotion behavior. ns. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
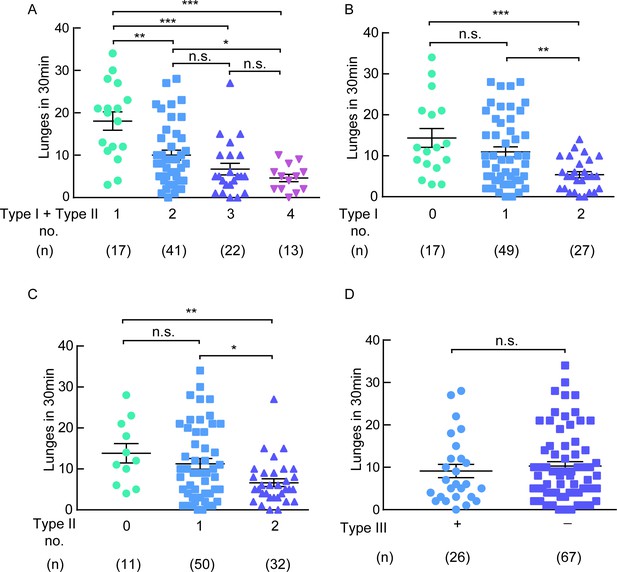
The type I and type II neurons are necessary for aggression.
(A) A negative correlation between the number of type I plus type II neurons inhibited and the lunge numbers of individual flies. (B) A negative correlation between the number of type I neurons inhibited and the lunge numbers of individual flies. (C) A negative correlation between the number of type II neurons inhibited and the lunge numbers of individual flies. (D) Lunge frequency in males with type III neurons silenced was not significantly different from that in males without type III neurons silenced. The flies of hs-FLP; UAS > stop > Kir2.1eGFP/DskGAL4 were heat-shocked for 90 min at 37°C during the mid- to late-larval stage. *p<0.05, **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
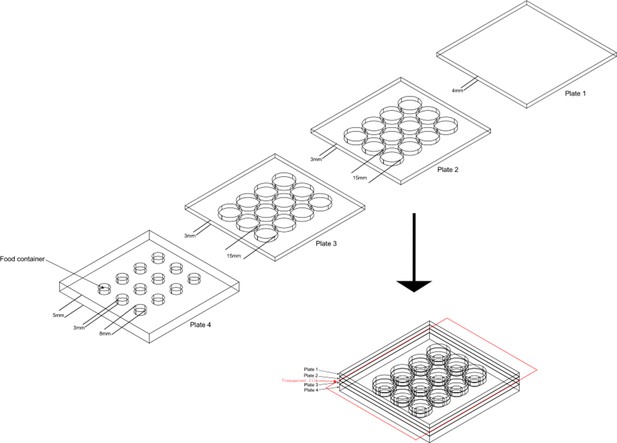
The design of aggression chamber.
The aggression chamber consists of four acrylic plates. The bottom plate (Plate 4) contains 12 wells for food patches (diameter: 8 mm; depth: 3 mm). The lower (Plate 3) and upper plates (Plate 2) have 12 cylindrical arenas (diameter: 15 mm; height of each plates: 3 mm). A transparent film is sandwiched by Plate two and Plate three to separate the two flies and removal of the transparent film allows encounters of the two flies.
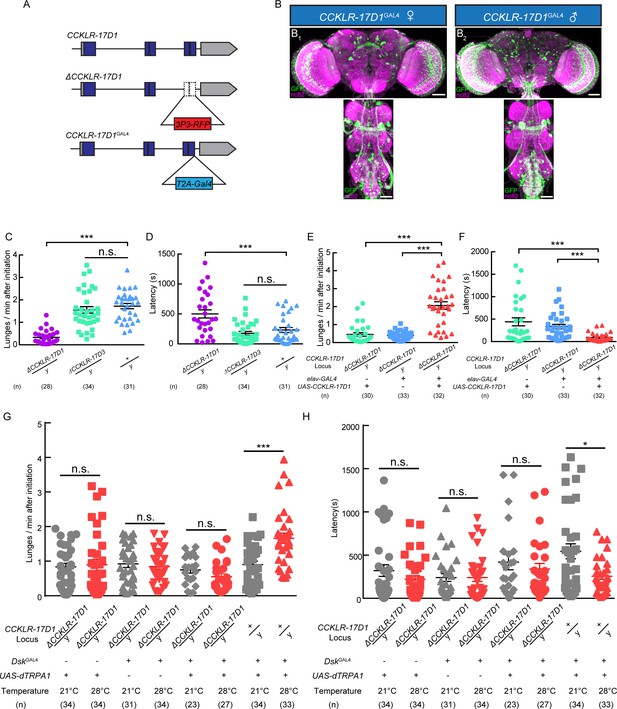
Dsk Receptor CCKLR-17D1 Is Necessary for Male-Male Aggression.
(A) Schematic view of CCKLR-17D1 gene locus and generation of ΔCCKLR-17D1 and CCKLR-17D1GAL4 Knock-in lines. The last two exons of CCKLR-17D1 gene were replaced by 3P3-RFP to generate ΔCCKLR-17D1. To generate CCKLR-17D1GAL4 Knock-in, T2A-GAL4 was fused to the end of ORF. (B) Anatomical analysis of CCKLR-17D1 expressing neurons revealed by CCKLR-17D1GAL4 driven UAS-mCD8:GFP expression. (B1) Adult female CNS stained with anti-GFP antibody (green) and nc82 antibody (magenta). (B2) Adult male CNS stained with anti-GFP antibody (green) and nc82 antibody (magenta). Scale bars represent 50 μm. (C) Number of lunges per minute after initiation of the indicated genotypes. (D) Fighting latency of the indicated genotypes. (E and F) Number of lunges per minute after initiation (E) and fighting latency (F) in ΔCCKLR-17D1 mutants rescued by elav-GAL4 driven UAS-CCKLR-17D1 expression. (G and H) Number of lunges per minute after initiation (G) and fighting latency (H) during thermogenetic activation of DskGAL4 neurons in the ΔCCKLR-17D1 mutant background. Thermogenetic activation of DskGAL4 neurons could not enhance aggression level in the ΔCCKLR-17D1 mutant background. *p<0.05, **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
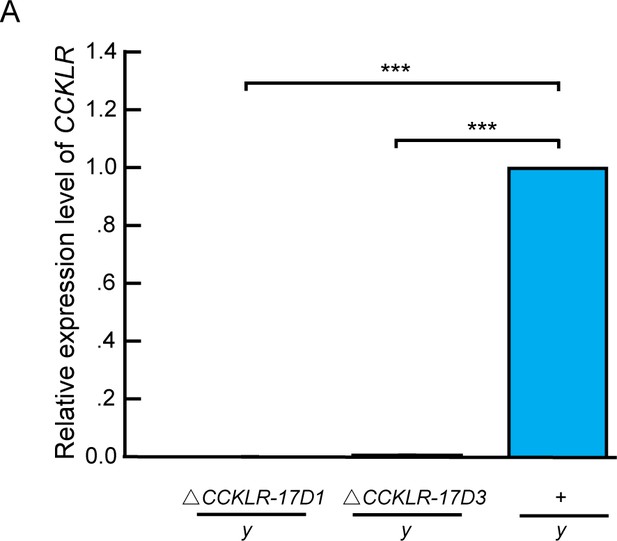
Molecular validation of mutants for Dsk receptors.
(A) Quantification of CCKLR-17D1 and CCKLR-17D3 mRNA by qRT-PCR in the CCKLR mutants and wildtypes. CCKLR-17D1 and CCKLR-17D3 transcripts were not detected in ΔCCKLR-17D1/y and ΔCCKLR-17D3/y mutants. Triplicate assays per cDNA sample were carried out independently, the results were obtained from three independent RNA samples. ***p<0.001 (one-way ANOVA and post-hoc Student’s T-test). Error bars indicate SEM.
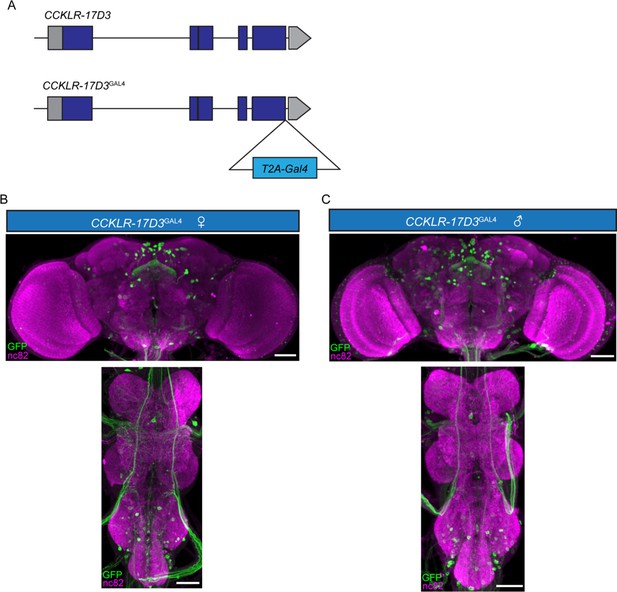
Expression patterns of CCKLR-17D3GAL4.
(A) Generation of CCKLR-17D3GAL4 Knock-in line. GAL4 was fused to the end of open reading frame (ORF) of CCKLR-17D3 with a T2A peptide linker. (B–C) Projection patterns of CCKLR-17D3GAL4 neurons in the central nervous system of female (B) and male (C) revealed by CCKLR-17D3GAL4 driving UAS-mCD8:GFP. Female and male central nervous system were immunostained with anti-GFP antibody (green) and the neuropil marker nc82 (magenta). Scale bars represent 50 μm.
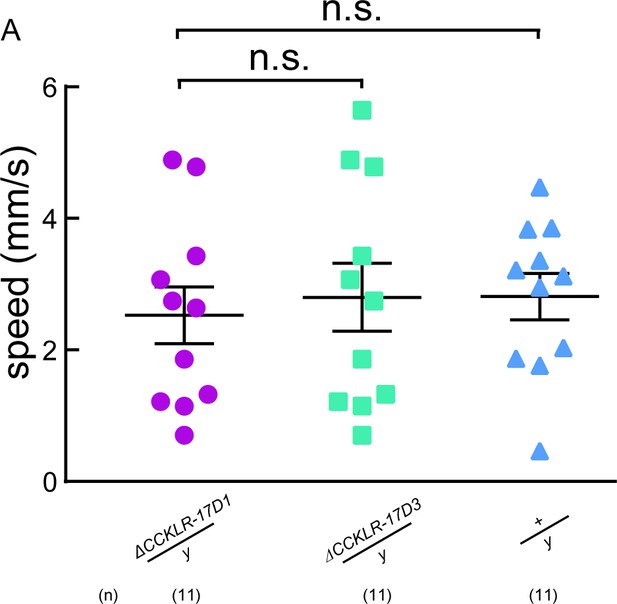
ΔCCKLR-17D1 does not affect locomotion behavior.
(A) The locomotive speed of ΔCCKLR-17D1 and ΔCCKLR-17D3 mutants. ns. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
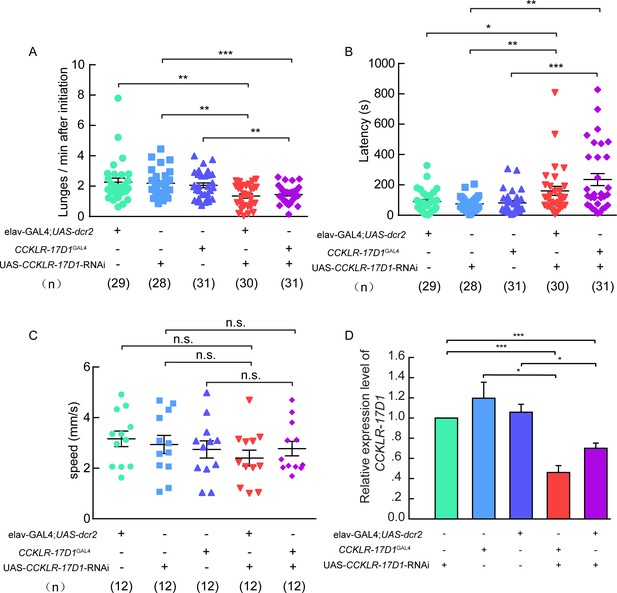
Aggression, locomotion behavior of CCKLR-17D1 RNAi in male flies.
(A–B) RNAi knock-down of CCKLR-17D1 decreases inter-male aggression. (A) Number of lunges per minute after initiation. (B) Fighting latency. elav-GAL4; UAS-dcr2 or CCKLR-17D1GAL4 drives CCKLR-17D1 RNAi to knock down the expression level of CCKLR-17D1. In both cases, RNAi knock-down of CCKLR-17D1 significantly reduces aggression. (C) The locomotion of CCKLR-17D RNAi no significant locomotion activity changes were observed. (D) Quantification of CCKLR-17D1 mRNA by qRT-PCR in the CCKLR-17D1 knock-down flies. Error bars indicate SEM. *p<0.05, **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests or post-hoc Student’s T-test).
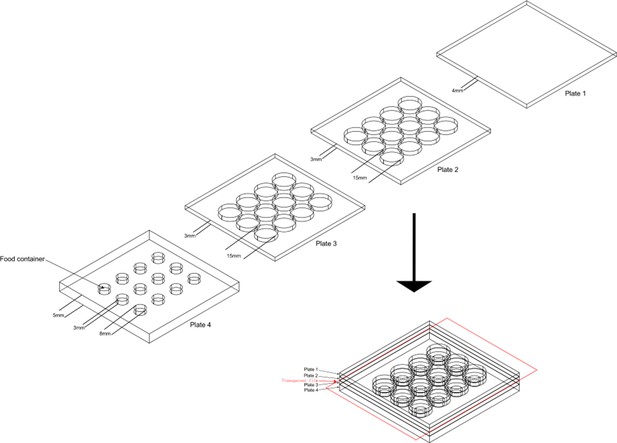
The design of aggression chamber.
The aggression chamber consists of four acrylic plates. The bottom plate (Plate 4) contains 12 wells for food patches (diameter: 8 mm; depth: 3 mm). The lower (Plate 3) and upper plates (Plate 2) have 12 cylindrical arenas (diameter: 15 mm; height of each plates: 3 mm). A transparent film is sandwiched by Plate two and Plate three to separate the two flies and removal of the transparent film allows encounters of the two flies.
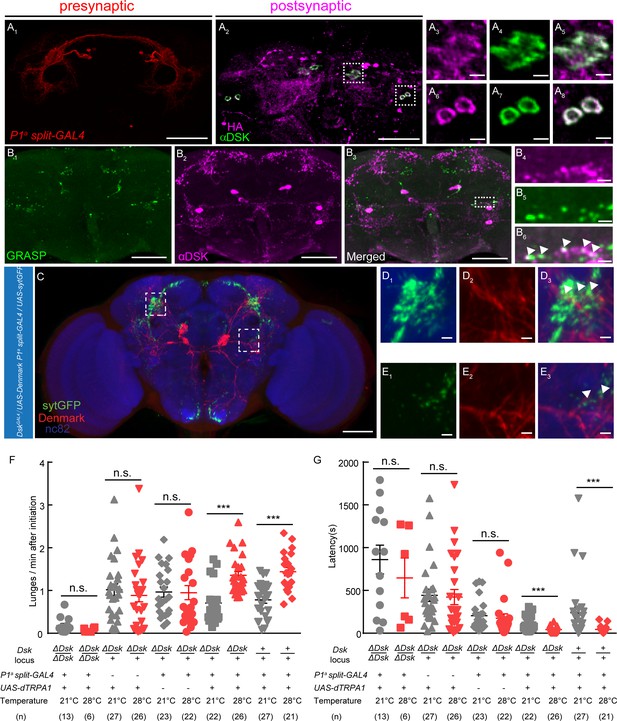
DSK Functions Downstream of a subset of P1 Neurons to Modulate Fighting Behavior.
(A) Transsynaptic labeling by trans-Tango method identifies DSK neurons as postsynaptic partners of a subset of P1 neurons. Expression of the trans-Tango ligand in a subset of P1 neurons (red) (A1) induces postsynaptic signals (anti-HA, magenta) (A2) in the central brain. Green, anti-DSK antibody staining (A2). (A3–A5) and (A6–A8) are enlargements of dashed boxes in (A2). Genotypes of a subset of P1 neurons (P1a split-GAL4): +/y; R15A01.AD/R15A01.AD; R71G01-GAL4.DBD/R71G01-GAL4.DBD. (B) GRASP signals (B1) reveal synaptic connections between R71G01-LexA labeled P1 Neurons and DSK neurons. spGFP1–10 is expressed by DskGal4 drivers; spGFP11 is driven by the R71G01-LexA. Magenta, anti-DSK antibody. (B4–B6) are enlargements of the dashed box in (B3). White arrowheads point to areas in which GRASP signal co-localized with synaptic boutons revealed by anti-DSK antibody staining (B6). Scale bars represent 50 μm in (A1–A2, B1–B3). Scale bars represent 5 μm (A3–A8, B4–B6). (C) Dendrites of DSK neurons revealed by DskGAL4 driven UAS-Denmark expression (red). Axons of a subset of P1 neurons revealed by P1a split-GAL4 driven UAS-sytGFP expression (green). nc82 antibody (blue) was used to label neuropils. Scale bars represent 50 μm. (D–E) Enlargements of dashed boxes in (C). White arrowheads point to areas in which a subset of P1 axons and DSK dendrites overlap. Scale bars represent 5 μm. (F and G) Number of lunges per minute after initiation (F) and fighting latency (G) during thermogenetic activation of a subset of P1 neurons in the ΔDsk mutant background. The aggression-promoting effect of activating a subset of P1 neurons is suppressed by the ΔDsk mutants. ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
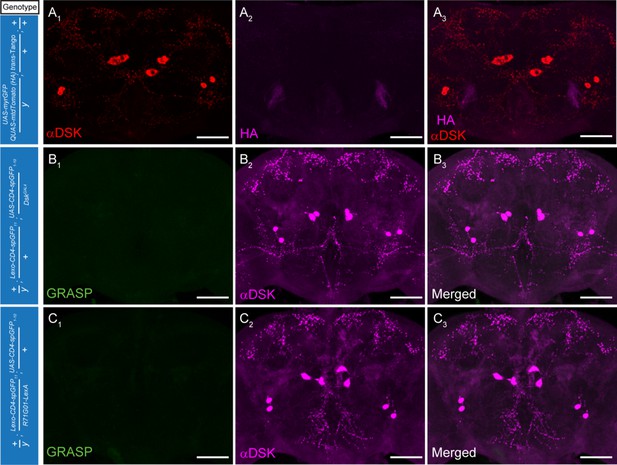
Controls for the trans-Tango and GRASP experiments.
(A) Negative control demonstrates lack of postsynaptic signals (anti-HA, magenta) (A2) for the trans-Tango experiment. Red, anti-DSK antibody staining (A1). (B–C) Negative controls for the GRASP experiments. Magenta, anti-DSK antibody (B2, C2). Scale bars represent 50 μm.
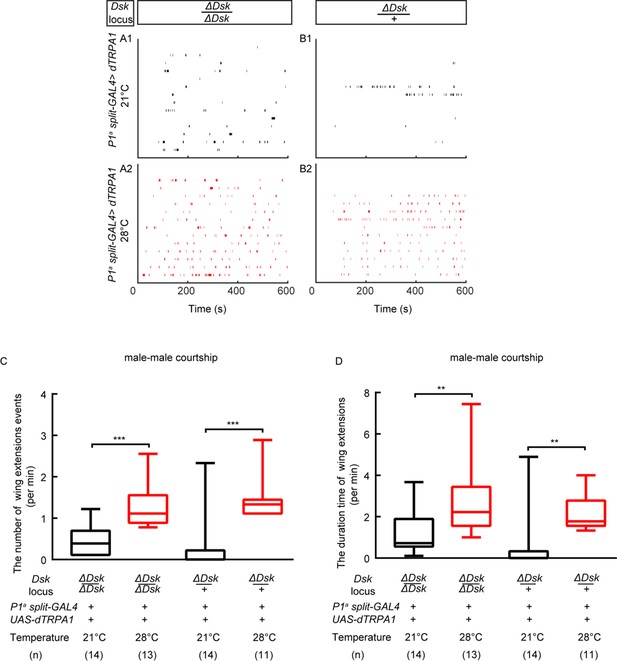
Activation of a subset of P1 neurons could promote courtship in the ΔDsk mutant background.
(A–B) Raster plots of unilateral wing extensions during 10 min. (C–D) The number (C) and total duration (D) of unilateral wing extensions in (A–B) during thermogenetic activation of a subset of P1 neurons in the ΔDsk mutant background (black: 21°C, red: 28°C). a subset of P1 neurons can promote courtship behavior in ΔDsk mutant background. **p<0.01, ***p<0.001 (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
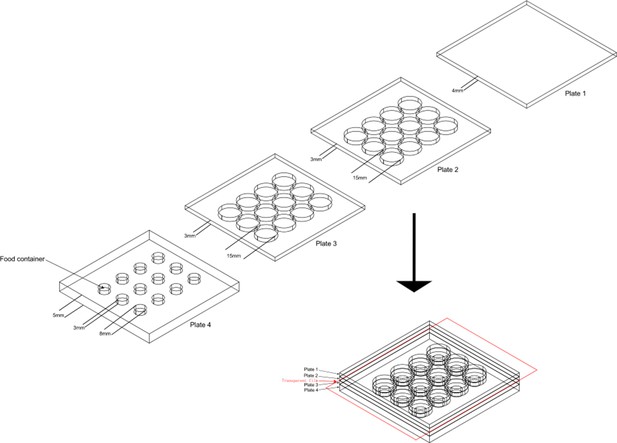
The design of aggression chamber.
The aggression chamber consists of four acrylic plates. The bottom plate (Plate 4) contains 12 wells for food patches (diameter: 8 mm; depth: 3 mm). The lower (Plate 3) and upper plates (Plate 2) have 12 cylindrical arenas (diameter: 15 mm; height of each plates: 3 mm). A transparent film is sandwiched by Plate two and Plate three to separate the two flies and removal of the transparent film allows encounters of the two flies.
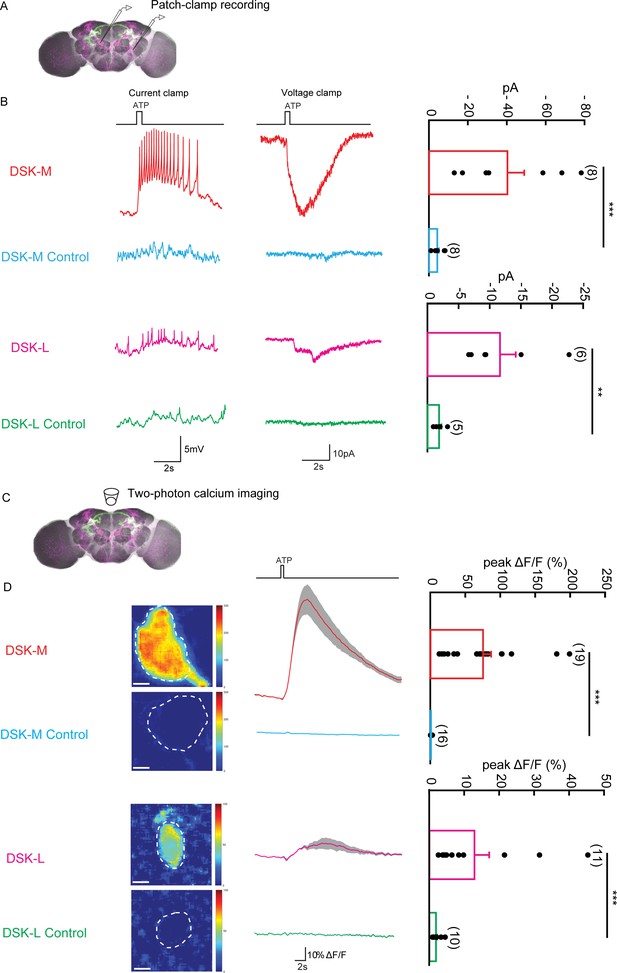
The Functional Connectivity Between DSK Neurons and R71G01-LexA labeled P1 Neurons.
(A) Illustration of patch-clamp recording on DSK neurons (magenta). ATP-gated ion channel P2X2 was expressed in R71G01-LexA labeled P1 neurons for chemogenetic activation of P1 neurons. Genotype: +/y; R71G01-LexA/+; DskGAL4/LexAop-P2 × 2,UAS-GCaMP6m. (B) The electrical responses of medial DSK neurons (DSK-M) and lateral DSK neurons (DSK-L) to ATP activation of R71G01-LexA labeled P1 neurons. ATP: 2.5 mM. Left: spikes firing (current clamp). Middle: current responses (voltage clamp). Right: quantification of absolute current responses. n = 8 from six flies for DSK-M, 8 from five flies for DSK-L, 6 from four flies for DSK-M control, 4 from four flies for DSK-L control. The electrical responses of medial DSK-M and DSK-L control to ATP activation. The Genotype of the control: +/y; +/+; DskGAL4/LexAop-P2 × 2,UAS-GCaMP6m. (C) Illustration of two-photon calcium imaging in DSK neurons. (D) Left: Calcium imaging of GCaMP6m in the cell bodies of medial DSK neurons (DSK-M) and lateral DSK neurons (DSK-L) with P2X2-expressing R71G01-LexA labeled P1 neurons activated by ATP. ATP: 2.5 mM. Middle: calcium responses of DSK neurons to ATP stimulation. Gray envelopes represent SEM. Right: quantification of peak ΔF/F in the DSK-M and DSK-L neurons. Scale bars represent 5 μm. n = 19 from four flies for DSK-M, 16 from four flies for DSK-L, 11 from four flies for DSK-M control, 10 from four flies for DSK-L control. Genotypes: +/y; R71G01-LexA/+; DskGAL4/LexAop-P2 × 2, UAS-GCaMP6m for DSK-M and DSK-L; +/y; +/+; DskGAL4/LexAop-P2 × 2, UAS-GCaMP6m for DSK-M control and DSK-L control. ***p<0.001 (Mann-Whitney U tests).
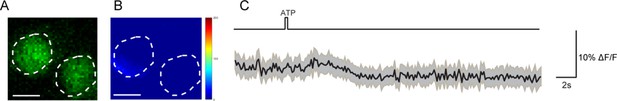
R71G01-LexA labeled P1 neurons do not respond to activation of DSK neurons.
(A–B) Calcium imaging of GCaMP6m in the cell bodies of R71G01-LexA labeled P1 neurons with P2X2-expressing DSK neurons activated by ATP. ATP: 2.5 mM. White dashed lines indicate ROI. Scale bars represent 5 μm. (C) Averaged traces of ΔF/F. The black lines represent the means, and the light gray envelopes represent SEM. n = 26 trials from 9 flies. Genotype: +/y; R71G01-LexA/+; DskGAL4/UAS-P2 × 2, LexAOP-GCaMP6m.
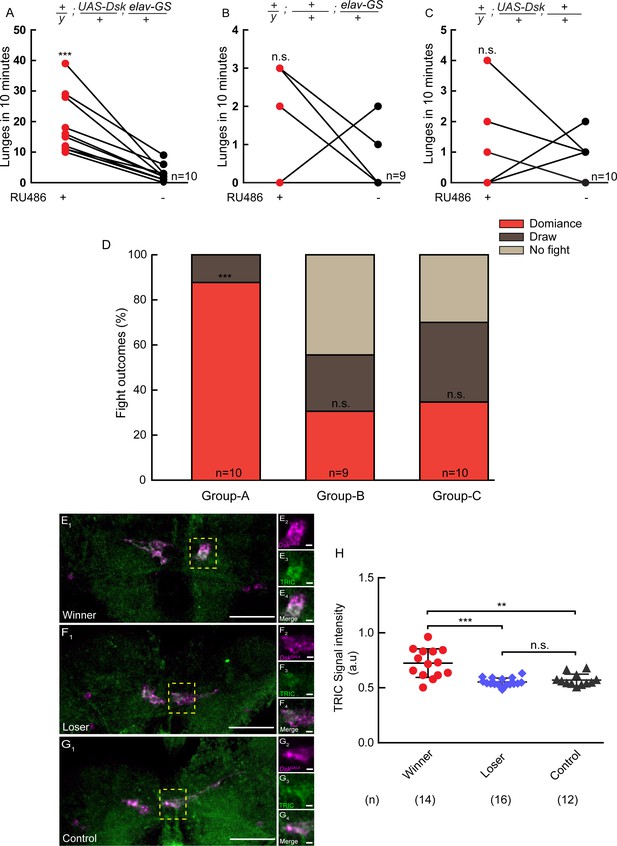
Conditional overexpression of DSK promotes winner effect and Winner Show Increased Calcium Activity of DSK-M Neurons.
(A–C) Conditionally overexpression of Dsk increases number of lunges compared to controls. Pairs of flies of the indicated genotypes were introduced into one chamber, one of which overexpressed DSK induced by RU486 while the other did not. (D) Conditionally overexpression of Dsk promotes social dominance. Fight outcomes of the RU486-induced flies of the indicated genotypes in (A–C) were classified into ‘dominance’, ‘draw’ and ‘no fight’. (E–G) Calcium activity of DSK-M neurons in winner (E), loser (F) and control (G) brains detected by the TRIC method. TRIC signal (green) in DSK-M neurons is increased in the winner brain (E3). (E2–E4), (F2–F4), (G2–G4) are within the dashed boxes in (E1), (F1) and (G1). Scale bars represent 50 μm. Genotype: UAS-IVS-mCD8::RFP, LexAop2-mCD8::GFP/y; nSyb-MKII::nlsLexADBDo/+; DskGAL4/UAS-p65AD::CaM. (H) Quantification of TRIC signals in the DSK-M neurons of winner, loser and control brains. **p<0.01, ***p<0.001, n.s. indicates no significant difference (Kruskal-Wallis and post-hoc Mann-Whitney U tests).
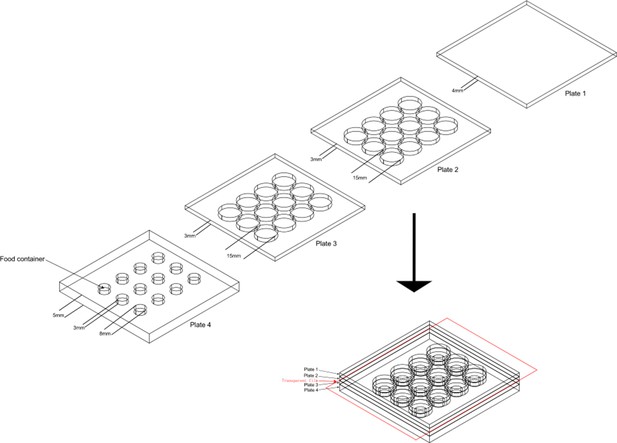
The design of aggression chamber.
The aggression chamber consists of four acrylic plates. The bottom plate (Plate 4) contains 12 wells for food patches (diameter: 8 mm; depth: 3 mm). The lower (Plate 3) and upper plates (Plate 2) have 12 cylindrical arenas (diameter: 15 mm; height of each plates: 3 mm). A transparent film is sandwiched by Plate two and Plate three to separate the two flies and removal of the transparent film allows encounters of the two flies.
Videos
Left, female aggression of ΔDsk mutants.
Right, female aggression of wildtypes. Female ΔDsk mutants show fewer aggressive encounters than wildtypes.
Thermogenetic activation of DskGAL4 neurons with UAS-dTRPA1 promotes inter-male aggression.
Chemogenetic activation of P1 neurons by ATP elicits calcium responses from DskGAL4 neurons.
Additional files
-
Supplementary file 1
Key resources table.
- https://cdn.elifesciences.org/articles/54229/elife-54229-supp1-v1.docx
-
Supplementary file 2
The number of labeled cells of mosaic flies.
- https://cdn.elifesciences.org/articles/54229/elife-54229-supp2-v1.xls
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/54229/elife-54229-transrepform-v1.docx





