Hippocampal low-frequency stimulation prevents seizure generation in a mouse model of mesial temporal lobe epilepsy
Figures
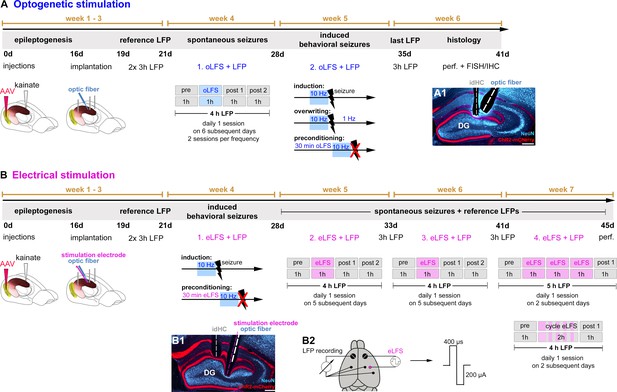
Experimental design for in vivo LFS.
Animals received intrahippocampal KA and a channelrhodopsin 2 (ChR2)-carrying virus into the entorhinal cortex to trigger epileptogenesis and the expression of ChR2-mCherry in entorhinal afferent fibers. After 16 days post-injection, recording electrodes, and (A) an optic fiber or (B) an optic fiber combined with a stimulation electrode were implanted. Following recovery from implantations, reference LFPs were recorded on 2 consecutive days for 3 hr each. (A) In the first group of experiments, the effect of oLFS on spontaneously occurring epileptiform activity was tested (week 4) in 4-hr recording sessions. A session consisted of 1 hr of ‘pre’ stimulus recording, followed by 1 hr of ‘oLFS’ pulses and 2 hr of post-stimulus recordings (‘post 1’ and ‘post 2’). Three different oLFS frequencies (1, 0.5, or 0.2 Hz) were applied on successive days in each animal (two sessions per animal). Next, generalized seizures were induced by optogenetic (10 Hz) stimulation. To test the effects of oLFS on seizures, oLFS (1 and 0.5 Hz) was applied either immediately after (overwriting) or before the pro-convulsive (10 Hz) stimulation (preconditioning) (week 5). (B) The second group of in vivo experiments assessed the effects of eLFS on epileptiform activity. First, we tested the effects of eLFS on optogenetically induced seizures (week 4, preconditioning, see above). In weeks 5 and 6, animals were stimulated daily for 1 hr (1 Hz, eLFS) following the same ‘pre’, ‘eLFS’,’ post 1’, post 2’ paradigm, as described above. In week 7, animals were stimulated twice (on 2 different days) over 3 hr continuously and twice in an on-off ‘cycle’ paradigm: after initial 30 min eLFS, eLFS stimulation was turned off for 10 min and then turned on again for 10 min. This was repeated four times, followed by another hour LFP recording (‘post 1’). (A1, B1) All animals were perfused after the last LFP recording and brain sections were processed for immunohistological procedures. (B2) Implantation scheme for eLFS. DG, dentate gyrus; FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; perf., perfusion.
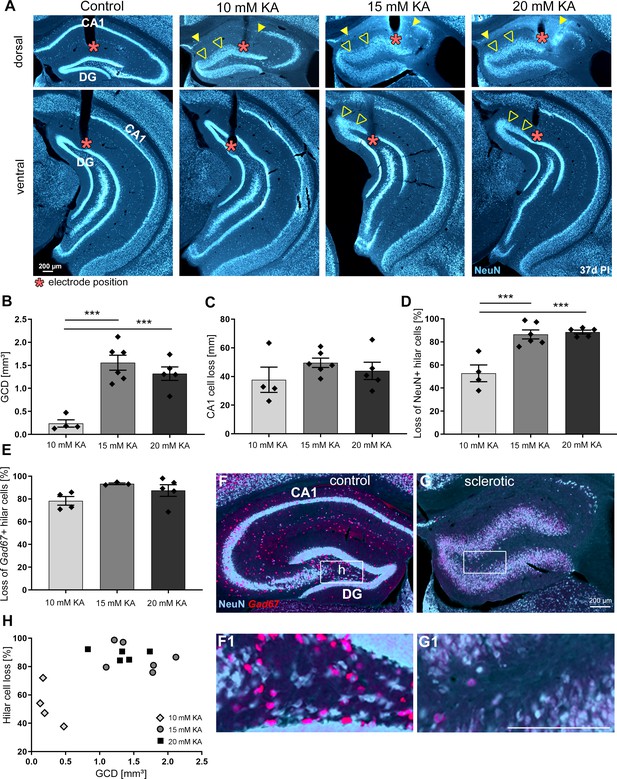
The degree of hippocampal sclerosis depends on KA concentration.
(A) Representative NeuN-labeled sections of dorsal and ventral hippocampal regions treated with different KA concentrations at 37 days post-injection (PI). In each section, the electrode position is marked with a red asterisk. Epileptic hippocampi show GCD in the dentate gyrus (open arrowheads) and cell loss in CA1 (the region between filled arrowheads). Comparing KA concentration groups with respect to different markers of hippocampal sclerosis by quantification of (B) GCL volume of dispersed regions (i.e. GCD), (C) total length of cell loss in CA1, (D) % loss of NeuN+ hilar cells, and (E) loss of Gad67+ hilar interneurons in the sclerotic vs. non-sclerotic hippocampus (i.e. (G, G1) ipsilateral vs (F, F1) contralateral). One-way ANOVA; Tukey’s multiple comparison test; *p<0.05, **p<0.01 and ***p<0.001. All values are given as mean ± standard error of the mean (SEM). (H) Animals injected with higher KA concentrations (15 and 20 mM KA) display stronger hilar cell loss along with a higher degree of GCD. Scale bars 200 µm.
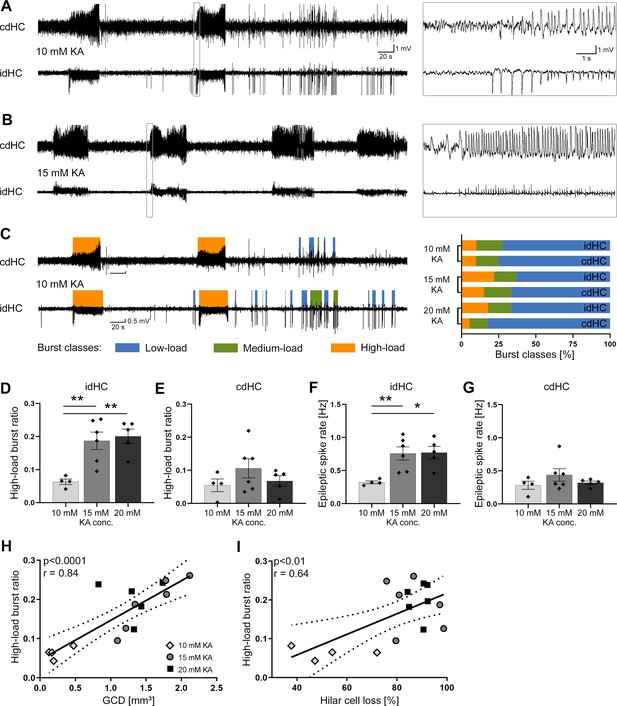
Variable severity of epileptiform activity elicited by different KA concentrations.
(A, B) Representative LFP traces for the 10 mM and 15 mM KA group (20 mM not shown) showing spontaneous epileptiform activity in the cdHC and idHC. (C) Automatic classification of epileptiform activity into low-load (blue), medium-load (green) and high-load bursts (orange). We used a custom algorithm as illustrated in Figure 3—figure supplement 1. In the 20 mM KA group, the percentage of high-load bursts in the cdHC is decreased, whereas for the 10 mM and 15 mM KA group the percentage of the burst classes is similar in both hippocampi. (D–G) Injections of 15 and 20 mM KA lead to an increased high-load burst ratio and a higher epileptic spike rate in the idHC but not in the cdHC. All values are given as mean ± SEM. Source data is provided in Figure 3—source data 1. (I, J) The high-load burst ratio is positively correlated with GCD and hilar NeuN+ cell loss ((I): p<0.0001, two-tailed; Pearson’s r = 0.84; (J): p<0.01, two-tailed; Pearson’s r = 0.64).
-
Figure 3—source data 1
Variable severity of epileptiform activity elicited by different KA concentrations.
(a) The occurrence of burst classes elicited by different KA concentrations. In the ipsilateral hippocampus, animals treated with high (15 and 20 mM) KA concentrations exhibit more high-load and fewer low-load events than animals treated with 10 mM KA. Compared to the ipsilateral hippocampus, animals treated with high (especially 20 mM KA) have less high-load seizures on the contralateral side. (b) Mean high-load burst ratio and epileptic spike rate of the different KA groups. The mean ratio of time spent in high-load bursts (mean high-load burst ratio) and epileptic spike rate were significantly lower in the idHC 10 mM KA group compared to the 15 and 20 mM KA group. The mean was calculated from the reference and ‘pre’ recording sessions. Values are given as mean ± SEM.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig3-data1-v2.docx
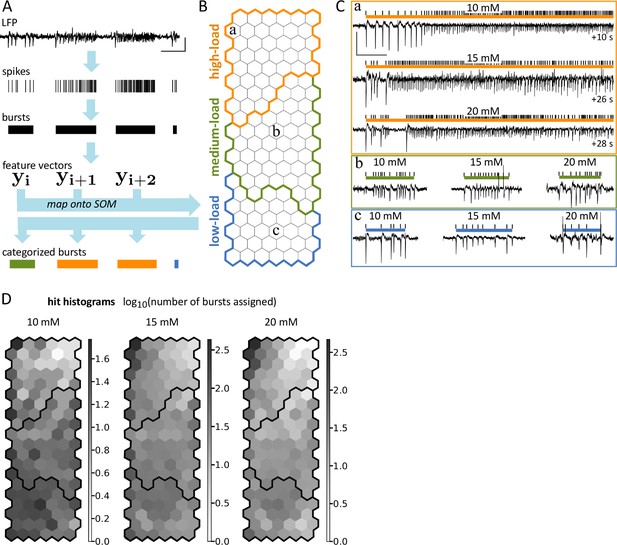
Analysis of epileptiform activity and comparison of different KA concentrations.
(A) The workflow of analysis. Spikes were detected in LFP traces and spikes closer than 2.5 s were grouped into the same burst. For each burst, a feature vector (yi) was extracted and mapped onto the SOM obtained from a reference dataset (see panel B). Each yiwas matched to the SOM’s node closest in feature space. Each burst inherited its category from this node. (B) SOM developed by Heining et al., 2019 was used to categorize bursts according to spike load. Nodes of the SOM are visualized as hexagons. (C) Exemplary bursts (ticks: detected spikes, colored bar: burst extent) matched to the hexagons marked a – c in B. For each hexagon, a representative burst from a mouse injected with 10, 15 or 20 mM KA is shown. Examples were truncated for visualization. They continued for the time indicated (+...s) with a similar activity pattern. (D) The normalized number of bursts (log10) matched to each node for mice injected with 10, 15 or 20 mM KA. We normalized by dividing the number of bursts by the total recording time analyzed for the respective dataset. Data shown in D is pooled across animals from all EEG and ‘pre’ recording sessions (10 mM: n = 35, 15 mM: n = 80, 20mM: n=58 sessions). Scale bars: 5 s; 4 mV.
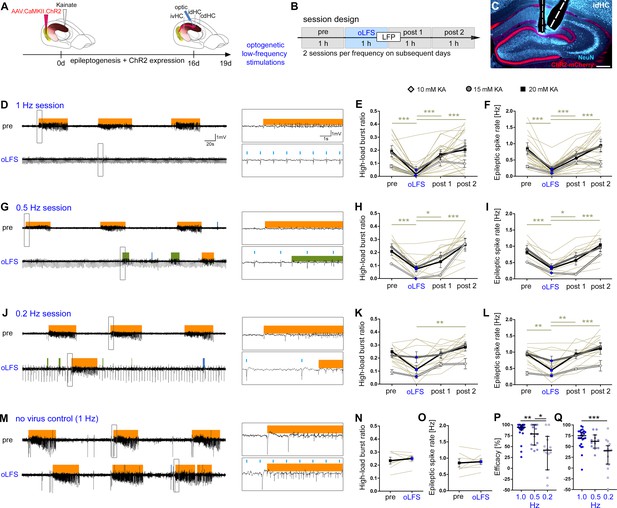
oLFS of entorhinal afferents interferes with spontaneous epileptiform activity in a frequency-dependent manner.
(A–C) Experimental design. We targeted ChR2-mCherry expression (C, red) to excitatory neurons in the medial entorhinal cortex using viral vectors. ChR2-mCherry expression pattern for all mice included in the study are shown in Figure 4—figure supplement 2. The electrode positions are shown in Figure 4—figure supplement 3. (B, C) We locally stimulated entorhinal afferents in the sclerotic idHC for 1 hr per day, twice at each frequency applying only one frequency per session (1, 0.5, or 0.2 Hz). (D, G, J, M) Representative LFP traces (15 mM KA, idHC electrode) for the ‘pre’ and ‘oLFS’ sub-sessions (1, 0.5, 0.2 Hz and no-virus control, 1 Hz) are shown. Automatic detection of epileptiform activity is marked for low-load (blue), medium-load (green), and high-load bursts (orange). (D) Photostimulation at 1 Hz effectively decreases spontaneous epileptiform activity in the idHC. (E, F) Automatic quantification of epileptiform activity shows that oLFS reduces the high-load burst ratio as well as the epileptic spike rate in all animals independently of the KA concentration (10 mM: light gray; 15 mM: dark gray; 20 mM: black) followed by a return to pre-stimulation levels within 2 hr (‘post 1’ and ‘post 2’). (G, J) oLFS with (H, I) 0.5 Hz or (K, L) 0.2 Hz has a weaker antiepileptic effect during stimulation. Single sessions (olive-green) were used to calculate the one-way ANOVA; Tukey’s multiple comparison test (all KA concentrations pooled); *p<0.05, **p<0.01, and ***p<0.001. All mice were video recorded and the running behavior was analyzed during each session as shown in Figure 4—figure supplement 5 with the source data provided in Figure 4—figure supplement 5—source data 1. (M–O) 1 Hz stimulation does not have any effect on epileptiform activity in no-virus controls (20 mM KA). All values are given as mean ± SEM. Analysis of the cdHC is shown in Figure 4—figure supplement 1 with the source data provided in Figure 4—figure supplement 1—source data 1. We noticed that local oLFS in the idHC leads to a delayed cellular responses in the cdHC as shown in Figure 4—figure supplement 4. (P, Q) Comparison of the stimulation frequencies in terms of suppression efficacy using the high-load burst ratio and epileptic spike rate (1-(‘oLFS’/‘pre’)*100)(one-way ANOVA; Dunns’s multiple comparison test (all KA concentrations pooled), mean ±95% CI; *p<0.05, **p<0.01, ***p<0.001). Source data is provided in Figure 4—source data 1.
-
Figure 4—source data 1
oLFS effect on ipsilateral epileptiform activity.
Burst ratios and epileptic spike rates of each sub-session are listed for the three KA concentrations (10, 15, 20 mM) for each oLFS frequency (1, 0.5, 0.2 Hz) and no-virus controls (Ctr.). (a, b) The bust ratio and epileptic spike rate are reduced in all KA groups during oLFS but recover within the first hour of post-recording (post 1). This effect is also observed after 0.5 and 0.2 Hz oLFS but less pronounced. No change is observed in no-virus control animals. (c, d) Summary for all KA groups merged. (e) Median (±95% CI) of the suppression efficacy for the three applied frequencies. oLFS at 1 Hz seems most effective for the suppression of epileptiform activity. Values are given as mean ± SEM.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig4-data1-v2.docx
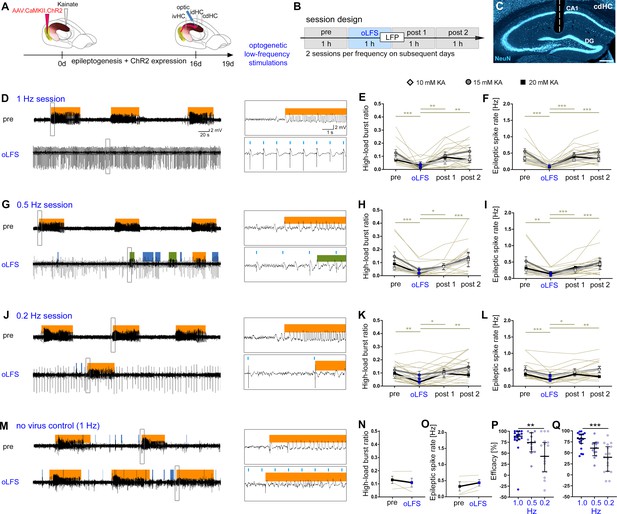
oLFS of entorhinal afferents in the idHC interferes with spontaneous epileptiform activity in the cdHC.
(A–C) Experimental design. We optogenetically stimulated ChR2-mCherry expressing entorhinal afferents in the sclerotic idHC for 1 hr/day, twice at each frequency applying only one frequency per session (1, 0.5, or 0.2 Hz) and recorded LFP in both hippocampi. (D, G, J, M) Representative LFP traces (15 mM KA, cdHC electrode) for the ‘pre’ and ‘oLFS’ sub-sessions (1, 0.5, 0.2 Hz and no virus control, 1 Hz) are shown. Automatic detection of epileptiform activity is marked blue for low-, green for medium- and orange for high-load bursts. (D) Photostimulation at 1 Hz effectively decreases spontaneous epileptiform activity in the cdHC. (E, F) Automatic quantification of epileptiform activity shows that oLFS reduces the burst ratio as well as the epileptic spike rate in all animals independently of the KA concentration (10 mM: light gray; 15 mM: dark gray; 20 mM: black) followed by a return to pre-stimulation levels within 2 hr (‘post 1’ and ‘post 2’). (G, J) oLFS with 0.5 Hz or 0.2 Hz has a weaker antiepileptic effect (H, I, and K, L). Single sessions (olive-green) were used to calculate the one-way ANOVA; Tukey’s multiple comparison test (all KA concentrations merged); *p<0.05, **p<0.01, and ***p<0.001. (M–O) 1 Hz stimulation does not have any effect in no-virus controls (20 mM KA); Paired t-test. All values are given as mean ± SEM. (P, Q) Comparison of the stimulation frequencies in terms of suppression efficacy using the high-load burst ratio and epileptic spike rate (1-(‘oLFS’/‘pre’)*100) (one-way ANOVA; Dunns’s multiple comparison test (all KA concentrations pooled), mean ± 95% CI; **p<0.01, ***p<0.001). Source data is provided in Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
oLFS effect on contralateral epileptiform activity.
Burst ratios and epileptic spike rates of each sub-session are listed for the three KA concentrations (10, 15, 20 mM) for each oLFS frequency (1, 0.5, 0.2 Hz) and no-virus controls (Ctr.). (a, b) The bust ratio and epileptic spike rate are reduced in all KA groups during oLFS but recover within the first hour of post-recording (post 1). This effect is also observed after 0.5 and 0.2 Hz oLFS but less pronounced. No change is observed in no-virus control animals. (c, d) Summary for all KA groups merged. oLFS at 1 Hz seems most effective for the suppression of ictal and interictal activity in the contralateral dorsal hippocampus. (e) Median (±95% CI) of the suppression efficacy for the three applied frequencies. oLFS at 1 Hz seems most effective for the suppression of epileptiform activity. Values are given as mean ± SEM.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig4-figsupp1-data1-v2.docx
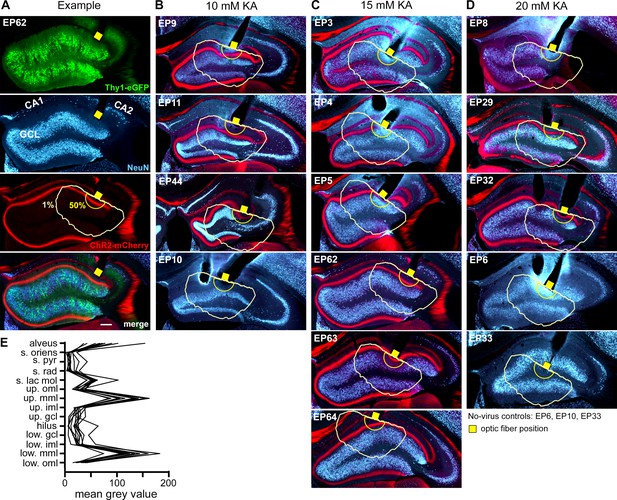
ChR2-mCherry expression pattern for all oLFS-stimulated mice.
(A–D) After oLFS experiments, Thy1-eGFP mice were perfused and immunostained (NeuN, blue) for the identification of hippocampal sclerosis and ChR2-mCherry (red) expression. Representative pictures at the position of the optic fiber (yellow square) are shown for all mice. Following injection of the viral construct into the medial entorhinal cortex ChR2-mCherry is expressed in the medial perforant path terminating in the mid-molecular layer of the dentate gyrus (in the upper (up.) and lower (low.) blade). Additionally, the temporoammonic path is weakly labeled. Taking into account the restricted penetration depth of 473 nm light in brain tissue and an output of 150 mW/mm² at the tip of our optic fiber, we estimated that in our case the radius of light emission with sufficient power to activate ChR2 (yellow 1% line) does not exceed 500 µm. (E) Measurements of the mean gray value across the dorsoventral axis confirm the expression pattern of ChR2-mCherry in entorhinal afferents of the sclerotic hippocampus. s., stratum; lac., lacunosum; pyr., pyramidale; rad., radiatum; mol., moleculare; oml., outer molecular layer; mml., mid molecular layer; iml., inner molecular layer; gcl., granule cell layer. Scale bar 200 µm.
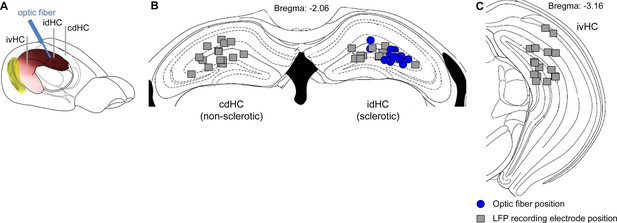
Positions of implanted electrodes and optic fibers for all animals included in the study.
(A) Implantation scheme for oLFS. Electrode positions for all animals (n = 16) were histologically validated and located at the three different recording sites: cdHC, contralateral dorsal hippocampus; idHC, ipsilateral dorsal hippocampus and ivHC, ipsilateral ventral hippocampus. The optic fiber was placed in a 30° angle at the idHC site. (B) Positions of the optic fiber (blue circle) and LFP electrodes (gray square) in the dorsal region. (C) Ipsilateral ventral electrode positions.
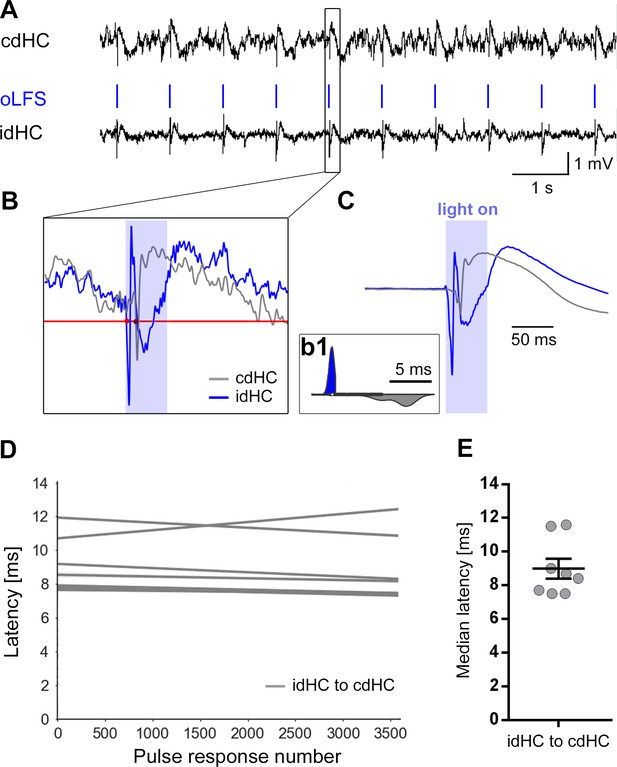
Local oLFS leads to delayed cellular responses in the cdHC.
(A) Representative LFP traces of the two dorsal electrodes (idHC and cdHC) during 1 Hz oLFS. Local stimulation of DGCs via entorhinal afferents in the idHC evokes population spikes also in the cdHC. (B) One representative example of a population spike. They occur first in the idHC (blue) then in the cdHC (gray). For identification of the spike time, each voltage curve of a pulse response was first filtered and z-scored as described in the Materials and methods. Then, a manually chosen threshold (red line) was used to extract the population spike onset (red dots) for each pulse response in both hippocampi and was kept for all animals. (b1) Distribution of population spike onsets for 3600 responses (one representative example of a 1 Hz oLFS sub-session) to visualize the delay from idHC to cdHC. (C) Mean of all population spikes of one 1 Hz oLFS sub-session. (D) Linear regression shows stable latencies from idHC to cdHC (n = 8 sessions from seven animals) over 1-hr oLFS. Individual latency data points are not shown for visualization. (E) Median latencies for each analyzed oLFS sub-session. Population spikes occur with a latency of 8–12 ms (mean ± SEM: 9 ± 0.6 ms) in the cdHC relative to the population spike onsets in the idHC. Calculations were performed in python 2.7 provided in Figure 4—figure supplement 4—source code 1.
-
Figure 4—figure supplement 4—source code 1
1 Hz oLFS response: idHC to cdHC delay calculation.
LFP data (smr files for idHC and cdHC channels) were imported, z-scored and filtered (4th order low pass Chebyshev filter type I with a cut-off frequency at 300 Hz). LFP-snippets of a defined time interval (−0.1 s, +0.2 s relative to stimulus onset) were used to identify the crossing of a previously identified threshold (this threshold was kept constant for all animals). The delay of the stimulation pulse response from idHC to cdHC was then calculated for each LFP-snippet.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig4-figsupp4-code1-v2.zip
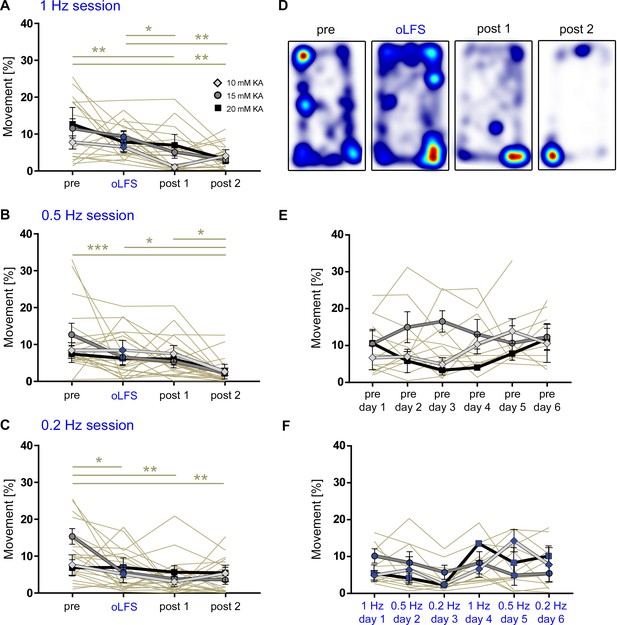
Movement analysis of chronically epileptic mice during LFP recordings.
Freely moving animals were video tracked. Running phases were automatically detected and quantified as time spent running (>4 cm/s). (A–C) Analysis of all sub-session reveals that the running behavior of all KA-treated mice is comparable and it declines gradually during the recording time of 4 hr. (D) Representative heat map of a 1 Hz session with the corresponding ‘pre’ and ‘post’ recordings. Warm colors indicate places of longer stays during each sub-session. (E, F) No statistically significant difference could be detected for the running behavior during oLFS experiments (6 days in a row) in the ‘pre’ or the ‘oLFS’ sessions. Hence, all experiments were performed under comparable conditions. Individual values are presented in Figure 4—figure supplement 5—source data 1. Single sessions (olive-green) are used to calculate the one-way ANOVA; Tukey’s multiple comparison test (all KA concentrations merged); *p<0.05, **p<0.01 and ***p<0.001. Values are given as mean ± SEM.
-
Figure 4—figure supplement 5—source data 1
oLFS effect on running behavior over time.
(a) Time spent running (>4 cm/s) of each sub-session is listed for the three KA concentrations (10, 15, 20 mM) for each oLFS frequency (1, 0.5, 0.2 Hz). The running behavior between KA groups is not significantly different. (b) During oLFS sessions, the movement is not impaired. The decrease in the movement over the 4 hr of LFP recording is most likely due to adaptation effects to the environment. Values are given as mean ± SEM.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig4-figsupp5-data1-v2.docx
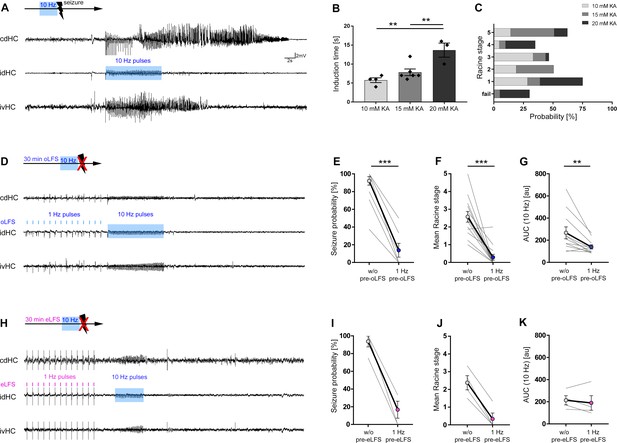
Preconditioning with LFS prevents optically evoked seizure generalization.
(A, D, H) Representative LFP traces at three recording sites (cdHC, idHC, and ipsilateral ventral hippocampus (ivHC)). A schematic of the respective stimulation procedure is shown above each cdHC trace. (A, B) Local 10 Hz photostimulation of entorhinal afferents reliably induces generalized seizures in all KA groups. Evoked, generalized seizures displayed electrographic features highly similar to spontaneous generalized seizures as shown in Figure 5—figure supplement 1. The time needed to induce a generalized seizure (induction time) is longer with increasing KA concentration. One-way ANOVA; Tukey’s multiple comparison test; **p<0.01. (C) Mice exhibit behavioral symptoms equivalent to RS stage 1–5, independently of the KA concentration. (D, E) 1 Hz oLFS as well as (H, I) eLFS for 30 min before the pro-convulsive stimulus significantly decreases the seizure probability in all animals. Wilcoxon rank test, matched-pairs; ***p<0.001 (oLFS, n = 13; eLFS, n = 4 animals). Preconditioning with 0.5 Hz was also able to interfere with the generation of evoked generalized seizures as shown in Figure 5—figure supplement 2. (F, J) Trials in which seizure generalization is not prevented completely, the ensuing seizure is associated with a milder behavioral phenotype (RS). Wilcoxon rank test, matched-pairs; ***p<0.001 (oLFS, n = 13; eLFS, n = 4 animals). (G, K) Cellular response to 10 Hz stimulation quantified as mean AUC. (G) The response is reduced after 1 Hz oLFS stimulation in sessions in which seizures have been successfully suppressed. (K) No significant reduction is visible for AUC values after eLFS. Paired t-test; **p<0.01 (oLFS, n = 13; eLFS, n = 4 animals, respectively). All values are given as mean ± SEM. AUC calculation was performed in python 2.7 provided in Figure 5—source code 1.
-
Figure 5—source code 1
AUC calculation of 10 Hz oLFS evoked responses.
LFP data (idHC) were imported to python 2.7 and filtered (4th order low-pass Chebyshev filter type I with a cut-off frequency at 300 Hz) to smoothen the signal. We calculated the AUC of evoked responses (integral of LFP traces in a defined time interval) during pro-convulsive optical stimulation (10 Hz). The time interval for AUC calculation was set relative to stimulus onset from −0.02 s to +0.06 s.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig5-code1-v2.zip
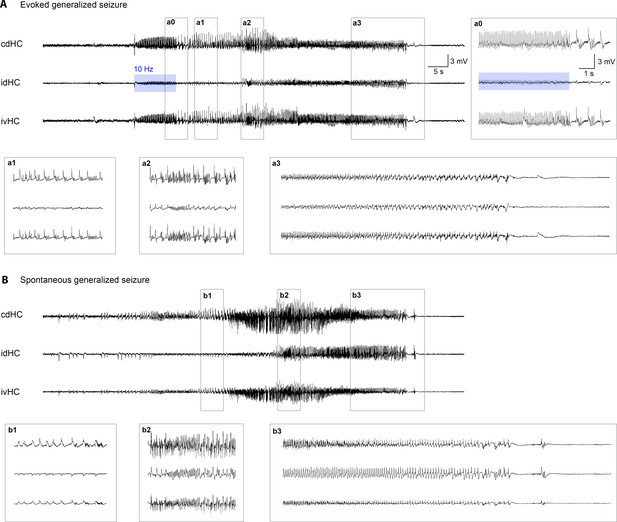
Comparison of spontaneous and evoked generalized seizures.
(A, B) Representative LFP traces of an optogenetically evoked and a spontaneous generalized seizure from the same animal. (a0) High-amplitude epileptic spikes emerge in addition to the evoked potentials during stimulation and rhythmic activity persists after 10 Hz stimulation has been stopped. Both seizures consist of the same building blocks [(a1, b1) spike-and-wave events; (a2, b2) fast discharges; (a3, b3) increasing inter-spike-intervals and subsequent termination] with similar dynamics. This is consistent for all mice (n = 5) in which both spontaneous and evoked generalized seizures were detected.
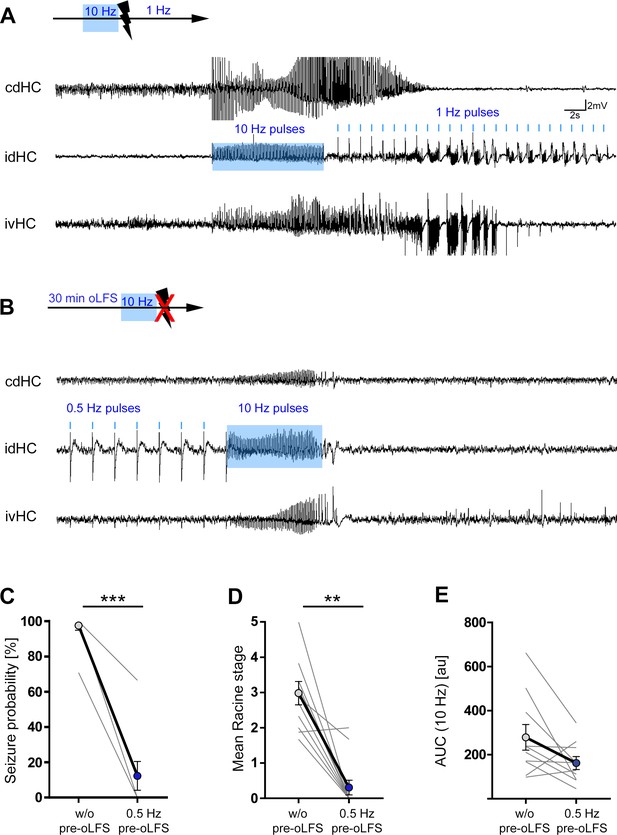
Preconditioning with 0.5 Hz prevents evoked generalized seizures.
(A) Representative LFP trace of an optogenetically evoked generalized seizure. Seizure generalization cannot be prevented by oLFS (1 Hz) applied directly after seizure induction. (B) oLFS at 0.5 Hz for 30 min prior to the pro-convulsive stimulus effectively prevents seizure generalization. (C) 0.5 Hz oLFS significantly decrease the seizure probability in all animals. Wilcoxon rank test, matched-pairs; ***p<0.001 (n = 10 animals). (D) Trials in which seizure generalization is not prevented completely, the ensuing seizure is associated with a milder behavioral phenotype (RS). Wilcoxon rank test, matched-pairs; **p<0.01, (n = 10 animals). (E) The cellular response to the 10 Hz stimulation (mean AUC) is reduced after 0.5 Hz oLFS stimulation in sessions in which seizures have been successfully suppressed. Paired t-test; *p<0.05 (n = 10 animals).
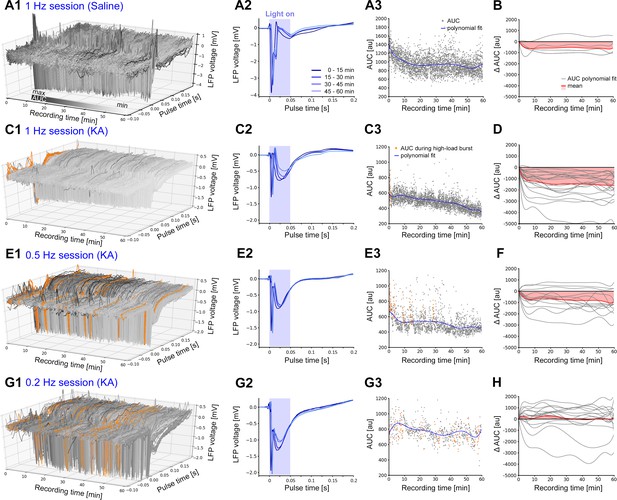
Evoked cellular responses decrease over time during continuous oLFS.
(A, C, E, G) Representative examples of evoked responses in the dentate gyrus of idHC following local photostimulation of entorhinal afferents for (A) non-epileptic control (1 Hz) and (C, E, G) a chronically epileptic mouse (1, 0.5, and 0.2 Hz). (A2, C2, E2, G2) Mean evoked responses (50 ms–long light pulse) across 15 min time windows. (A3, C3, E3, G3) For each evoked response, AUCs are calculated during a [−0.1, +0.2 s] interval relative to the onset of each light pulse. AUC values that are within high-load bursts are marked in orange and are excluded for the calculation of the polynomial fit (blue line). (B, D, F, H) Polynomial fits of AUC normalized to the first AUC value (delta AUC) for all stimulation sessions (gray) and mean changes (red). Calculations were performed in python 2.7 provided in Figure 6—source code 1.
-
Figure 6—source code 1
AUC calculation of 1 Hz oLFS evoked responses.
LFP data (idHC) were imported to python 2.7 and filtered (4th order low-pass Chebyshev filter type I with a cut-off frequency at 300 Hz) to smoothen the signal. We calculated the AUC of evoked responses (integral of LFP traces in a defined time interval = LFP snippet) during oLFS (1, 0.5, 0.2 Hz). The time interval for AUC calculation was set relative to stimulus onset from −0.1 s to +0.2 s. For responses co-occurring with high-load bursts, the AUC was not determined. Using a representative example, we plotted a mean stimulation response across 15 min time windows and individual AUC values for 1-hr oLFS. Polynomial fits of AUC values normalized to the first AUC value (delta AUC) for all stimulation sessions were then calculated and plotted.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig6-code1-v2.zip
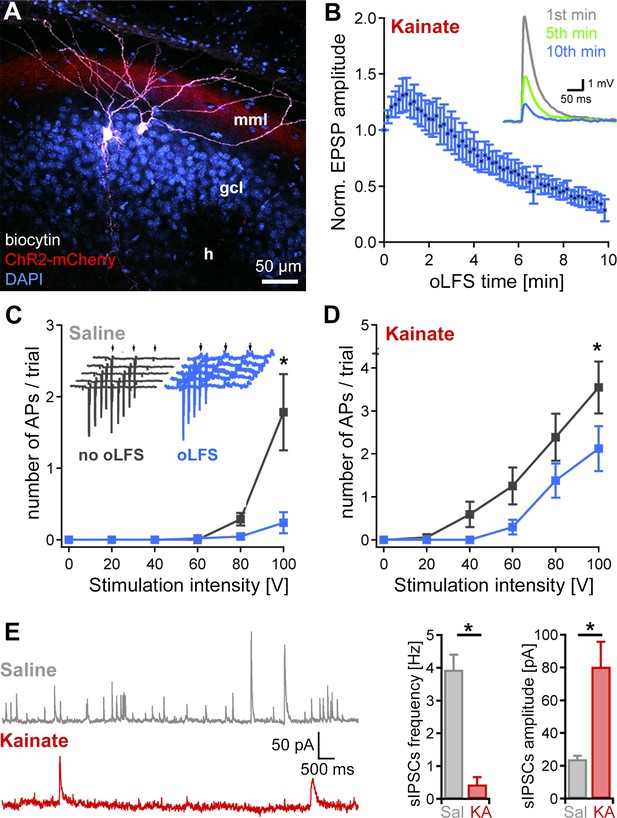
Decrease of single-cell EPSPs and discharge probability after 10 min oLFS.
(A) Representative confocal projection of a dentate gyrus slice from a KA- (15 mM) and AAV-treated mouse 28 days after SE. The two biocytin-filled DGCs (white) were recorded in this section. ChR2-expressing entorhinal fibers are visible in the middle molecular layer (red, mml). Cell bodies were stained with DAPI (blue). h, hilus; scale bar 50 µm. (B) Pulsed blue light delivery reliably induces EPSPs, which decline strongly during a 10-min stimulation protocol (50 ms pulses at 1 Hz). (C, D) Extracellular electrical stimulation (trial = 5 pulses, 50 Hz, arrows) of entorhinal fibers induces action potentials (APs) measured in DGCs (inset, gray traces, recorded in loose-patch). Photostimulation at 1 Hz over 10 min (inset, blue traces) significantly reduces the discharge probability of DGCs in epileptic KA (n = 19 cells) and saline (n = 11 cells) control slices. ANOVA on ranks with Dunn’s Bonferroni post-hoc correction; *p<0.05. Values are given as mean ± SEM. (E) Spontaneous IPSCs in DGCs of saline (n = 6 cells) and KA (n = 7 cells) slices were analyzed for frequency and amplitude. The number of sIPSCs is strongly reduced in ‘epileptic’ DGCs compared to control but those that occur have a larger amplitude. Mann-Whitney Rank Sum Test; *p<0.05 both.
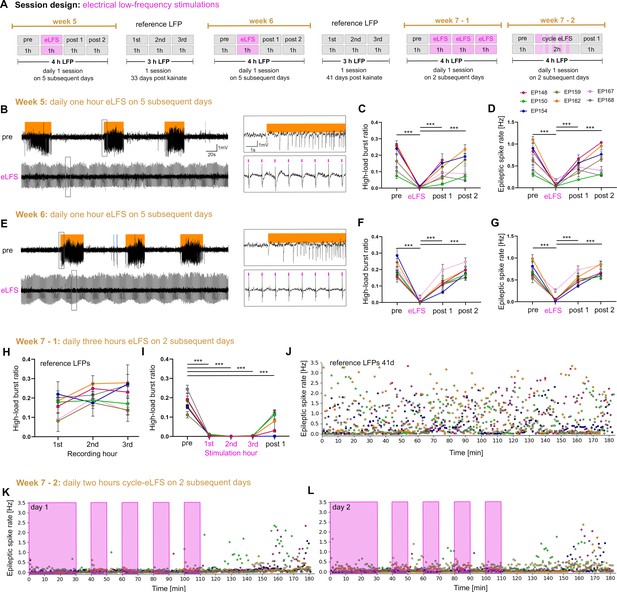
Stable suppression of epileptiform activity through eLFS in the sclerotic hippocampus.
(A) Session design. We locally stimulated the dentate gyrus in the sclerotic idHC for 1 hr/day (1 Hz) over 2 weeks. In the third week, we stimulated animals 3 hr continuously and 2 hr in a 10 min ‘on-off’ manner on 4 successive days. (B, E) Representative LFP traces (15 mM KA, idHC electrode) for the ‘pre’ and ‘eLFS’ sub-sessions (weeks 5 and 6) are shown. Automatically detected high-load bursts are indicated by an orange bar. (C, D, F, G) Quantification of epileptiform activity shows that eLFS nearly extinguishes epileptiform activity in all animals followed by a return to pre-stimulation levels within 2 hr (‘post 1’ and ‘post 2’). Single sessions were used to calculate the RM one-way ANOVA; Tukey’s multiple comparison test ***p<0.001. Animals with a misplaced stimulation electrode did not show this remarkable suppression of epileptiform activity as shown in Figure 8—figure supplement 1. The correct positions of electrodes were confirmed by histology as shown in Figure 8—figure supplement 2. (H) Animals have a stable high-load burst ratio over 3 hr of reference recordings. (I) 3 hr continuous eLFS effectively reduces the high-load burst ratio over the whole stimulation period, reoccurring in a lower manner within 1 hr after stimulation (Two-way ANOVA; Tukey’s multiple comparison test ***p<0.001). All values are given as mean ± SEM. Source data is provided in Figure 8—source data 1. (J) Epileptic spike rates for each minute (one data point, color-coded for each animal) in a 3-hr recording. (K, L) An initial 30 min eLFS and 10 min ‘on-off’ stimulation protocol abolishes epileptic spikes during the stimulation cycle. Approximately 40 min after the last 10 min eLFS the epileptic spike rate starts to rise again.
-
Figure 8—source data 1
eLFS effect on ipsilateral epileptiform activity over time.
(A, B) High-load burst ratios and epileptic spike rates of each sub-session for the first and the second week of daily 1 Hz eLFS are listed. The high-load burst ratio and epileptic spike rate are reduced during eLFS but recover within the first hour of post-recording (post 1). (C) High-load burst ratios of reference LFP recordings (days 33 and 41 after SE) for 3 hr of individual animals. (D) High-load burst ratios for individual animals that went into 3-hr continuous eLFS experiments. Epileptiform activity is effectively reduced during ongoing eLFS but returns in a reduced manner within the first hour in some animals. The implant of EP167 broke after the last reference LFP recording and was therefore excluded. Values are given as mean ± SEM.
- https://cdn.elifesciences.org/articles/54518/elife-54518-fig8-data1-v2.docx
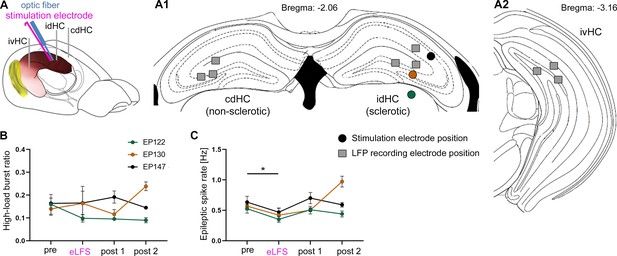
eLFS outside of the dentate gyrus is ineffective in suppressing spontaneous epileptiform activity.
(A) Implantation scheme. (A1) Dorsal electrode (square) and optic fiber/stimulation electrode (dot) positions for three animals in which the stimulation electrode was misplaced during the implantation surgery. (A2) Ventral electrode positions. (B) In the idHC, the high-load burst ratio was not reduced during 1-hr eLFS (two sessions per animal). (C) The epileptic spike rate was slightly reduced compared to the ‘pre’ session. Single sessions were used to calculate the one-way ANOVA; Tukey’s multiple comparison test *p<0.05 (n = 3 animals).
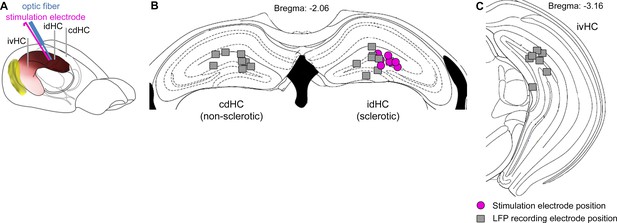
Positions of implanted electrodes for all animals included in the study.
(A) Implantation scheme for eLFS combined with optogenetic seizure induction. The stimulation electrode was glued to the optic fiber and both were implanted in a 30° angle at the idHC site. (B) Positions of the stimulation electrode/optic fiber (pink circle) and LFP electrodes in the dorsal region (n = 8). (C) Ipsilateral ventral electrode positions.
Additional files
-
Supplementary file 1
Quantitative summary of statistically tested parameters.
- https://cdn.elifesciences.org/articles/54518/elife-54518-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/54518/elife-54518-transrepform-v2.pdf