The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy
Figures
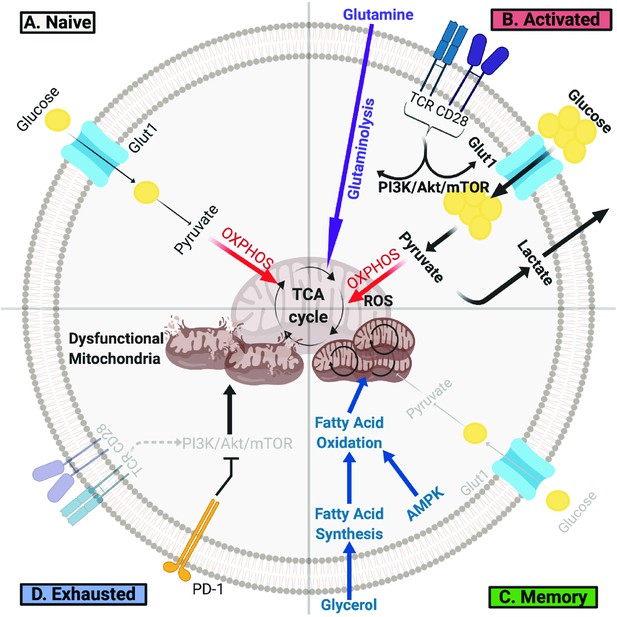
T Cells Undergo Metabolic Rewiring in Different Stages of Their Life.
(A) Naïve T cells uptake sufficient amounts of glucose to fuel oxidative phosphorylation and survive as they survey antigens. (B) Upon encountering cognate antigen, activated T cells rapidly uptake glucose and glutamine to fuel their bioenergetic needs. Activated T cells perform aerobic glycolysis, which shunts products of glycolysis to biosynthetic processes necessary for proliferation and effector function and generates lactate as a byproduct. (C) Once the antigen is cleared, T cells can form long-lived memory cells in which AMPK signaling stimulates fatty acid oxidation. Memory T cells also increase their mitochondrial mass and spare respiratory capacity to prepare for future encounter with cognate antigen. (D) T cells can become exhausted if they fail to clear antigens such as during chronic infections or cancer. T lymphocytes isolated from tumors display elevated levels of PD-1, which decreases PI3K/Akt/mTOR signaling and glycolysis. Exhausted TILs rely on fatty acid oxidation, though they often have dysfunctional mitochondria and decreased mitochondrial mass as well.
Tables
Hostile Conditions in The Tumor Microenvironment Impair T Cell Metabolism and Anti-Tumor Immunity.
Cancer cell metabolism, improper blood vessel formation, and extracellular vesicles all contribute to a toxic milieu deficient in key nutrients, such as glucose and oxygen, and high in waste products, such as lactate. Consequently, TILs entering the TME are deprived of key nutrients, disturbing metabolic processes critical for their anti-tumor functions.
| Component of the TME | Impact on T Cell Metabolism | Effect on Anti-Tumor Immunity |
|---|---|---|
| Hypoxia | • Stabilizes HIF-1α • Increases pyruvate dehydrogenase kinase, blocking the conversion of pyruvate to acetyl-CoA and thus mitochondrial respiration and ROS production • Increases lactate dehydrogenase A expression and inactivates pyruvate dehydrogenase, shunting pyruvate to lactate | • Increases granzyme B packaging into granules, leading to rejection of B16 tumors in mice • Upregulates PD-L1 expression on MDSCs • Decreases T cell infiltration |
| Depletion of Glucose | • Reduces aerobic glycolysis • Decreases levels of phosphoenolpyruvate, which regulates calcium and NFAT signaling | • Suppresses TIL effector function • Reduces EZH2 expression, decreasing T cell polyfunctionality |
| Accumulation of Lactate | • Impedes lactic acid export from CD8+ T cells, which slows down glycolysis and reduces ATP levels • Decreases NFAT levels and translocation to the nucleus | • Inhibits T cell proliferation, activation, and function • Induces T and NK cell apoptosis |
| Tumor-derivedExtracellular Vesicles (EVs) | • Modulates the metabolism of tumor associated macrophages and other cell types. • Effects of EVs on T cell metabolism are currently unknown | • Suppresses TIL anti-tumor function. • However, blocking EV biogenesis induces T cell activation, proliferation, and effector function. |





