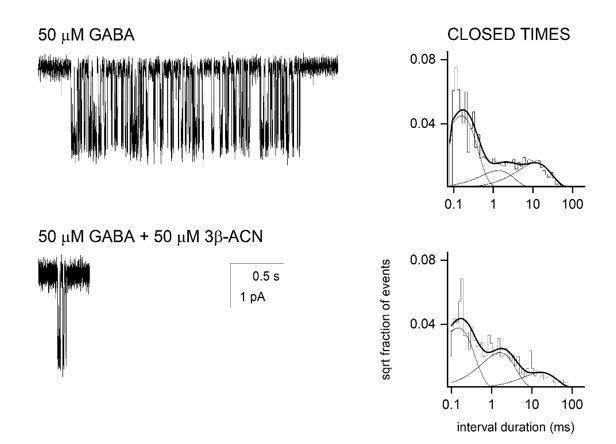
Site-specific effects of neurosteroids on GABAA receptor activation and desensitization
Figures
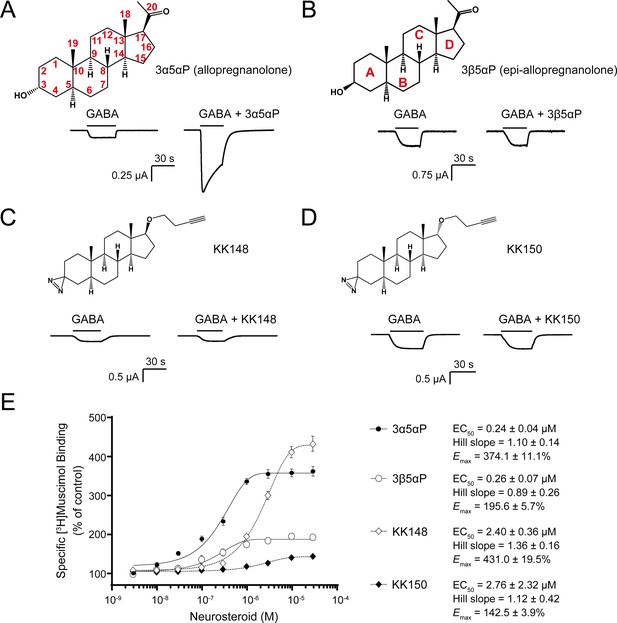
Distinct neurosteroid effects on potentiation of GABAAR currents and modulation of [3H]muscimol binding.
(A) Structure of allopregnanolone (3α5αP) with carbon atoms numbered and sample current traces from α1β3 GABAAR activated by 0.3 μM GABA showing potentiation by 10 μM 3α5αP. The traces were recorded from the same cell. (B), (C) and (D) Structures of epi-allopregnanolone (3β5αP) with steroid rings labeled, neurosteroid analogue photolabeling reagents KK148 and KK150, respectively, and sample current traces from α1β3 GABAAR activated by 0.3 μM GABA showing the absence of potentiation by 10 μM neurosteroids. Each pair of traces was recorded from the same cell. (E) Concentration-response relationship for neurosteroid modulation of [3H]muscimol binding to α1β3 GABAAR. 3 nM–30 μM neurosteroids modulate [3H]muscimol (3 nM) binding in a concentration-dependent manner. Data points, EC50, Hill slope and maximal effect value [Emax (% of control): 100% means no effect] are presented as mean ± SEM (n = 6 for 3α5αP and KK148; n = 3 for 3β5αP and KK150).
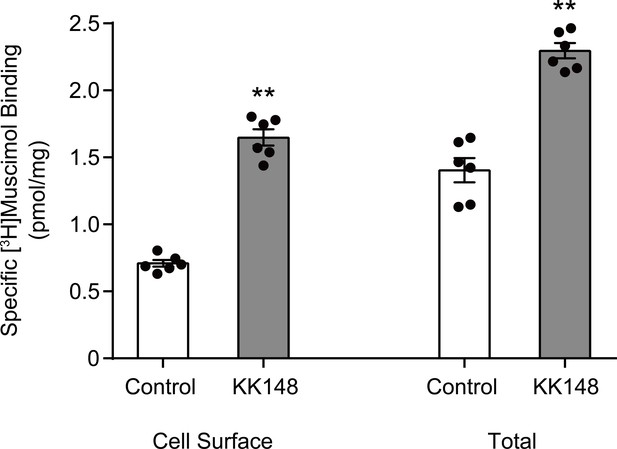
Neurosteroid modulation of muscimol binding to intact cells.
Enhancement of specific [3H]muscimol (3 nM) binding to α1β3 GABAARs on intact HEK cell surfaces (left bars) and total receptors (cell surface receptors + intracellular receptors, right bars) by 10 μM KK148. The larger amount of control binding in total vs. cell surface demonstrates the distribution of GABAAR between plasma membrane and intracellular membrane. Statistical differences are compared using unpaired t-test (n = 6,± SEM). **p<0.01 vs. control.
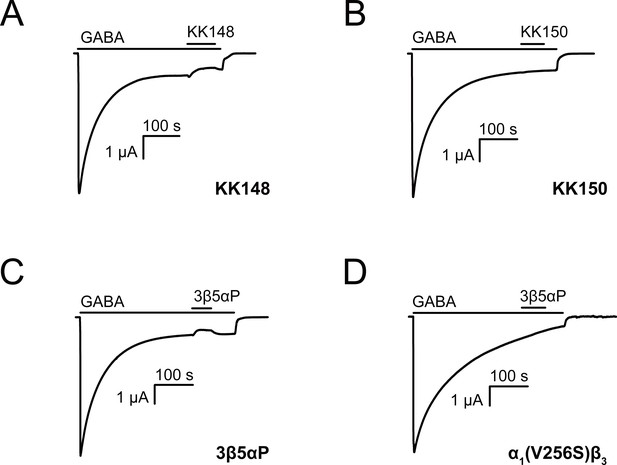
Neurosteroids promote steady-state desensitization of α1β3 GABAARs.
Representative traces showing the effects of KK148, KK150 and epi-allopregnanolone (3β5αP) on maximal steady-state GABA-elicited currents. α1β3 GABAARs expressed in Xenopus laevis oocytes were activated with 1 mM GABA to maximally activate GABAAR current. (A–C) The effect of KK148 (10 μM), KK150 (10 μM) and 3β5αP (3 μM) on steady-state current. (D) The effect of 3β5αP (3 μM) on steady-state current in α1β3 GABAARs containing the α1V256S mutation, known to eliminate NS-induced desensitization. The results show that 3β5αP and KK148 reduce steady-state currents, consistent with enhanced desensitization, whereas KK150 does not. The effect of 3β5αP on steady-state currents is eliminated by the α1V256S mutation, consistent with 3β5αP enhancing desensitization rather than producing channel block.
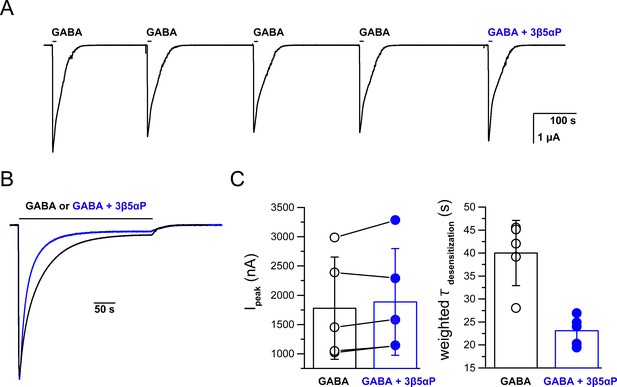
Co-application of epi-allopregnanolone with a saturating concentration of GABA.
(A) Representative experiment showing serial brief applications of 1 mM GABA to oocytes expressing α1β3 GABAARs, followed by application of GABA + 3 µM epi-allopregnanolone (3β5αP). 3β5αP does not reduce the peak current amplitude. The horizontal bars above the tracings indicate the duration of drug application. The data are summarized in Panel C left. (B) Representative experiment of prolonged co-application of 1 mM GABA + 3 µM 3β5αP showing that 3β5αP increases the decay rate of GABA-elicited currents. The traces are from different cells. The amplitudes have been adjusted to precisely overlay the peak responses. The data are summarized in Panel C right. (C) (left) Five paired samples showing that 3β5αP has no significant effect of on the amplitude of GABA-elicited current. Peak currents were compared using a paired two-tailed t-test (n = 5,± SD). (right) Weighted time constants (τ) of current decay (from a two-exponential fit) between GABA and GABA + 3β5αP. The time constants were compared using a paired two-tailed t-test; p=0.001 (n = 5,± SD).
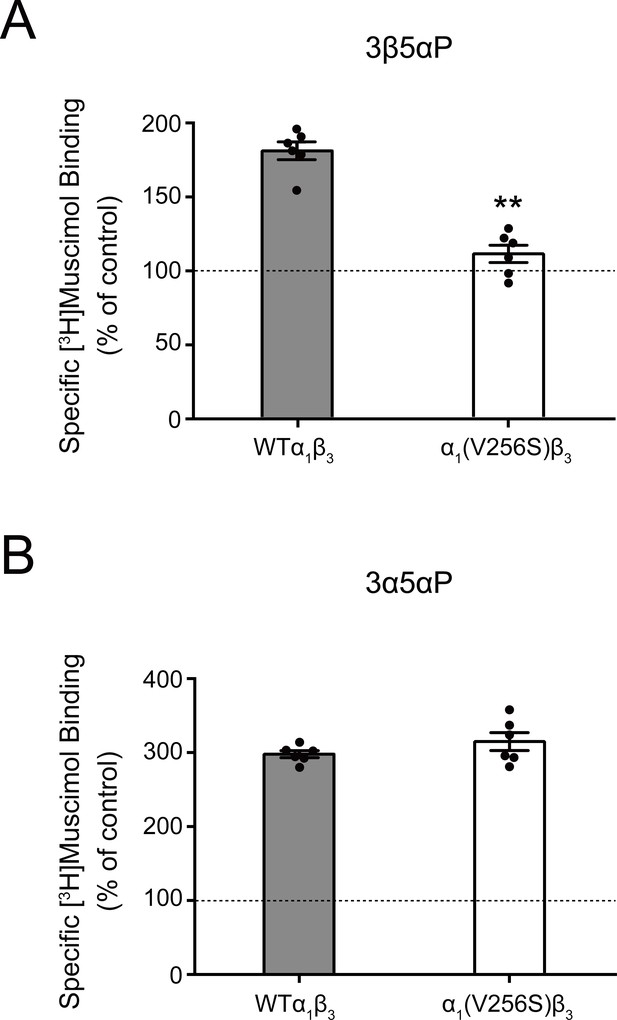
Effect of α1(V256S)β3 mutation on neurosteroid enhancement of [3H]muscimol binding.
(A) Enhancement of specific [3H]muscimol (3 nM) binding to α1β3 GABAAR WT by 10 μM epi-allopregnanolone (3β5αP) is absent in α1(V256S)β3 GABAAR. (B) Enhancement of [3H]muscimol binding by 10 μM allopregnanolone (3α5αP) is unaffected by the α1V256S mutation. These data indicate that 3β5αP enhancement of orthosteric ligand binding requires receptor desensitization, whereas 3α5αP does not. Statistical differences are compared using unpaired t-test (n = 6,± SEM). **p<0.01 vs. WT.
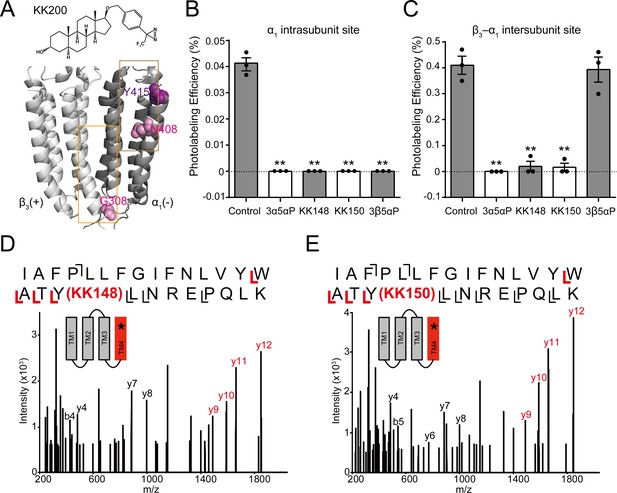
Competitive prevention of neurosteroid photolabeling at an intersubunit and intrasubunit site.
(A) Structures of the neurosteroid photolabeling reagent KK200 and the α1β3 GABAAR-TMDs highlighting the residues G308 in the β3(+)–α1(-) intersubunit site and N408 in the α1 intrasubunit site previously identified by KK200 photolabeling in pink. Shown in purple is Y415 in the α1 intrasubunit site, which is photolabeled by KK148 and KK150. Adjacent β3(+) and α1(-) subunits are shown and the channel pore is behind the subunits. (B) Photolabeling efficiency of α1 subunit TM4 (α1 intrasubunit site) in α1β3 GABAAR by 3 μM KK200 in the absence or presence of 30 μM allopregnanolone (3α5αP), KK148, KK150, and epi-allopregnanolone (3β5αP). Statistical differences are analyzed using one-way ANOVA with Bonferroni's multiple comparisons test (n = 3,± SEM). **p<0.01 vs. control. (C) Same as (B) for β3 subunit TM3 [β3(+)–α1(-) intersubunit site, n = 3,± SEM]. (D) HCD fragmentation spectrum of the α1 subunit TM4 tryptic peptide photolabeled by 30 μM KK148. Red and black indicate fragment ions that do or do not contain KK148, respectively. The schematic highlight in red identifies the TMD being analyzed and the asterisk denotes the approximate location of KK148. (E) Same as (D) photolabeled by 30 μM KK150.
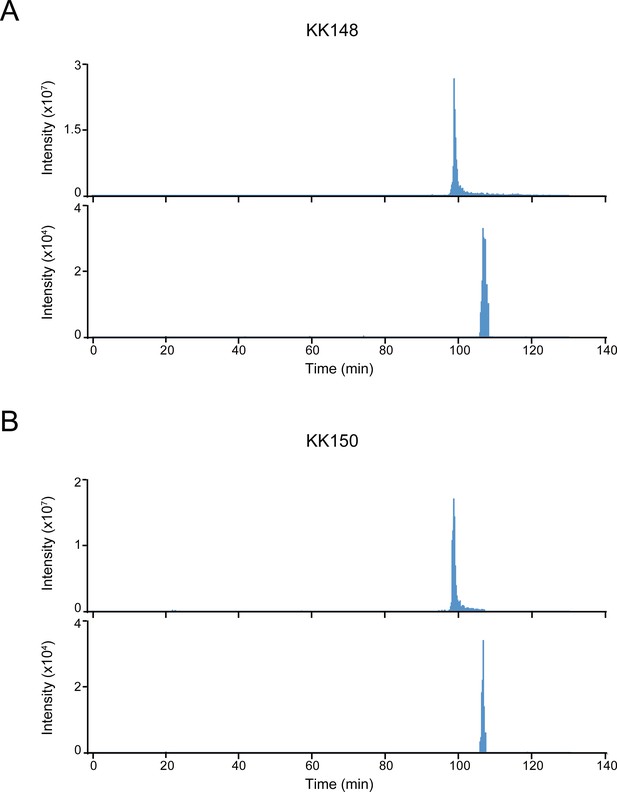
Extracted ion chromatograms of labeled and unlabeled β3 subunit TM4 peptides.
(A) Extracted ion chromatogram (XIC) of the β3 subunit TM4 tryptic peptide in the α1β3 GABAAR. The upper and lower XIC show representative unlabeled β3 subunit TM4 peptide and the peptide labeled with 30 μM KK148, respectively. (B) Same as (A) for KK150.
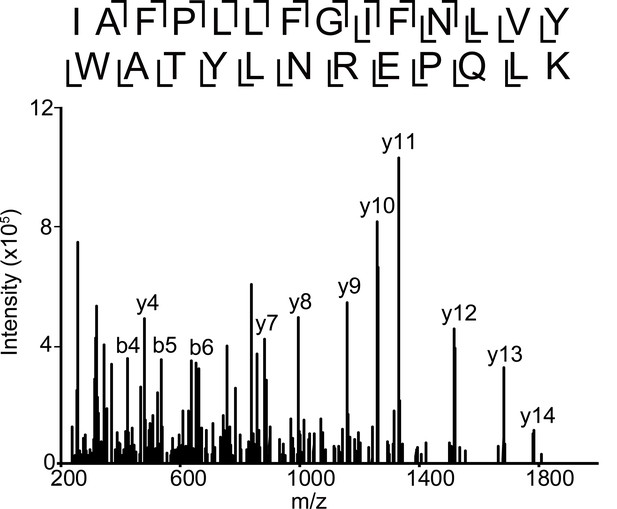
Fragmentation spectrum of unlabeled α1 subunit TM4 peptide.
HCD fragmentation spectrum of the α1 subunit TM4 unlabeled tryptic peptide in the α1β3 GABAAR.
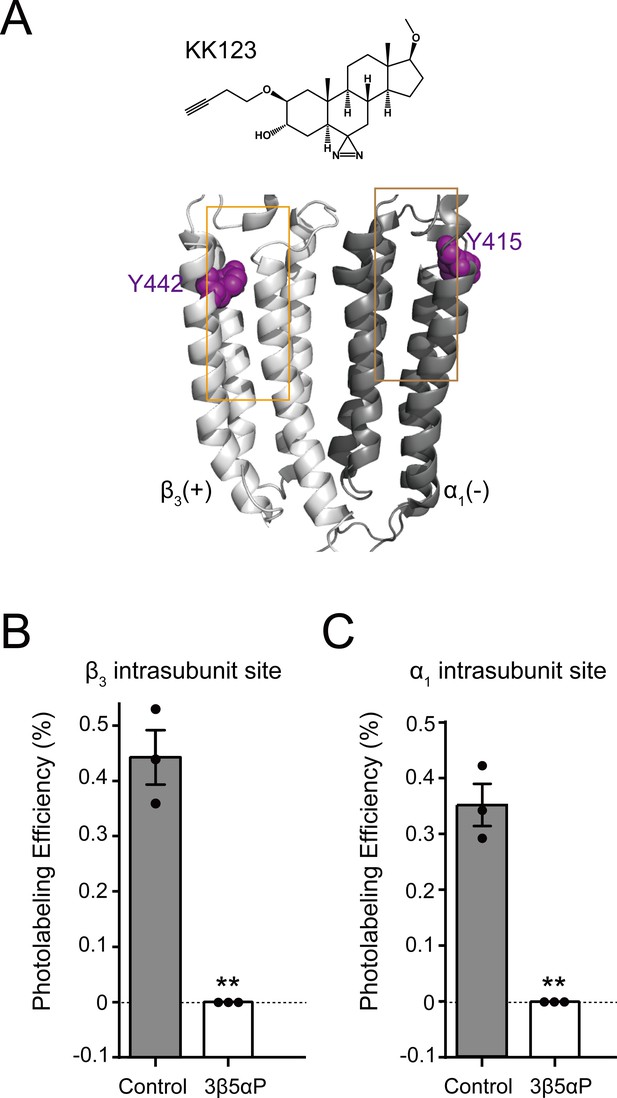
Epi-allopregnanolone prevents neurosteroid photolabeling at the α1 and β3 intrasubunit sites.
(A) Structures of the neurosteroid photolabeling reagent KK123 and the α1β3 GABAAR-TMDs highlighting the residues Y442 in the β3 intrasubunit site and Y415 in the α1 intrasubunit site previously identified by KK123 photolabeling in purple. Adjacent β3(+) and α1(-) subunits are shown and the channel pore is behind the subunits. (B) Photolabeling efficiency of β3 subunit TM4 (β3 intrasubunit site) in α1β3 GABAAR by 3 μM KK123 in the absence or presence of 30 μM epi-allopregnanolone (3β5αP). Statistical differences are compared using unpaired t-test (n = 3,± SEM). **p<0.01 vs. control. (C) Same as (B) for α1 subunit TM4 (α1 intrasubunit site, n = 3,± SEM).
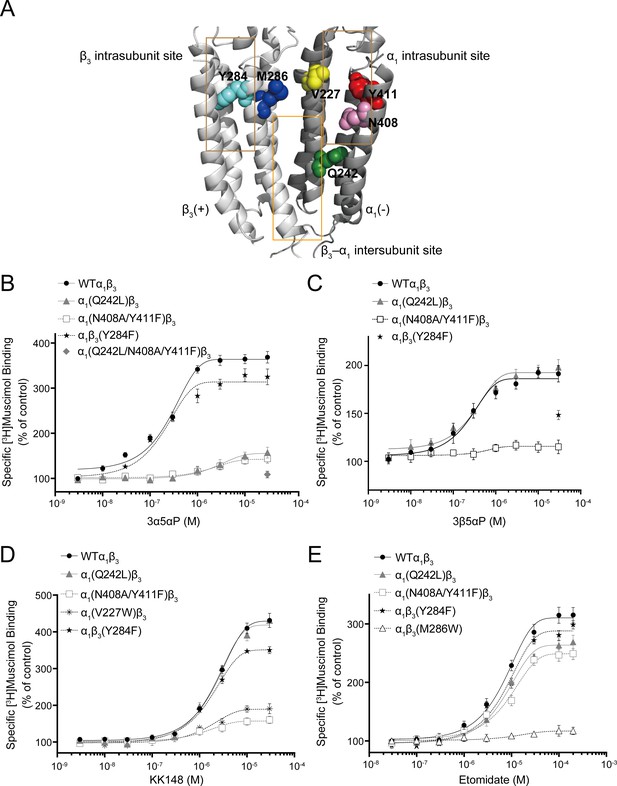
Effect of mutations in α1β3 GABAAR on neurosteroid modulation of [3H]muscimol binding.
(A) Structure of the α1β3 GABAAR-TMD highlighting the residues where mutations were made in putative binding sites for neurosteroids (Q242-green for β3–α1 intersubunit site; V227-yellow, N408-pink and Y411-red for α1 intrasubunit site; Y284-cyan for β3 intrasubunit site) and M286-blue for etomidate. Adjacent β3(+) and α1(-) subunits are shown and the channel pore is behind the subunits. (B) Concentration-response relationship for the effect of 3 nM–30 μM allopregnanolone (3α5αP) on [3H]muscimol (3 nM) binding to α1β3 GABAAR WT and indicated mutants. Data points represent mean ± SEM (n = 6). (C), (D) and (E) Same as (B) for 3 nM–30 μM epi-allopregnanolone (3β5αP) (n = 3), KK148 (n = 6) and 30 nM–200 μM etomidate (n = 6), respectively. The data for WT in panels 6B and 6D is a replot of the same data shown in Figure 1E.
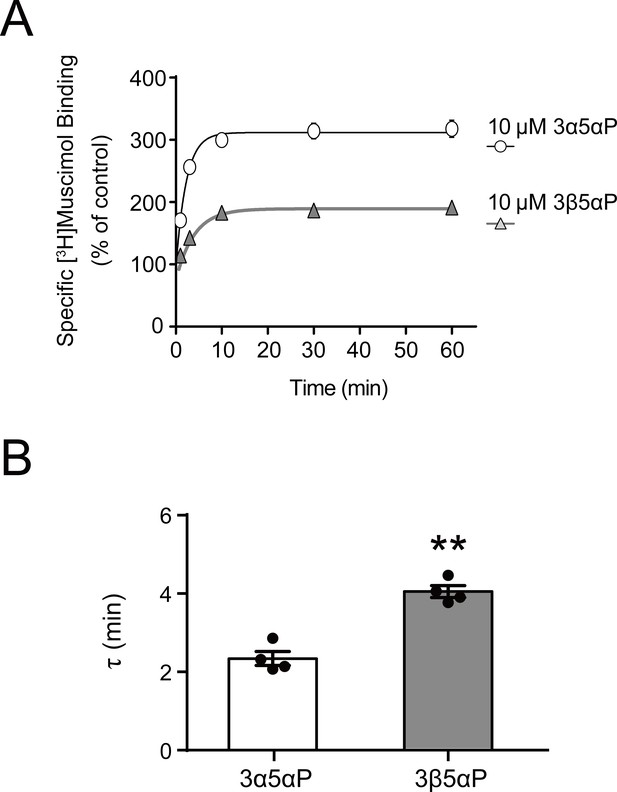
Time course of neurosteroid modulation of muscimol binding.
(A) Time course of [3H]muscimol binding enhancement by 10 µM allopregnanolone (3α5αP) and epi-allopregnanolone (3β5αP). Neurosteroids were added to α1β3 GABAAR membranes that had been fully equilibrated with 3 nM [3H]muscimol and binding was measured as a function of time (n = 4,± SEM). (B) Time constants for neurosteroid-induced enhancement of [3H]muscimol binding. The statistical difference is compared using unpaired t-test (n = 4,± SEM). **p<0.01 vs. 3α5αP.
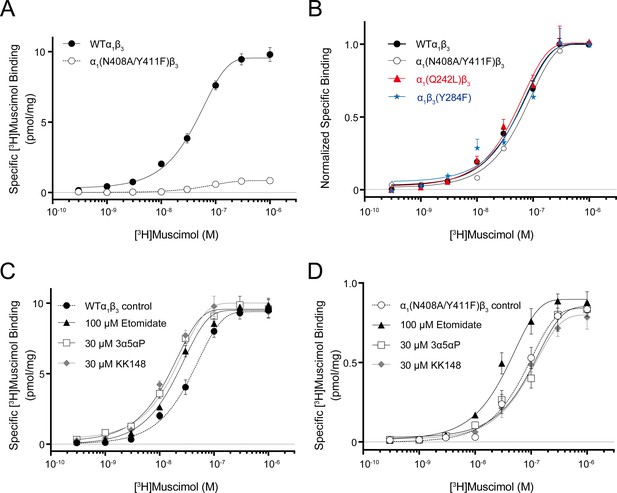
Neurosteroid effect on [3H]muscimol binding isotherms in α1β3 WT and α1(N408A/Y411F)β3 GABAARs.
(A) [3H]muscimol binding isotherms (0.3 nM–1 μM) for α1β3 GABAAR WT and α1(N408A/Y411F)β3 GABAAR. Data points are presented as mean ± SEM (n = 3). (B) Normalized curves of [3H]muscimol binding isotherms (0.3 nM–1 μM) for α1β3 GABAAR WT and representative mutated receptors for each neurosteroid binding site [i.e. α1(Q242L)β3 for the β3–α1 intersubunit site; α1(N408A/Y411F)β3 for the α1 intrasubunit site; α1β3(Y284F) for the β3 intrasubunit site]. Each data point represents mean ± SEM (n = 6 for WT; n = 3 for mutated receptors). (C) Effect of 100 μM etomidate, 30 μM allopregnanolone (3α5αP) and 30 μM KK148 on [3H]muscimol binding isotherms in the α1β3 GABAAR WT. (D) Same as (C) in the α1(N408A/Y411F)β3 mutant. Each data point represents mean ± SEM (n = 3).
-
Figure 7—source data 1
Properties of [3H]muscimol binding isotherms in α1β3 WT and mutant GABAARs.
Kd and Bmax for [3H]muscimol binding isotherms in the α1β3 GABAARs WT and mutated receptors. Kd and Bmax values are compared using one-way ANOVA with Bonferroni's multiple comparisons test. Data are presented as mean ± SEM (n = 6 for WT; n = 3 for mutated receptors).
- https://cdn.elifesciences.org/articles/55331/elife-55331-fig7-data1-v2.docx
-
Figure 7—source data 2
Properties of neurosteroid effect on [3H]muscimol binding isotherms in α1β3 GABAAR WT and α1(N408A/Y411F)β3 mutant.
Kd and Bmax for the [3H]muscimol binding isotherms in Figure 7C–D. Kd values are compared using unpaired t-test. Statistical differences between the whole curves are analyzed using two-way ANOVA. Data are presented as mean ± SEM (n = 3).
- https://cdn.elifesciences.org/articles/55331/elife-55331-fig7-data2-v2.docx
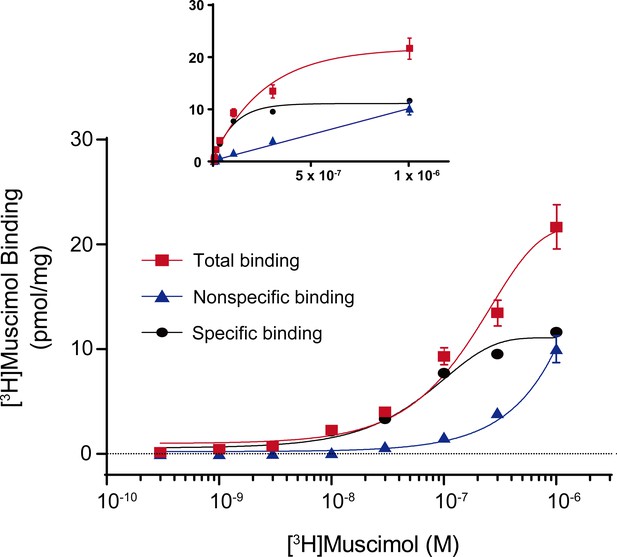
Total, nonspecific and specific [3H]muscimol binding curves.
Representative total, nonspecific and specific binding curves to experimentally determine a [3H]muscimol binding isotherm (0.3 nM–1 μM) for WT α1β3 GABAARs. Data points are from a single experiment with triplicate determinations for total and nonspecific binding (± SEM). Inset shows curves using identical data set plotted on a linear scale for abscissa.
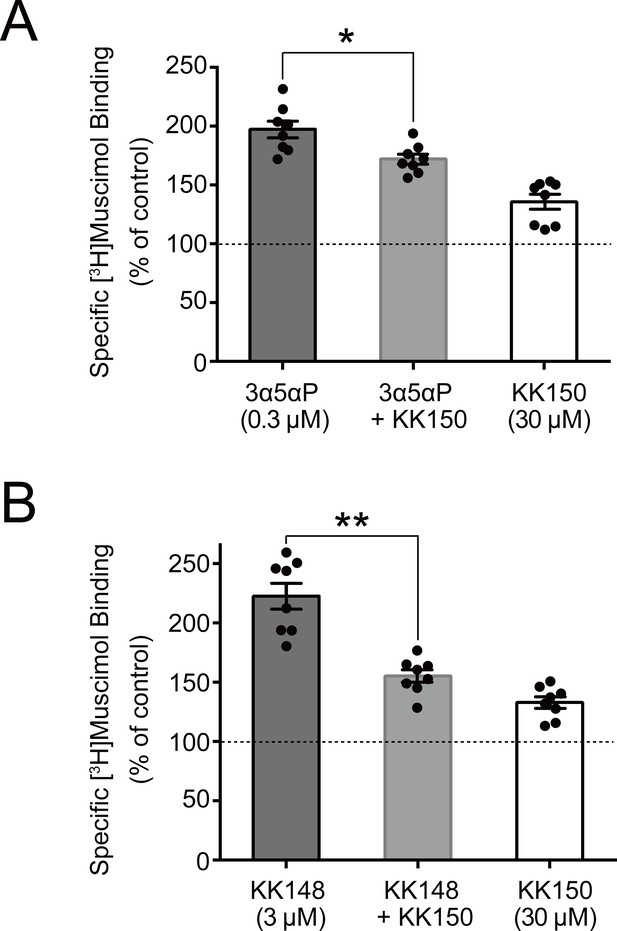
KK150 prevents neurosteroid-induced muscimol binding enhancement.
(A) Enhancement of specific [3H]muscimol (3 nM) binding to α1β3 GABAAR by 0.3 μM allopregnanolone (3α5αP) in the absence (black bar) or presence (grey bar) of 30 μM KK150 and KK150 alone (white bar). Statistical differences are analyzed using one-way ANOVA with Bonferroni's multiple comparisons test (n = 8,± SEM). *p<0.05 vs. 0.3 μM 3α5αP alone. (B) Same as (A) for 3 μM KK148 (n = 8,± SEM). **p<0.01 vs. 3 μM KK148 alone.
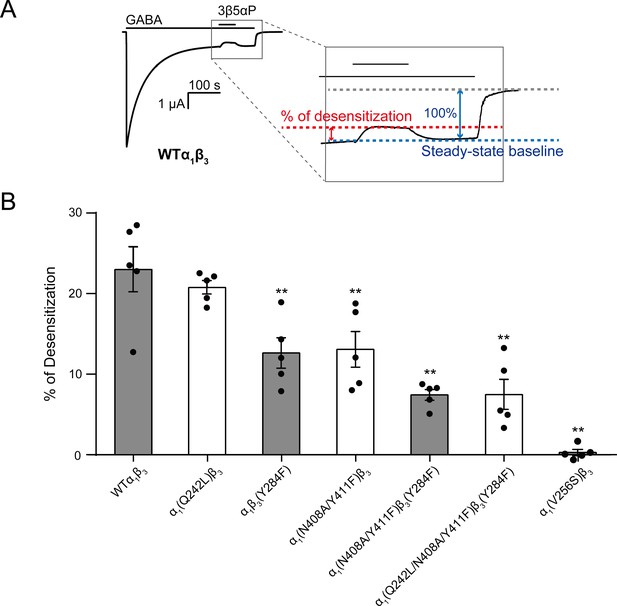
Mutations in intrasubunit sites prevent desensitization by epi-allopregnanolone.
(A) Sample current trace showing the effect of 3 μM epi-allopregnanolone (3β5αP) on steady-state current elicited by continuous administration of 1 mM GABA to α1β3 GABAAR expressed in oocytes. A zoomed-in box shows neurosteroid-induced desensitization of the steady-state GABA current. (B) Percent desensitization of the steady-state α1β3 GABAAR currents (WT and mutants) by 3 μM 3β5αP during continuous application of 1 mM GABA [for WT, α1(Q242L)β3, α1β3(Y284F), α1(N408A/Y411F)β3 and α1(V256S)β3 GABAARs] or 1 mM GABA + 25 μM pentobarbital (PB) [for α1(N408A/Y411F)β3(Y284F) and α1(Q242L/N408A/Y411F)β3(Y284F) GABAARs]. The combination of GABA and PB is essential for some mutated receptors to obtain a high, consistent peak open probability. Statistical differences are analyzed using one-way ANOVA with Bonferroni's multiple comparisons test (n = 5,± SEM). **p<0.01 vs. WT.
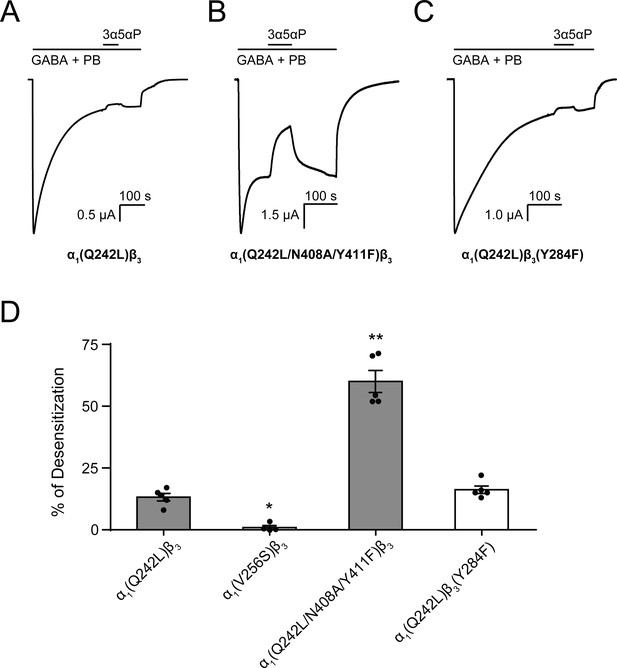
Allopregnanolone desensitizes GABAAR currents via binding to the β3 intrasubunit site.
(A) Sample current trace showing the effect of 3 μM allopregnanolone (3α5αP) on α1(Q242L)β3 GABAAR activated by 1 mM GABA co-applied with 40 μM pentobarbital (PB). (B), (C) Same as (A) for α1(Q242L/N408A/Y411F)β3 GABAAR and α1(Q242L)β3(Y284F) GABAAR, respectively. (D) Percent desensitization of the steady-state currents elicited by 1 mM GABA with 40 μM PB in α1β3 GABAAR with specified mutations. Statistical differences are analyzed using one-way ANOVA with Bonferroni's multiple comparisons test [n = 4 for α1(V256S)β3; n = 5 for others,± SEM]. *p<0.05; **p<0.01 vs. α1(Q242L)β3, respectively.
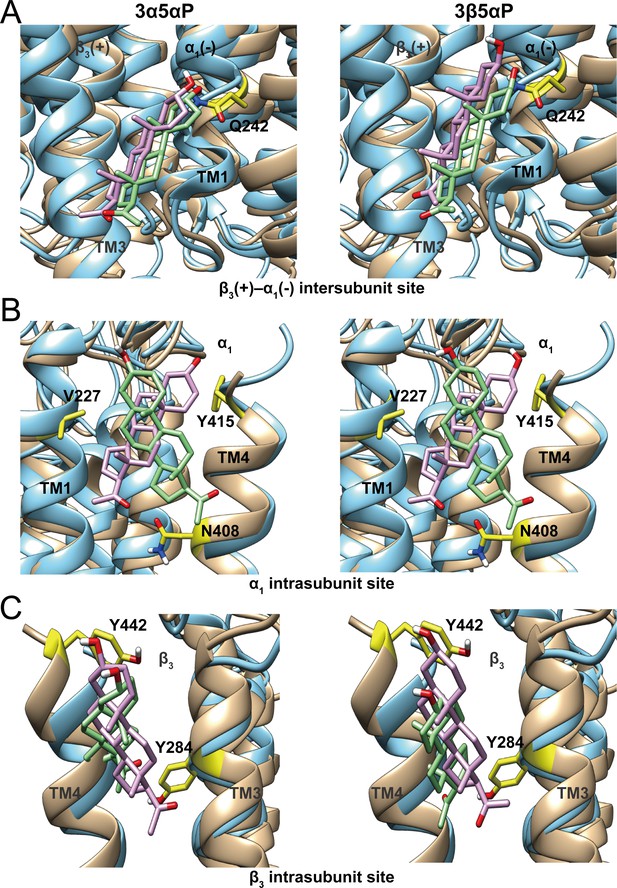
Comparison of allopregnanolone and epi-allopregnanolone docking poses within three neurosteroid binding pockets of the modeled α1β3 GABAAR TMD and the cryo-EM structure of an α1β3γ2 GABAAR (PDB ID: 6I53).
The two structures were read into UCSF Chimera and mutually aligned using MatchMaker. The α1β3 model is shown in tan, while the α1β3γ2 structure is in cyan. (A) Representative poses for allopregnanolone (3α5αP) and epi-allopregnanolone (3β5αP) docked within the β3(+)–α1(-) intersubunit site, the poses for the α1β3 model are in pink, while those for the α1β3γ2 structure are in light green. The α1Q242 side chain is shown in yellow. (B) Same as for (A) for the α1 intrasubunit site; also shown are the sidechains V227, Y415, and N408. (C) Same as (A) for the β3 intrasubunit site; also shown are the sidechains Y284 and Y442. The Vina docking scores for 3α5αP and 3β5αP at each site in the α1β3 model and the α1β3γ2 structure are shown in Figure 11—source data 1.
-
Figure 11—source data 1
Vina docking scores for allopregnanolone and epi-allopregnanolone at each site in α1β3 model and α1β3γ2 GABAAR structure.
- https://cdn.elifesciences.org/articles/55331/elife-55331-fig11-data1-v2.docx
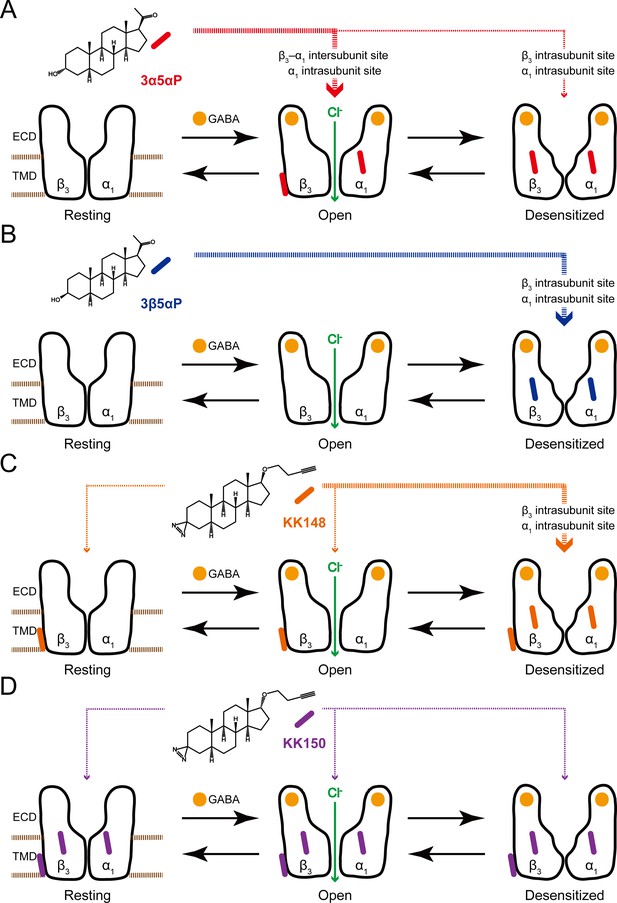
Neurosteroids preferentially stabilize GABAAR in different states.
(A) Model showing three fundamental conformational states that depict the channel function in the GABAAR: a resting state; an open state; and a desensitized state. Agonist (GABA: ) binding shifts the equilibrium towards high-affinity states (open and desensitized). Allopregnanolone (3α5αP: ) allosterically stabilizes the high-affinity states (an open state through the β3–α1 intersubunit and the α1 intrasubunit sites; a desensitized state through the β3 intrasubunit site). The width of red arrows indicates relative affinities of 3α5αP for the open or desensitized state of the receptor. (B) Same as (A) for epi-allopregnanolone (3β5αP: ). 3β5αP stabilizes a desensitized state through the β3 and α1 intrasubunit sites. (C) Same as (A) for KK148 ( ). KK148 allosterically stabilizes a desensitized state through the β3 and α1 intrasubunit sites, and equally stabilizes all three states of the receptor through the β3–α1 intersubunit site. The width of orange arrows indicates relative affinities of KK148 for each state of the receptor. (D) Same as (A) for KK150 ( ). KK150 equally stabilizes all three states of the receptor through the β3 and α1 intrasubunit sites, and the β3–α1 intersubunit site.
Tables
Effects of mutations on neurosteroid modulation of [3H]muscimol binding.
EC50, Hill slope and maximal effect values [Emax (% of control): 100% means no effect] for the concentration-response curves in Figure 6B–E. Statistical differences are analyzed using one-way ANOVA with Bonferroni's multiple comparisons test (*p<0.05 vs. WT; **p<0.01 vs. WT). Data are presented as mean ± SEM.
| 3α5αP | EC50 (μM) | Hill slope | Emax (% of control) | N |
|---|---|---|---|---|
| WTα1β3 | 0.24 ± 0.04 | 1.10 ± 0.14 | 374.1 ± 11.1 | 6 |
| α1(Q242L)β3 | **2.66 ± 0.51 | 1.16 ± 0.37 | **159.8 ± 10.9 | 6 |
| α1(N408A/Y411F)β3 | **2.30 ± 0.48 | 0.87 ± 0.44 | **146.0 ± 9.3 | 6 |
| α1β3(Y284F) | 0.19 ± 0.04 | 0.87 ± 0.16 | 342.3 ± 13.9 | 6 |
| α1(Q242L/N408A/Y411F)β3 | - | - | **105.9 ± 7.3 | 6 |
| 3β5αP | ||||
| WTα1β3 | 0.25 ± 0.08 | 0.84 ± 0.23 | 195.1 ± 6.7 | 3 |
| α1(Q242L)β3 | 0.27 ± 0.09 | 0.77 ± 0.21 | 204.3 ± 4.5 | 3 |
| α1(N408A/Y411F)β3 | 0.61 ± 0.26 | 2.25 ± 0.92 | **124.3 ± 2.6 | 3 |
| α1β3(Y284F) | - | - | **148.6 ± 4.9 | 3 |
| KK148 | ||||
| WTα1β3 | 2.40 ± 0.36 | 1.36 ± 0.16 | 431.0 ± 19.5 | 6 |
| α1(Q242L)β3 | 2.20 ± 0.31 | 1.24 ± 0.12 | 434.5 ± 5.6 | 6 |
| α1(N408A/Y411F)β3 | 1.63 ± 0.53 | 0.73 ± 0.23 | **161.7 ± 3.5 | 6 |
| α1(V227W)β3 | 1.73 ± 0.68 | 0.76 ± 0.31 | **209.2 ± 7.4 | 6 |
| α1β3(Y284F) | 1.79 ± 0.44 | 1.35 ± 0.13 | **357.2 ± 8.1 | 6 |
| Etomidate | ||||
| WTα1β3 | 7.24 ± 1.18 | 1.07 ± 0.17 | 331.1 ± 9.9 | 6 |
| α1(Q242L)β3 | 7.50 ± 0.95 | 1.35 ± 0.20 | **277.8 ± 10.9 | 6 |
| α1(N408A/Y411F)β3 | 9.14 ± 2.20 | 1.07 ± 0.26 | **268.2 ± 5.9 | 6 |
| α1β3(Y284F) | 7.71 ± 1.10 | 0.90 ± 0.11 | 303.5 ± 5.8 | 6 |
| α1β3(M286W) | *22.5 ± 6.17 | 0.50 ± 0.16 | **128.6 ± 7.8 | 6 |