Remyelination alters the pattern of myelin in the cerebral cortex
Figures
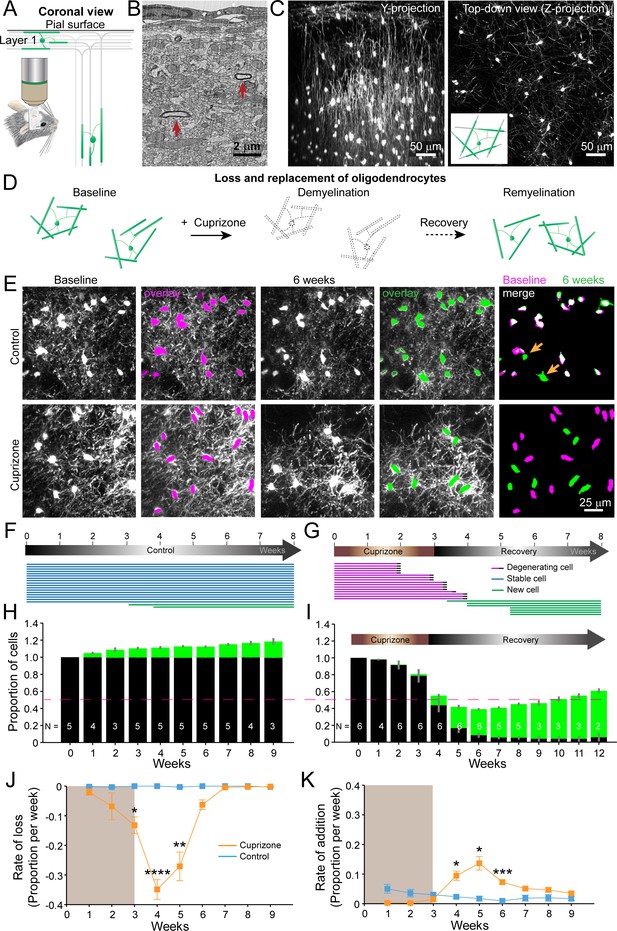
An in vivo platform to monitor loss and replacement of oligodendrocytes in the cerebral cortex.
(A) In vivo two photon microscopy through chronic cranial windows over the somatosensory cortex of Mobp-EGFP mice (coronal view), showing myelinated fibers in cortical layer I parallel to pial surface and in deeper layers oriented perpendicularly. (B) Electron micrograph reconstruction of adult mouse visual cortex (from Bock et al., 2011) illustrating low density of myelinated fibers (arrows) in the upper layers of cortex. (C) Maximum intensity y-projection (coronal view, 425 μm x 150 μm x 550 μm) and z-projection (top-down view, 425 μm x 425 μm x 100 μm) example regions from Mobp-EGFP mice with chronic cranial windows. (D) Schematic illustrating longitudinal course of loss (demyelination) and replacement (remyelination) of cortical oligodendrocytes. (E) Examples of maximum intensity projection images of the same region (156 μm x 156 μm x 84 μm) imaged repeatedly from an adult sham- (control, top row) or a cuprizone-treated (bottom row) mouse are shown with overlay of cell bodies from baseline (magenta) and after 6 weeks (green). Merge of baseline and 6 week overlays show where new cells are added to the region (arrows). (F-G) Individual cells (represented by magenta, blue or green lines) were tracked longitudinally in somatosensory cortex from mice fed control (F; from region in top row of E) or cuprizone diet (G; from region in bottom row of E). (H-K) The same cortical volume (425 μm x 425 μm x 300 μm) was imaged repeatedly in mice given either control or cuprizone diet, and individual cells present at baseline (black) or formed at later time points (green) were tracked over time. Shown are the average cell counts depicted as a proportion of baseline number of cells, (H, N = 5 control mice; I, N = 6 cuprizone mice, I; number of mice imaged at each time point indicated). (J-K) The average rate of loss (J) or addition (K) of oligodendrocytes per week in control-treated (blue) v. cuprizone-treated mice (orange) relative to the baseline population of oligodendrocytes. Treatment with sham or cuprizone-supplemented chow denoted by shaded background. In cuprizone-treated mice, there was a higher rate of oligodendrocyte loss over weeks 3–5 and addition of new cells between 4–6 weeks compared to control. Data is presented as means with standard error of the mean bars. See Supplementary file 1 for statistical tests and significance level not otherwise noted.
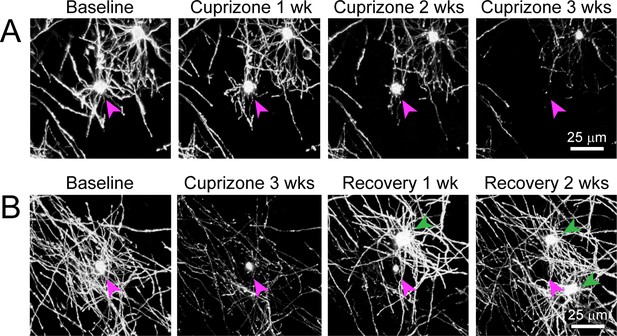
Degeneration of oligodendrocytes in cuprizone-treated Mobp-EGFP mice.
Shown are two examples of individual oligodendrocytes tracked longitudinally using two-photon in vivo imaging through chronic cranial windows in cuprizone-fed adult Mobp-EGFP mice. (A) Example of an oligodendrocyte present at baseline (cell body denoted with magenta arrowhead) that loses EGFP fluorescence in processes and myelin sheaths and eventually the cell body by 3 weeks of cuprizone treatment (maximum intensity projection of 156 μm x 156 μm x 45 μm volume). (B) Example of an oligodendrocyte present at baseline that loses EGFP fluorescence in processes and myelin sheaths over a much longer time course than the cell in A, eventually disappearing at 3 weeks of recovery after new oligodendrocytes (green arrowheads) are formed during recovery period (maximum intensity projection of 156 μm x 156 μm x 55 μm volume).
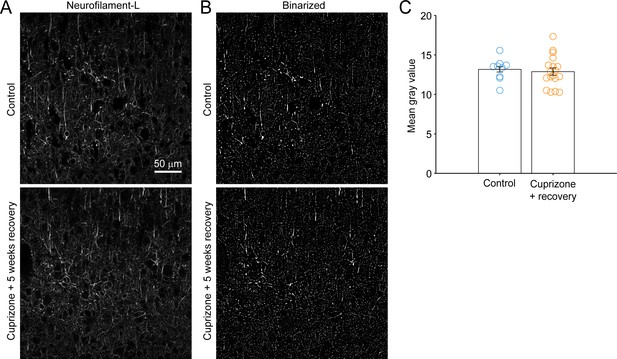
Preservation of cortical axons following cuprizone treatment.
(A) Images of neurofilament-L immunoreactivity in single 7 µm-thin coronal sections of LII/III-IV of somatosensory cortex from control (top) or cuprizone-treated mice after 5 weeks recovery (bottom). Each image is 2048 x 2048 pixels, 319.45 µm x 319.45 µm. (B) Binarized versions of images from (A) (see Methods). (C) Quantification of mean gray values in the image field, (Control: 13.2 ± 0.33 arbitrary units, N = 9 fields from three mice; Cuprizone + recovery: 12.9 ± 0.46 arbitrary units, N = 18 fields from six mice; p=0.7043, not significant by two-sample unpaired t-test).
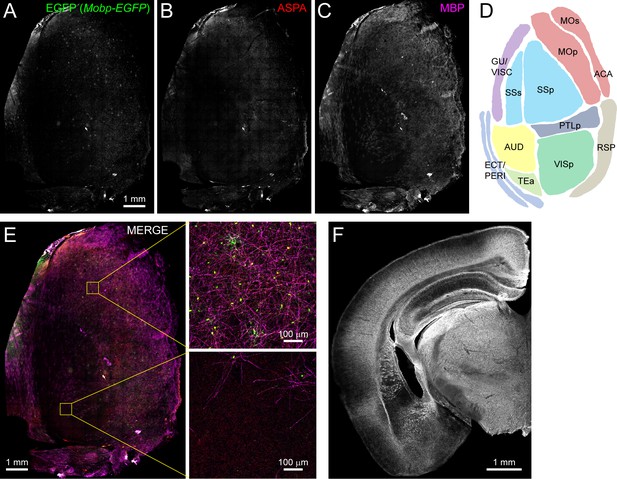
Non-uniform distribution of myelin in the adult rodent cortex.
(A-C) Left hemi-cortex from an adult Mobp-EGFP mouse flattened and immunostained for EGFP (A), ASPA (B, oligodendrocytes) and MBP (C, myelin), merged together (E). Individual regions (schematized map in D) are expanded to illustrate that some cortical areas have a higher density of MBP+ myelin sheaths and EGFP+/ASPA+ oligodendrocytes (top, primary somatomotor cortex) than others (bottom, auditory cortex). (F) Coronal section from a 6-month-old wild-type mouse immunostained for MBP shows the regional heterogeneity of myelin across the cortical mantle.
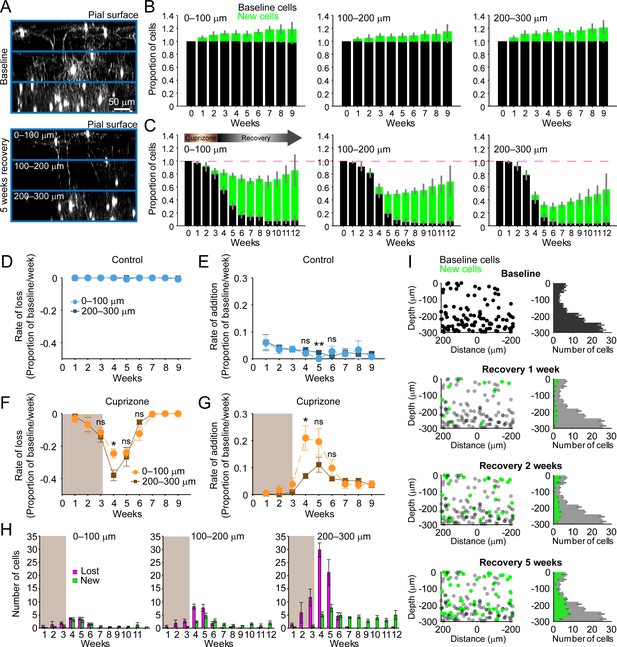
Regeneration of oligodendrocytes declines with cortical depth.
(A) Fate of individual oligodendrocytes over time were determined within the same cortical volume (425 μm x 425 μm x 300 μm) that was divided into 0–100, 100–200, or 200–300 μm zones. Images show maximum intensity Y-projection (400 μm x 118 μm x 300 μm) at baseline (top) and later at 5 weeks recovery (bottom). B-C: Histograms showing fate of existing (black) or newly generated oligodendrocytes (green) in mice fed normal (B) or cuprizone-supplemented diet (C). Average cell counts per volume depicted as proportion of baseline cells (B: N = 5 control mice; C: N = 6 cuprizone-treated mice; same number of mice imaged at each time point as shown in Figure 1H,I). (D-G) Rate of cell loss (D, F) or addition (E,G) relative to baseline oligodendrocytes in each zone depicted for each imaging time-point as a function of cortical depth (0–100 v. 200–300 μm zones), over 9 weeks of imaging in control (D–E) or cuprizone-treated (F–G) mice. Treatment with cuprizone denoted by shaded background (F–G). Cells are rarely lost in control regions (D), and the rate of cell addition in the 0–100 (light blue circles) v. 200–300 μm (dark blue squares) zones are similar (except @ week 5). In the bottom 200–300 μm zone (F-G, in cuprizone-treated mice (brown squares), the rate of oligodendrocyte loss (F) is significantly greater at week 4 relative to the top (0–100 μm, orange circles) zone, and the rate of addition is significantly lower (G). H: Mean number of oligodendrocytes lost (magenta) and added (green) at each imaging time-point, between 0–100 μm and 200–300 μm zones. I: Distribution of baseline (gray) and new (green) oligodendrocyte cell bodies within one volume (left-side panels; 425 μm x 425 μm x 300 μm) and for all regions (right-side panels; mean values from N = 6 mice) at recovery weeks 1, 2 and 5 (corresponding to weeks 4, 5, and 8 of imaging weeks from A-H). Mean values depicted with error bars as standard error of the mean. See Supplementary file 1 for statistical tests and significance level for each comparison.
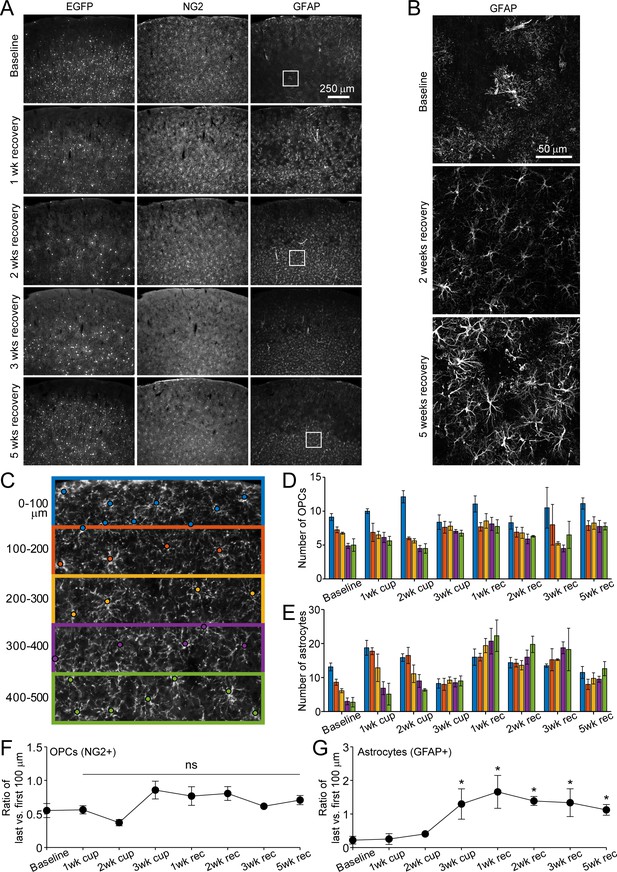
Distribution of astrocytes and oligodendrocyte precursor cells over the course of cuprizone treatment and recovery.
(A) Example coronal images from brains of young adult Mobp-EGFP mice euthanized at baseline at 1, 2, 3 or 5 weeks recovery. Sections were immunostained for EGFP (oligodendrocytes), NG2 (OPCs) and GFAP (astrocytes). By 2 weeks of recovery, the relatively sparse distribution of EGFP+ cells represent newly formed cells (as demonstrated by in vivo imaging in Figure 1), with increasing number of new EGFP+ cells in lower cortical layers in later weeks of recovery. NG2+ OPC distribution remains constant over the course of damage and repair. GFAP+ cells increase in number after cuprizone and remain elevated in lower cortical regions over several weeks of recovery. (B) Example maximum intensity projections of coronal sections from Mobp-EGFP mice euthanized at baseline, 2 weeks recovery or 5 weeks recovery, immunostained for GFAP (213 μm x 213 μm x 35 μm, from A). GFAP+ astrocytes exhibit reactivity after exposure to cuprizone and maintain reactive morphology at 5 weeks of recovery. (C) Example somatosensory cortex coronal section from an Mobp-EGFP mouse at baseline, immunostained for NG2+, and divided into 100 μm zones from the pial surface to 500 μm in depth. Cell body location marked with a circle. Each 100 μm zone color coded from pial surface corresponds to bar colors in D and E. D-E: Quantification of cortical NG2+ cell distribution (D) and GFAP+ astrocytes (E) from brains of adult Mobp-EGFP mice euthanized at baseline (N = 4), 1 week of cuprizone (N = 4), 2 weeks of cuprizone (N = 4), 3 weeks of cuprizone (N = 4), 1 week of recovery (N = 8), 2 weeks of recovery (N = 5), 3 weeks of recovery (N = 5 for NG2, N = 4 for GFAP) and 5 weeks of recovery (N = 4). F-G. Ratio of cell number in the last (400–500 μm, green in C) versus first (0–100 μm, blue in C) zone for NG2+ OPCs (F) and GFAP+ astrocytes (G). Compared to baseline, the relative proportion of OPCs in top vs. bottom regions is stable over the course of cuprizone-treatment and recovery (F), whereas GFAP+ astrocytes significantly increase in the bottom zone after 2 weeks of cuprizone and remain elevated (G). Error bars are standard error of the mean. See Supplementary file 1 for statistical tests and significance levels for each comparison.
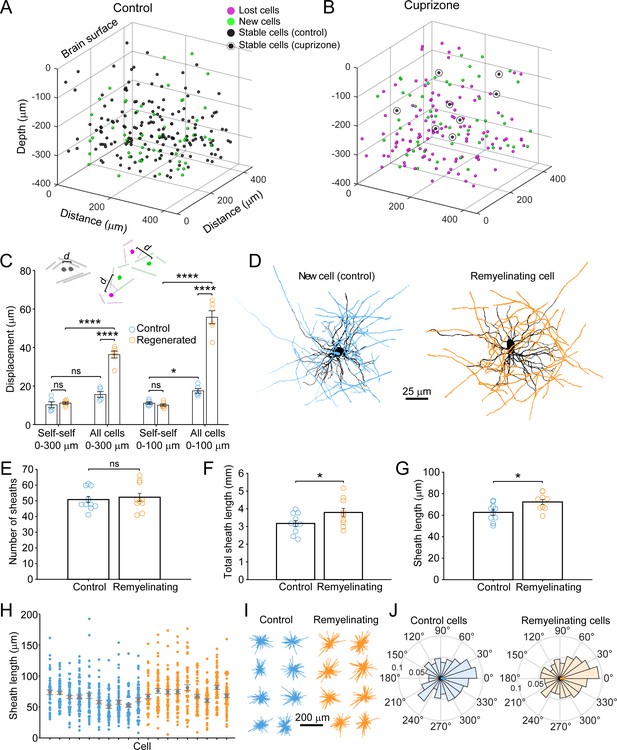
Remyelinating oligodendrocytes appear in novel locations but exhibit normal morphological characteristics.
(A-B) Location of oligodendrocyte cell bodies within the same cortical volume (425 μm x 425 μm x 300 μm) at baseline and after 8 weeks of two photon in vivo imaging are plotted and overlaid in 3-D for control (A) and cuprizone-treated (B) cortex. Cell fate is designated as stable (black), lost (magenta), or new (green) (see Video 4). (C) Histogram showing average displacement (Euclidean distance) of regenerated oligodendrocytes at 5 weeks recovery relative to the nearest oligodendrocyte at baseline. Self-self illustrates the minor movement of cells that survived over the entire 8 weeks (schematized above by two gray oligodendrocytes displaced by small distance d). All cells indicates the nearest neighbor distance between regenerated and baseline cells for cuprizone-treated mice (schematized above by the green and magenta oligodendrocytes displaced by larger distance d), and for both stable and newly generated oligodendrocytes in Control. ns, not significant. (D) Examples of maximum intensity projections of rendered pseudocolored tracings of newly appearing oligodendrocytes in cortical layer I (top zone). Complete reconstructions of cell bodies (black), processes (black) and myelin sheaths are shown for control (blue, 10 cells, N = 3 mice) and cuprizone treated mice (orange, 9 cells, N = 3 mice). E-F: Histograms comparing myelin sheath number (E), total length of myelin (F) and average length of individual myelin sheaths (G) between newly formed control and remyelinating oligodendrocytes. H: Graph showing distribution of sheath lengths for individual cells 12–14 days from first appearance (Control, blue; Remyelinating, orange, with mean and SEM in gray). I: 2D montage of oligodendrocyte morphologies (cell bodies and cytoplasmic processes) illustrating process orientation. Vectors were calculated from the cell body extending to each paranode of a reconstructed oligodendrocyte at days 12–14 and x and y vector components were summed and oriented to same direction of the vector sum. J: Vector plots showing the average orientation of oligodendrocyte processes for Control (N = 10 cells from three mice) and cuprizone treated mice (N = 9 cells from three mice). Oligodendrocyte process orientations were not significantly different from uniformly radial (J, Control, –0.047 ± 1.30 (std) rad; Regenerated, 0.076 ± 1.30 (std) rad), and exhibited similar degrees of circularity. See Supplementary file 1 for statistical tests and significance level for each comparison.
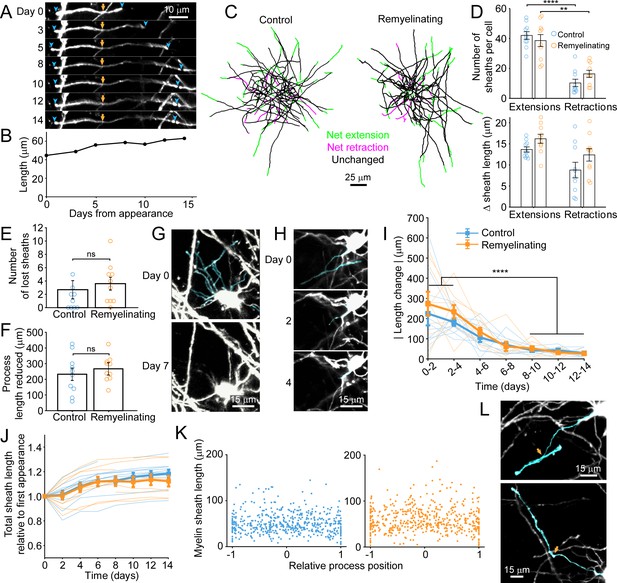
Dynamics of oligodendrocyte maturation in adult Mobp-EGFP mice.
Every cell body, and associated myelin sheath belonging to newly appearing oligodendrocytes in cortical layer I were traced using Simple Neurite Tracer (Image J) on day of appearance and imaged every 1–3 days for up to 14 days. (A) An individual myelin sheath (demarcated by blue arrowheads at the paranodal tips) at day 0 was followed over 14 days. The left-side paranode extends (through day 5) and then retracts and the right-side paranode extends to encounter a neighboring sheath and subsequently flanks a node of Ranvier (day 14). The yellow arrow marks the position where the oligodendrocyte process connects to the myelin sheath. (B) The overall length of the individual sheath shown in A increases over 14 days. Length was calculated in three dimensions. (C) Example maximum intensity projections of traced processes and myelin sheaths from newly formed cells in control or cuprizone-treated mice on day of appearance, that were followed over 14 days and length of individual traced sheaths are denoted as either unchanged (black) or exhibiting net extension (green) or retraction (magenta). (D) There were significantly more myelin sheaths undergoing extension than retraction in newly formed control and remyelinating cells (top) but no difference in net length change of extensions or retractions (bottom), and no significant difference between control or remyelinating cells. (E-F) There were low numbers of myelin sheaths lost in newly formed oligodendrocytes (E) and cytoplasmic processes were retracted (F), but there were no significant differences between control or remyelinating cells (control N = 10 cells, remyelinating N = 10 cells)). (G-H) Examples of cytoplasmic processes and myelin sheaths (cyan) present at day 0 in a newly formed remyelinating cell, that are no longer present at day 7 (G) or day 4 (H). In H, the myelin sheath is dissolved first (day 2) and then the process connecting it to the cell body retracts completely by day 4. (I-J) The absolute value of net total change in myelin sheath length over time is plotted for newly formed traced cells in control (blue) and cuprizone-treated (orange) cortex (thick line depicts means, thin lines represent individual cells). The majority of the length changes occur in the first 4 days after appearance. (J) Summed total length of all myelin sheaths per newly formed cell plotted as a proportion of the total length at day of appearance. The overall trend is extension of myelin for both control and remyelinating cells. (K) The length of individual myelin sheaths across all reconstructed cells plotted against the contact point of the cytoplasmic process to the myelin sheath, where 0 represents the center of an individual sheath (example in L, top panel, cyan process intersecting the cyan sheath towards the center of the sheath at orange arrow) and 1 or −1 are the distal tips of the sheath (example in L, bottom panel, cyan process intersecting at the paranode of the cyan sheath at orange arrow). See Supplementary file 1 for statistical tests and significance levels for each comparison.
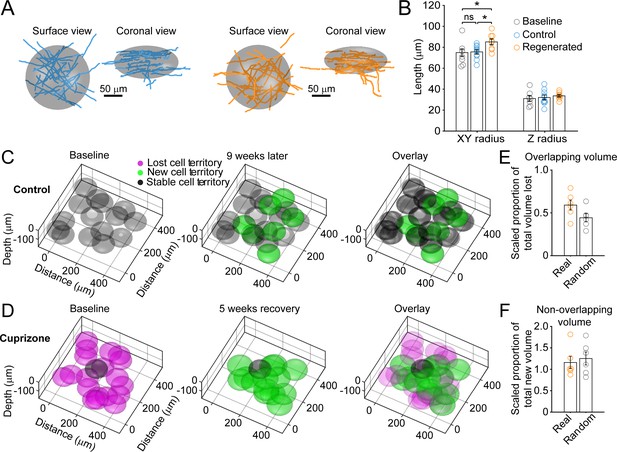
Remyelinating oligodendrocytes myelinate distinct cortical territories.
(A-B) Schematic showing best fit ellipsoids that encompass 80% of all myelin sheaths of oligodendrocytes from Control (blue) and Remyelinating (orange) cells in the 0–100 μm zone. B: Histogram comparing ellipsoid lengths for x-y and z radius for Control (blue, N = 10 cells from three mice), Cuprizone (orange, N = 9 cells from three mice) and Baseline (gray, N = 7 cells from four mice). Circles represent individual cells. Remyelinating cells were significantly wider (x-y radius) than newly generated oligodendrocytes in Control. (C-D: Average best-fit ellipsoids for Control (C) or Remyelinating cells (D) calculated in (B) plotted for top 0–100 μm of cortex (425 μm x 425 μm x 100 μm) based on location of cell bodies. E-F: Histograms showing proportion of overlap (total territory volume) between baseline and regenerated oligodendrocytes (E) (scaled to account for differences in number of baseline and regenerated cells; see Materials and methods), and additional volume encompassed by regenerated oligodendrocytes relative to total baseline volume (F), for 0–100 μm regions in cuprizone-treated mice (orange, N = 6 mice) compared to volumes predicted if the same number of regenerated cells appeared at random (gray). Regenerated oligodendrocyte territories partially overlap with baseline volume (E; 59.1%) and encompass novel territory (F; 115%), at a similar proportion to regenerated cells placed at random. See Supplementary file 1 for statistical tests and significance level for each comparison.
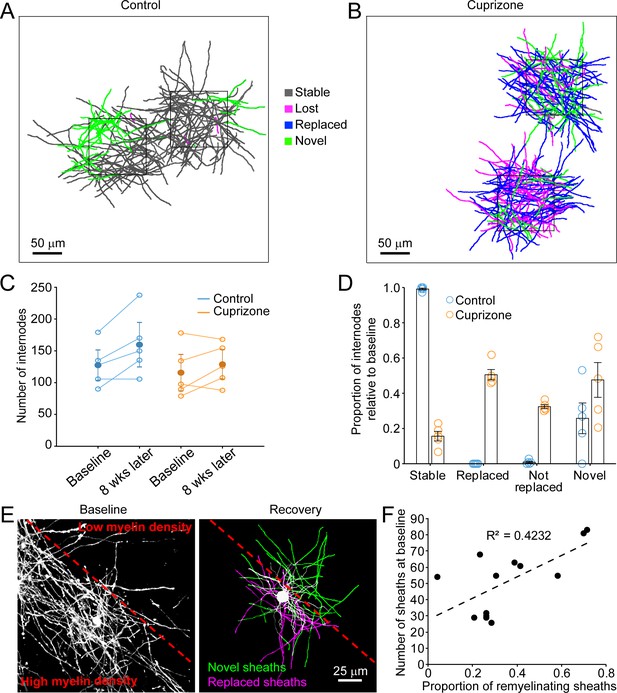
Oligodendrocyte regeneration results in a new pattern of cortical myelin.
(A–B) 2D rendering of individual myelin sheaths that passed through a 100 μm x 100 μm x 100 μm volume within the top 0–100 μm zone. Fate of each sheath from control (A) and cuprizone-treated (B) cortex shown as stable (black), lost (magenta), replaced (blue) or novel (green) across the time series. (C) Graph of total number of traced internodes at baseline and after 8 weeks of imaging (circles represent means for individual mice, with line connecting two time-points; mean for all mice is filled circle with SEM) for control (blue, N = 5), and cuprizone-treated (orange, N = 5) mice. (D) Histogram of internode fates from cuprizone-treated (orange, N = 5) and control (blue, N = 5) mice (circles represent mean proportional values relative to baseline from individual mice). Error bars are standard error of the mean. (E) Left panel, maximum intensity projection image (226 μm x 226 μm x 60 μm) illustrating myelin sheaths at baseline. Dashed red line demarcates an area of higher (lower left) and lower myelin density (top right). Right panel, rendering of a regenerated oligodendrocyte that appeared at 3 days recovery, which formed myelin sheaths that either replace those lost (magenta) or are novel (green). (F) Plot showing proportion of replaced sheaths per new oligodendrocyte relative to the number of baseline myelin sheaths present within the territory (average remyelinating ellipsoid, see Figure 4A,B) of the new cells (13 remyelinating oligodendrocytes from N = 4 mice), correlation co-efficient R2 = 0.4232.
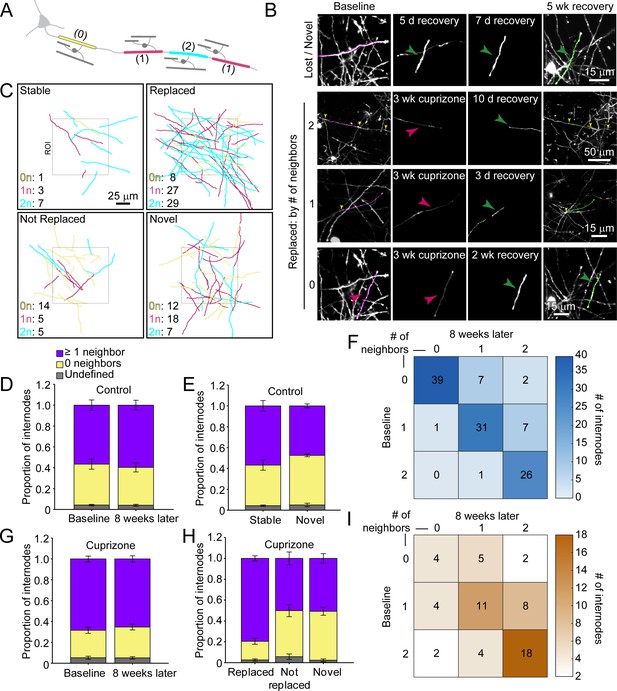
Regenerated oligodendrocytes preferentially remyelinate extensively myelinated axons.
(A) Schematic illustrating intermittent myelination of cortical axons, designating each internode by the number of flanking myelin sheaths (0, yellow; 1, magenta; or 2, cyan). (B) Example maximum intensity projections of lost (magenta) and new (green) myelin sheaths from cuprizone-treated mice. Top row, example of a lost, but not replaced, sheath, and a novel sheath not present at baseline (green arrowhead; also see Video 8). Remaining rows are examples of lost (magenta sheaths and arrowheads) and replaced internodes (green sheaths and arrowheads) that had 0 (see also Video 9), 1, or two neighboring sheaths at baseline. Yellow arrowheads in the two neighbor example indicate nodes of Ranvier. (C) 2D rendering of traced myelin sheaths that passed through a 100 μm x 100 μm x 100 μm volume within the top 0–100 μm zone. The fate of each sheath within the volume was determined as in Figure 5. Sheath color illustrates whether it had 0, 1 or two neighbors as in A. (D, G) Comparison of mean proportion of internodes with at least one neighbor (lavender), isolated (yellow), or undefined (gray) within a 100 μm x 100 μm x 100 μm volume at baseline and 8 weeks later, from control (D, N = 5) and cuprizone-treated (G, N = 5) mice. Volumes from both control and cuprizone-treated conditions have the same relative proportion of isolated vs. ≥1 neighboring internode at both time-points. (E, H) Comparison of the mean proportion of internodes with 0 or ≥1 neighbor that are stable or novel (control, (E) or replaced, not replaced, or novel (cuprizone, (H). There is no significant difference in the proportion of isolated vs. ≥1 neighbor population between stable and novel sheaths in control (E), and between lost and novel sheaths in cuprizone (H), but relatively more internodes with ≥1 neighbor were replaced in cuprizone-treated cortex (H). F, I: Myelination matrix illustrating average number of internodes categorized by number of neighbors at baseline and at final imaging time-point for control (F), and cuprizone-treated mice (I). Internodes with more neighbors at baseline are more likely be replaced (largest average # of internodes in bottom right of matrix). See Supplementary file 1 for statistical tests and significance levels for each comparison.
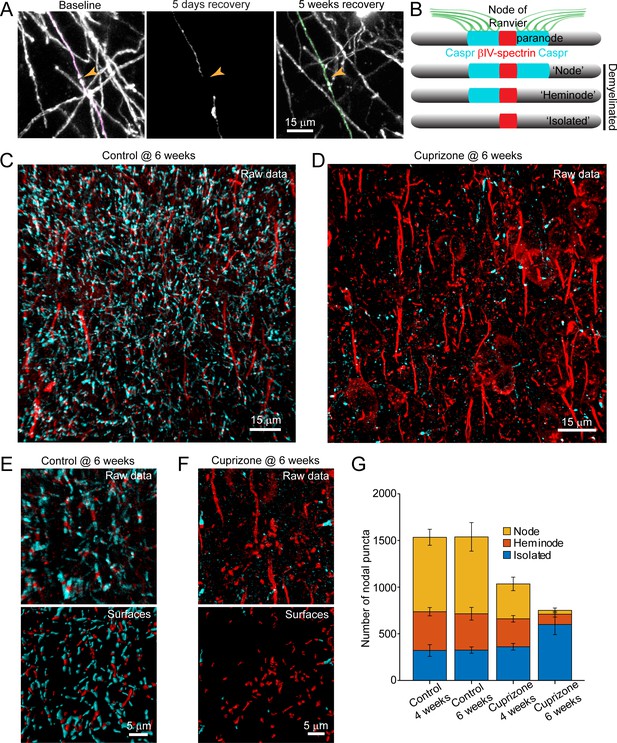
Structural components of the node of Ranvier persist after demyelination.
(A) Images showing fate of myelin sheaths along a single axon in a cuprizone treated mouse, illustrating the loss (magenta, baseline) and regeneration of its sheaths (green, 5 weeks recovery). Pseudocolored sheaths are overlaid on maximum intensity projections from a longitudinally imaged somatosensory cortical region. Orange arrowhead denotes location of node of Ranvier at baseline and at 5 weeks of recovery (see Video 10). (B) Schematic depicting axonal regions of a myelinated axon where βIV-spectrin (node of Ranvier, red) and Caspr (paranode, cyan) localize. After demyelination, βIV-spectrin can be found with two flanking Caspr puncta (‘Nodes’), one flanking Caspr punctum (‘Heminode’), or no nearby Caspr puncta (‘Isolated’). (C-D) Images βIV-spectrin and Caspr immunoreactivity in coronal sections from mice fed cuprizone-supplemented diet (D) or sham chow (C) for 6 weeks. E-F: Magnified views of βIV-spectrin and Caspr immunoreactivity (top panels, Raw data) from control (E) and cuprizone-treated (F) brains. Examples of post-processed (lower panels, Surfaces) images used to calculate nearest neighbor distances between βIV-spectrin puncta and Caspr puncta for images in E and F. Axon initial segments (AIS) were excluded from surface rendering. There was no significant difference in AIS between control (54.3 ± 2.4) and cuprizone-treated (59.3 ± 3.7) mice (p=0.354, two-tailed unpaired t-test). (G) Histogram showing total βIV-spectrin puncta categorized as either Node, Heminode or Isolated. Fewer βIV -spectrin puncta were observed after cuprizone, but isolated puncta become more numerous with increasing duration of cuprizone exposure (control @ 4 weeks, N = 3 mice; control @ 6 weeks, N = 3 mice; cuprizone @ 4 weeks, N = 4 mice; cuprizone @ 6 weeks, N = 4 mice). Bars are SEM.
Videos
Loss and replacement of oligodendrocytes.
Longitudinal in vivo imaging of demyelination and remyelination. This is a 392 μm x 392 μm x 100 μm volume shown as a maximum intensity projection that was repeatedly imaged through a chronic cranial window over the somatosensory cortex in an adult Mobp-EGFP mouse, at baseline, over 3 weeks of cuprizone administration, and then through 5 weeks of recovery. Scale bar is 50 μm.
New oligodendrocytes are added in the upper cortical layers in adult mice.
Longitudinal imaging of an adult Mobp-EGFP mouse with a chronic cranial window fed sham diet. Region corresponds to images shown in Figure 1E, top row. Scale bar is 25 μm.
Oligodendrocytes are lost and new cells appear after cuprizone-treatment.
Longitudinal imaging of an adult Mobp-EGFP mouse with a chronic cranial window fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery. Region corresponds to images shown in Figure 1E, bottom row. Scale bar is 25 μm.
New oligodendrocyte cell bodies appear in locations that are different than those of lost oligodendrocytes.
Longitudinal imaging of an adult Mobp-EGFP mouse with a chronic cranial window fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery. All oligodendrocytes from the example region in Figure 4B (cortical volume: 425 μm (X) x 425 μm (Y) x 300 μm (Z)) are shown in three dimensions at each imaging time point, with a rotation around the Z (depth) axis. The final image is an overlay of pseudocolored cells present at baseline (magenta) or 5 weeks recovery (green), illustrating that remyelinating cells appear in distinct locations from those present at baseline.
Myelin sheaths are lost and not replaced over time.
An example volume (100 μm x 100 μm x 100 μm) within the top 0–100 μm zone from an adult Mobp-EGFP mouse with a chronic cranial window who was fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery and longitudinally imaged (same region as depicted in Figure 6C; Video 6 and 7). Twenty-four individual myelin sheaths present at baseline and lost by 5 weeks of recovery are pseudocolored in magenta and overlaid with high-resolution images of EGFP-signal (white) at baseline, 3 days recovery (peak demyelination) and 5 weeks recovery time-points. For each time-point, the movie starts at the pial surface and proceeds to a depth of 100 μm in 1 μm steps. Note that magenta pseudocolored sheaths overlay EGFP+ myelin sheaths at baseline, but not at 5 weeks of recovery.
Novel myelin sheaths are generated during remyelination.
An example volume (100 μm x 100 μm x 100 μm) within the top 0–100 μm zone from adult Mobp-EGFP mouse with a chronic cranial window who was fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery and longitudinally imaged (same region as depicted in Figure 6C; Video 5 and 7). Thirty-seven individual myelin sheaths newly formed by 5 weeks of recovery, but not present at baseline, are pseudocolored in green and overlaid with high-resolution images of EGFP-signal (white) at baseline, 3 days recovery (peak demyelination) and 5 weeks recovery time-points. For each time-point, the movie starts at the pial surface and proceeds to a depth of 100 μm in 1 μm steps. Note that green pseudocolored sheaths overlay EGFP+ myelin sheaths 5 weeks of recovery, but not at baseline.
Individual myelin sheaths are replaced following demyelination.
An example volume (100 μm x 100 μm x 100 μm) within the top 0–100 μm zone from an adult Mobp-EGFP mouse with a chronic cranial window who was fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery and longitudinally imaged (same region as depicted in Figure 6C; Video 5 and 6). Sixty-four individual myelin sheaths which were present at baseline, lost, and then replaced by 5 weeks of recovery are pseudocolored in blue and overlaid with high-resolution images of EGFP-signal (white) at baseline, 3 days recovery (peak demyelination) and 5 weeks recovery time-points. For each time-point, the movie starts at the pial surface and proceeds to a depth of 100 μm in 1 μm steps. Note that blue pseudocolored sheaths overlay EGFP+ myelin sheaths at baseline and at 5 weeks of recovery.
Myelin sheaths are lost and novel sheaths are formed after cuprizone-treatment.
Longitudinal imaging of an adult Mobp-EGFP mouse with a chronic cranial window fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery. A myelin sheath at baseline (traced and pseudocolored magenta, overlaid in maximum intensity projection of longitudinally-imaged region), degenerates over time (only the traced sheath from baseline is shown in subsequent time-points, and is lost by 1 week of recovery). At 5 days of recovery, a novel isolated sheath (not present at baseline, traced and pseudocolored in green in the 5 week recovery time-point overlay) appears, formed by a remyelinating oligodendrocyte not present at baseline (cell in 5-week recovery time-point overlay). Scale bar is 15 μm.
Isolated myelin sheaths are replaced.
Longitudinal imaging of an adult Mobp-EGFP mouse with a chronic cranial window fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery. An isolated myelin sheath at baseline (traced and pseudocolored magenta, overlaid in maximum intensity projection of longitudinally imaged region), degenerates over time (only the traced sheath from baseline is shown in subsequent time-points, and is lost by 3 days of recovery). At 5 days of recovery a replacement isolated sheath appears (traced and pseudocolored in the 5 week recovery time-point overlay), formed by a remyelinating oligodendrocyte not present at baseline (cell in 5-week recovery time-point overlay). Scale bar is 15 μm.
Neighboring myelin sheaths with at least one neighbor are replaced and form a node of Ranvier in close proximity to one present at baseline.
Longitudinal imaging of an adult Mobp-EGFP mouse with a chronic cranial window fed 3 weeks of a cuprizone-supplemented diet followed through 5 weeks of recovery. Two neighboring myelin sheaths (pseudocolored magenta in baseline time-point), flank an unlabeled node of Ranvier (paranodal loops of myelin accumulate cytoplasmic EGFP in Mopb-EGFP mice [Hughes et al., 2018]); these sheaths were traced over each imaging time-points, and degenerate after cuprizone-treatment. Remyelinating oligodendrocytes (one shown in 5-week recovery time-point) form replacement myelin sheaths (pseudocolored green in 5-week recovery time-point flank an unlabeled node of Ranvier in a similar position as that observed at baseline. Scale bar is 15 μm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Stain, strain background (M. musculus) | STOCK Tg(Mobp-EGFP) IN1Gsat/Mmucd | MMRC | RRID:MMRRC_030483- UCD | Hughes et al., 2018 |
| Stain, strain background (M. musculus) | C57BL/6NCrl | Charles River | RRID:IMSR_CRL:27 | |
| Antibody | Rabbit anti-Aspartoacylase (ASPA) | Genetex | RRID:AB_2036283 | 1:1500 |
| Antibody | Goat anti-GFP | Cell signalling | RRID:AB_10705523 | 1:500 |
| Antibody | Chicken anti-GFP | Aves lab | RRID:AB_2307313 | 1:1500 |
| Antibody | Rabbit anti-GFP | Richard Huganir lab | Gift of R. Huganir | 1:1000 |
| Antibody | Mouse anti-MBP | Sternberger | RRID:AB_2564741 | 1:2000 |
| Antibody | Chicken anti-MBP | Aves | RRID:AB_2313550 | 1:500 |
| Antibody | Guinea pig anti-NG2 | Generated in D.E. Bergles lab against entire NG2 protein Kang et al., 2013 | 1:10,000 | |
| Antibody | Rabbit anti- βIV spectrin | Generated by M. Rasband Lab | Gift from M. Rasband | 1:300 |
| Antibody | Chicken anti- βIV spectrin | Generated by M. Rasband Lab | Gift from M. Rasband | 1:100 |
| Antibody | Rabbit anti-Ankrin G | Generated by M. Rasband Lab | Gift from M. Rasband | 1:200 |
| Antibody | Guinea pig anti-Caspr | Generated by M. Bhat Lab | Gift from M. Bhat | 1:1500 |
| Antibody | Rabbit anti-Neurofilament-L | Cell Signaling | RRID:AB_823575 | 1:1000 |
| Antibody | Donkey anti-Rabbit conjugated to Alexa 488, Cy3 or Cy5 | Jackson Immuno | RRID:AB_2340619 RRID:AB_2313568 RRID:AB_2340625 | 1:2000 |
| Antibody | Donkey anti- Mouse conjugated to Alexa 488, Cy3 or Cy5 | Jackson Immuno | RRID:AB_2340849 RRID:AB_2340817 RRID:AB_2340820 | 1:2000 |
| Antibody | Donkey anti- Guinea pig conjugated to Alexa 488, Cy3 or Cy5 | Jackson Immuno | RRID:AB_2340454 RRID:AB_2340461 RRID:AB_2340477 | 1:2000 |
| Antibody | Donkey anti- Goat conjugated to Alexa 488 or Cy3 | Jackson Immuno | RRID:AB_2340430 RRID:AB_2340413 | 1:2000 |
| Antibody | Donkey anti- Chicken conjugated to Alexa 488 or Cy5 | Jackson Immuno | RRID:AB_2340376 RRID:AB_2340347 | 1:2000 |
| Chemical compound, drug | bis(cyclohexanone) oxaldihydrazone (Cuprizone) | Sigma-Aldrich | Catalog # C9012 | |
| Software, algorithm | ZEN Blue/Black | Zeiss | RRID:SCR_013672 | |
| Software, algorithm | Fiji | http://fiji.sc | RRID:SCR_002285 | |
| Software, algorithm | ImageJ | https://imagej.nih.gov/ij/ | RRID:SCR_003070 | |
| Software, algorithm | Adobe Illustrator CS6 | Adobe | RRID:SCR_014198 | |
| Software, algorithm | MATLAB | Mathworks | RRID:SCR_001622 | |
| Software, algorithm | SyGlass | IstoVisio | RRID:SCR_017961 | |
| Software, algorithm | Imaris | Bitplane | RRID:SCR_007370 |
Additional files
-
Source data 1
Source data for figures.
- https://cdn.elifesciences.org/articles/56621/elife-56621-data1-v3.xlsx
-
Supplementary file 1
Summary of statistical tests and significance level for comparisons by comparison for each referenced figure panel.
- https://cdn.elifesciences.org/articles/56621/elife-56621-supp1-v3.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/56621/elife-56621-transrepform-v3.pdf





