Retrieval practice facilitates memory updating by enhancing and differentiating medial prefrontal cortex representations
Figures
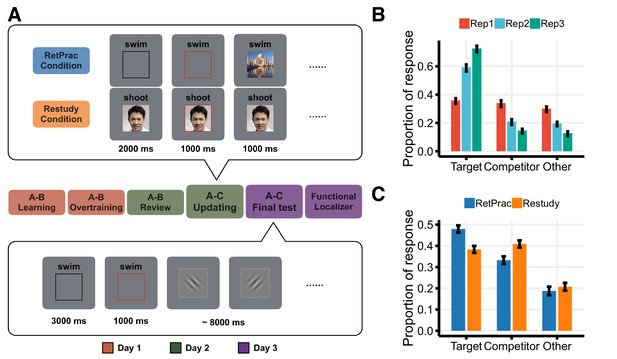
Experimental design and behavioral results.
(A) Experimental design. On Day 1, subjects were over-trained with 144 pairs of word (A) – picture (B) associations. On Day 2, subjects were introduced to 144 new A-C associations (B and C were always from different visual categories) and asked to replace the old associations with the new ones before entering the scanner. In the scanner, each new A-C association was studied three times under one of the two updating conditions: Retrieval Practice (RetPrac) vs Restudy. Each trial started with a recall phase showing the cue word A paired either with a black rectangle (RetPrac) or with the associated picture C (Restudy). Two seconds after the recall phase, a red rectangle lasting for 1 s was shown and subjects needed to judge the category of picture C within this response window. Then the correct picture C was shown on the screen for 1 s as a feedback. On Day 3, subjects performed the A-C memory test while being scanned. The recall phase lasted for 3 s followed by a 1 s response window. Then subjects were asked to perform a perceptual orientation judgment task for 8 s. (B) Proportions of responses of targets (correctly choosing A-C), competitors (wrongly choosing A-B), and 'others' as shown according to each of the three study repetitions during updating. (C) The proportions of recalled targets, competitors, and 'other' categories during the final memory test one day after updating practice.
-
Figure 1—source data 1
Memory performance during Updating (Exp1).
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig1-data1-v2.csv
-
Figure 1—source data 2
Memory performance during final test (Exp1).
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig1-data2-v2.csv
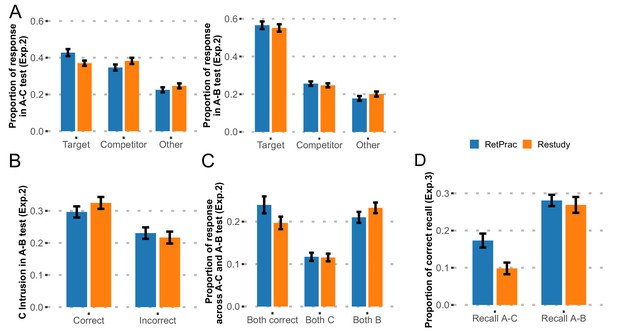
Results of follow-up behavioral Experiments 2 and 3.
(A) Behavioral results from Exp. 2. The proportions of recalled targets, competitors, and 'other' categories during the final A-C and A-B memory tests one day after updating practice. Note, the targets and competitors were referred to as A-C and A-B memory in the A-C memory test, but as A-B and A-C memory in A-B memory test. (B) The proportions of A-C memory intrusions during A-B recall, as determined by whether the correct A-C memory was recalled during the A-C test. (C) Joint analysis for both A-B and A-C memories revealed memory differentiation. (D) Behavioral results from Exp. 3. The proportions of correct item recall during the final A-C and A-B memory tests one day after updating practice.
-
Figure 2—source data 1
Memory performance during final test (Exp2).
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig2-data1-v2.csv
-
Figure 2—source data 2
Memory performance during final test (Exp3).
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig2-data2-v2.csv
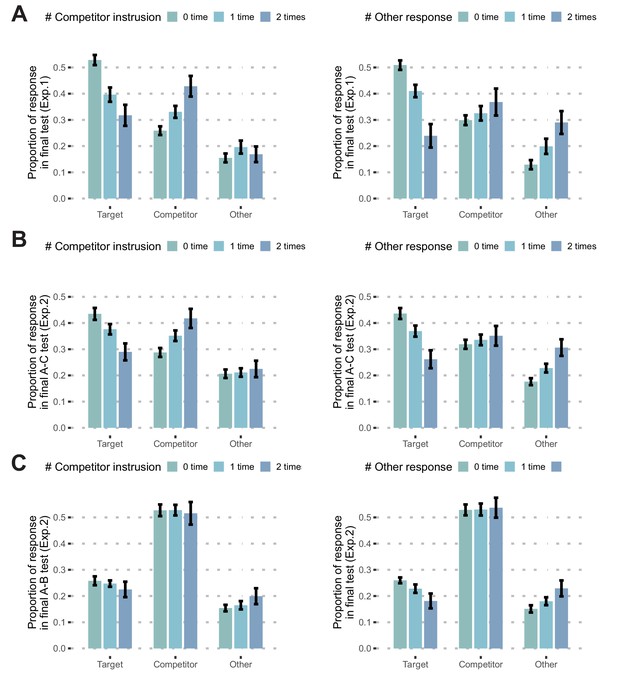
Incorrect responses during updating and memory performance during the final test.
(A) Proportion of responses during the final test in Exp. 1 by the pair’s learning history during updating. The left panel shows that pairs with more competitor intrusions had fewer target responses (2 times vs 0 times, t(18) = −4.07, p<0.001), more competitor responses (t(18) = 4.06, p<0.001), and comparable 'other' responses (t(18) = 0.55, p=0.59) during the final memory test. For the number of 'other' responses (right panel), pairs with more 'other' responses during updating had fewer target responses (t(18) = −5.86, p<0.001), more 'other' responses (t(18) = 3.85, p=0.001), and comparable competitor responses (t(18) = 1.38, p=0.18) during the final test. There were more A-B intrusions than 'other' responses during the final test (t(18) = 4.47, p<0.001), even when the number of responses during updating was matched, suggesting strong A-B interference. (B) Proportion of responses during the final A-C memory test in Exp. 2. The results replicated those of Exp. 1. A greater number of competitor intrusions was related to fewer target responses (t(45) = −4.24, p<0.001), more competitor responses (t(45) = 3.79, p<0.001), and comparable 'other' responses (t(45) = 0.52, p=0.60) during the final A-C memory test. Again, a greater number of 'other' responses was related to fewer target responses (t(45) = −4.63, p<0.001), more 'other' responses (t(45) = 3.85, p<0.001), and comparable competitor responses (t(45) = 0.88, p=0.38) during the final A-C memory test. There were many more A-B intrusions than 'other' responses during the final test (t(45) = 5.46, p<0.001). (C) Proportion of responses during the final A-B memory test in Exp. 2. In terms of the memory for A-B pairs, neither the number of competitor intrusions nor that of 'other' responses during updating was related to A-B memory performance (competitor intrusions: t(45) = −0.25, p=0.80; 'other' responses: t(45) = 0.24, p=0.81). Error bars indicate within-subject standard error.
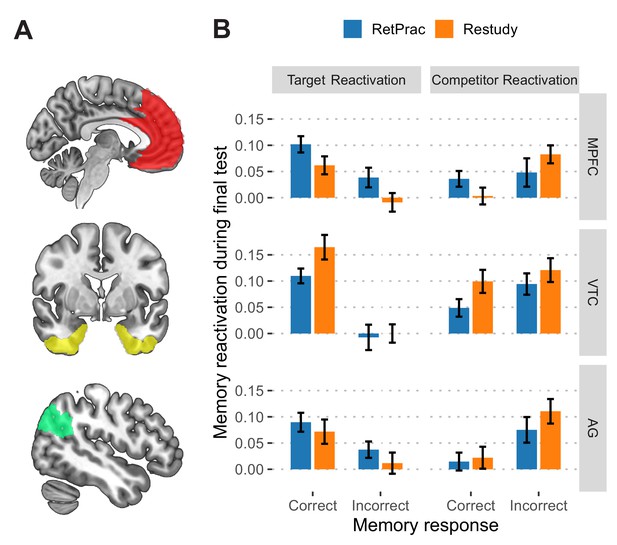
Neural reactivation during the final memory test.
(A) Depiction of the anatomical ROIs used in the main analysis. All ROIs consisted of regions from both hemispheres. (B) The reactivation of target (picture C) and competitor (picture B) during the final test as a function of updating method and memory outcome, based on classifier outputs (after subtracting 'other' evidence). Error bars indicate within-subject standard errors.
-
Figure 3—source data 1
Classifier evidence during the final test.
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig3-data1-v2.csv
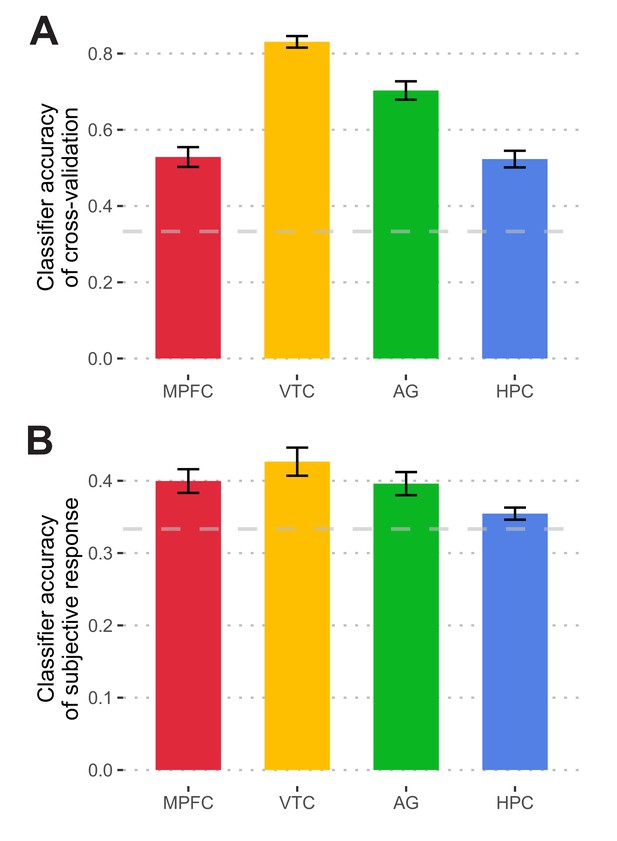
Classifier performances.
(A) Cross-validation accuracy for functional localizer data. To examine the performance of the classifier, a leave-one-run-out cross-validation procedure was conducted on functional localizer data. The average accuracy was significantly above the chance level (ranging from 52.3% to 83.1%, all p-values <0.001, survived FDR correction). (B) Classifier accuracy in predicting subjective responses during the final test. Classifier outputs predicted subjects’ subjective responses significantly better than the chance level in the MPFC, VTC, and AG (ranging from 39.6% to 42.6%, all p-values <0.001, with FDR correction), but not in HPC (35.5%, t(18) = 0.96, p=0.35). Error bars indicate within-subject standard errors. The dashed line indicates the chance level.
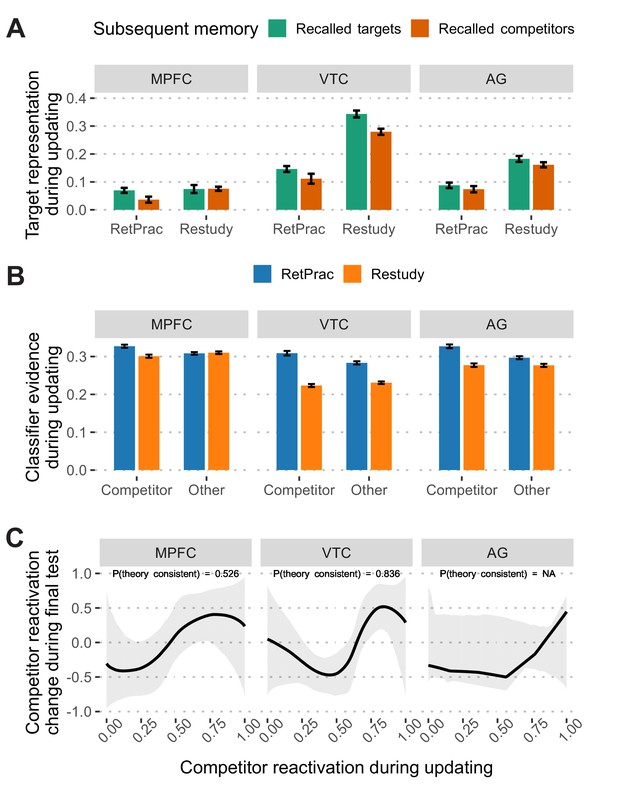
Memory reactivation during updating and its change in the final test.
(A) Target memory representation (after subtracting 'other' evidence) during updating as a function of subsequent memory performance (correctly recalled targets vs incorrectly recalled competitors) during the final memory test. (B) Classifier evidence of competitor and other categories during the A-C updating phase under the Restudy and RetPrac conditions. Restudy was associated with weaker competitor reactivation. (C) Model fitting of the nonmonotonic plasticity hypothesis under the RetPrac condition. Only VTC showed the hypothesized pattern in which modest competitor reactivation (normalized into [0, 1] range) weakened, and strong competitor reactivation enhanced later competitor memory reactivation. Error bars indicate within-subject standard errors.
-
Figure 4—source data 1
Classifier evidence during memory updating by subsequent memory performance.
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig4-data1-v2.csv
-
Figure 4—source data 2
Classifier evidence during memory updating.
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig4-data2-v2.csv
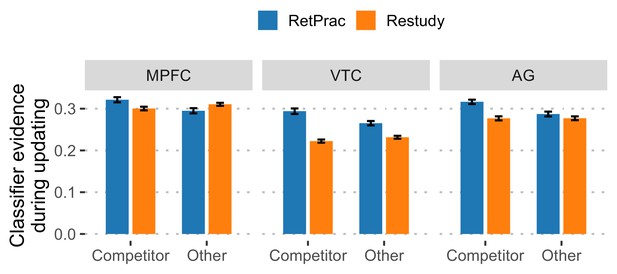
Classifier evidence of competitor and 'other' categories for correct trials during the A-C updating phase.
A similar pattern was found when only correct (target) trials were included. There was greater competitor than 'other' evidence under the RetPrac condition (all p-values <0.017), but comparable levels of evidence under the Restudy condition (all p-values >0.07). Direct comparisons also revealed significantly stronger competitor reactivation under the RetPrac than under the Restudy condition in all regions (all p-values <0.005).
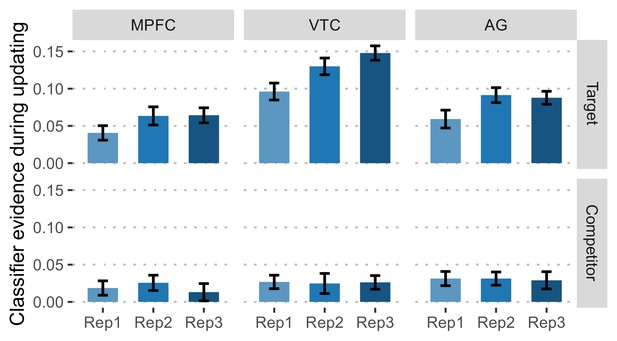
Change of competitor and target evidence with training under the RetPrac condition.
Target and competitor evidence as a function of repetition under the RetPrac condition during memory updating. Error bars indicate within-subject standard errors.
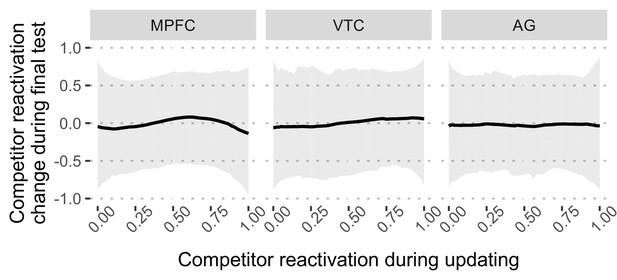
Nonmonotonic plasticity model fitting the results for the Restudy condition.
Under the Restudy condition, all three regions failed on the model fitting procedure, resulting in unreliably fitted curves. This may be due to the relatively weak competitor reactivation during restudy, which did not cover the full range of the nonmonotonic plasticity curve.
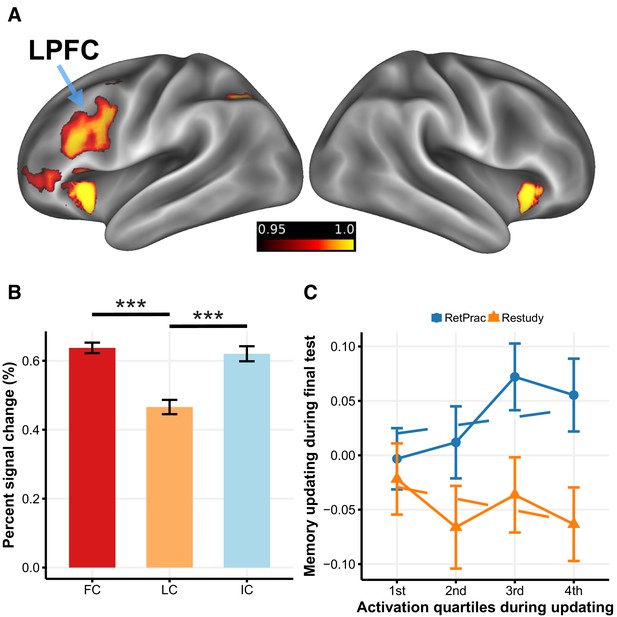
LPFC activity and memory updating under the RetPrac condition.
(A) Brain regions that showed greater activation during memory updating under the RetPrac than under the Restudy condition. The color bar indicates one minus the P-value (corrected). (B) Activity in the LPFC was sensitive to pairs’ updating performance. The failed recall (IC) and the successfully recalled the first time (first correct, FC) trials showed greater activation than the successfully recalled the second or the third time (later correct, LC) trials, indicating that the LPFC was involved in inhibiting competitive memories. (C) MPFC memory updating (target representation minus competitor representation) as a function of LPFC activation during updating (divided by quartiles). Error bars indicate within-subject standard errors.
-
Figure 5—source data 1
Percent signal change by conditions during memory updating.
- https://cdn.elifesciences.org/articles/57023/elife-57023-fig5-data1-v2.csv
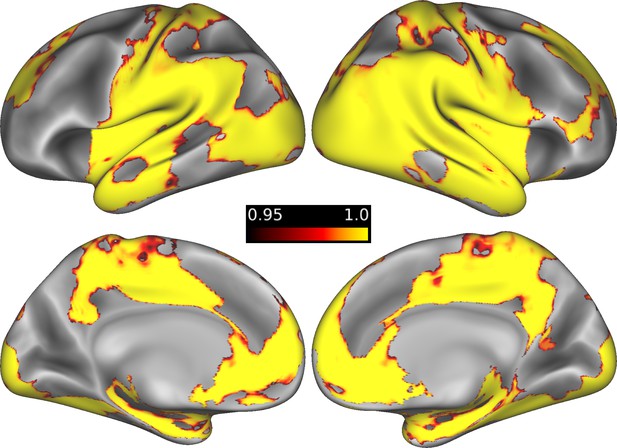
Brain regions showing greater activation for restudy than retrieval practice.
Regions showing greater activation during memory updating under the Restudy condition than under the RetPrac condition. Stronger activation was found in the medial prefrontal cortex (MPFC), bilateral hippocampus (HPC), ventral visual regions, medial parietal cortex, and lateral parietal cortex (which extended to the lateral temporal cortex). The color bar indicates one minus the P value (corrected). Please refer to Supplementary file 3b for details.
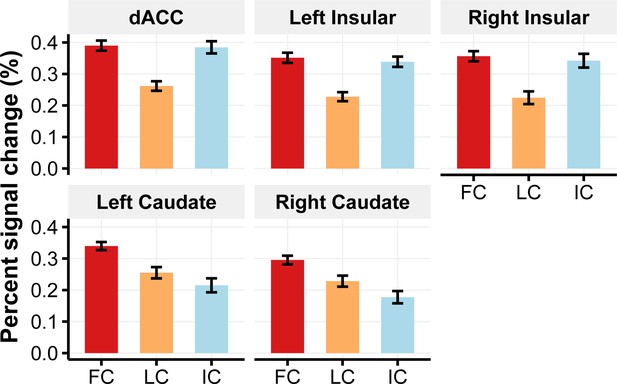
Brain activity as a function of memory performance.
During A-C updating, the dACC, bilateral anterior insular cortex, and caudate showed greater activities (percent signal change) under the RetPrac condition than under the Restudy condition. Further analysis indicated that activations in these regions were differentially modulated by updating the performance of each item. Incorrectly recalled trials (IC) and the successfully recalled trials for the first time for a given item (first correct, FC) showed greater activation than items corrected recalled againfor a second or third time (later correct, LC) in the dACC and bilateral anterior insular cortex, indicating their role in conflict monitoring. By contrast, the caudate showed greater activity for FC trials than for all other trials, suggesting its role in positive prediction error. Error bars indicate within-subject standard errors. Please refer to Supplementary file 3c for statistical details.
Additional files
-
Supplementary file 1
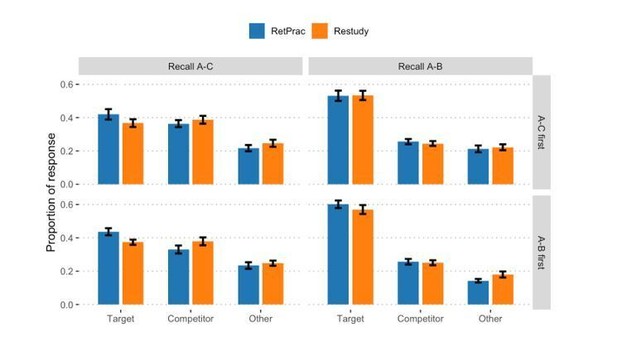
Update method (RetPrac, Restudy) x Test order (A-C first, A-B first) two-way ANOVA table by Memory test type and Response type.
- https://cdn.elifesciences.org/articles/57023/elife-57023-supp1-v2.docx
-
Supplementary file 2
ANOVA tables of classifier evidence tests.
(a) Updating method (RetPrac, Restudy) x Classifier evidence (Target, Competitor) x Memory outcome (Correct, Incorrect) three-way ANOVA table. (b) Updating method (RetPrac, Restudy) x Memory outcome (Correct, Incorrect) two-way ANOVA table by Classifier evidence type.
- https://cdn.elifesciences.org/articles/57023/elife-57023-supp2-v2.docx
-
Supplementary file 3
Tables of univariate activation contrasts.
(a) Regions showing greater activation during updating under the RetPrac condition than under the Restudy condition. (b) Regions showing greater activation during updating under the Restudy condition than under the RetPrac condition. (c) Pairwise comparisons of brain activity between trials with different updating performance.
- https://cdn.elifesciences.org/articles/57023/elife-57023-supp3-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/57023/elife-57023-transrepform-v2.docx