Activation of astrocytes in hippocampus decreases fear memory through adenosine A1 receptors
Figures
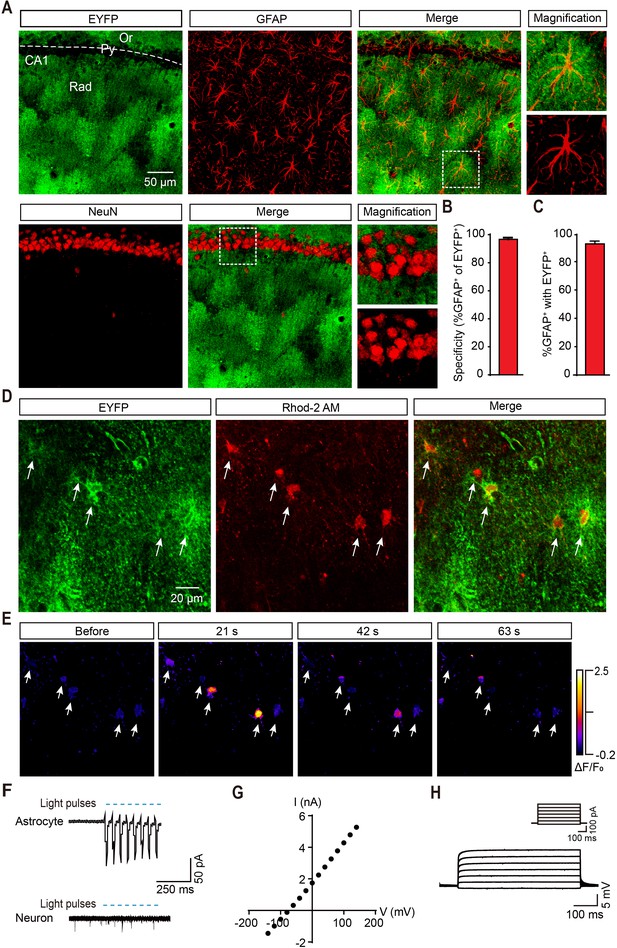
Specific ChR2 expression and light-induced Ca2+ elevation in CA1 astrocytes of GFAP-ChR2-EYFP rats.
(A) Immunohistochemistry (IHC) confocal images showing co-localization of EYFP labeling with the specific astrocytic marker GFAP, but not with the neuronal marker NeuN (right, higher magnification images; scale bar, 50 µm; Or, stratum oriens; Py, stratum pyramidale; Rad, stratum radiatum). (B and C) Quantification of the percentages of co-localization cells in astrocytes positive for EYFP (148/153 cells from six rats) and GFAP (148/160 cells from six rats). (D) Confocal images showing ChR2-expressing astrocytes loaded with the Ca2+ fluorescent dye Rhod-2 AM. (E) Example time-lapse images of Ca2+ signals before and 21, 42, and 63 s after the termination of light stimulation (488 nm, 10 s). (F) Light pulses (blue bars, 473 nm, 20 ms, 10 Hz) reliably induce inward currents in the ChR2-expressing astrocytes but not in neurons in the hippocampal slice. (G) Linear current-voltage relationship of an astrocyte in voltage-clamp mode. (H) Voltage responses from an astrocyte evoked by currents in steps of 120 pA from –100 pA to +680 pA. This figure has one figure and one video supplements.
-
Figure 1—source data 1
Data for Figure 1.
Quantification of the percentages of co-localization cells in astrocytes positive for EYFP and GFAP; values for current and voltage of an astrocyte in voltage-clamp mode.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig1-data1-v2.xlsx
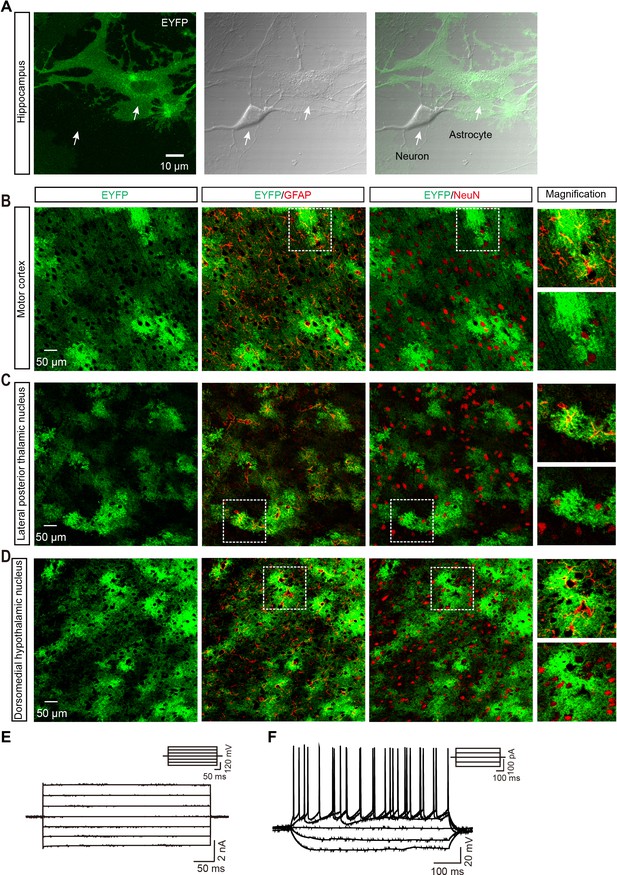
ChR2-EYFP is specifically expressed in astrocytes.
(A) Example images showing co-localization of ChR2-EYFP with astrocytes co-cultured with neurons in the hippocampus. (B–D) Specific ChR2 expression in astrocytes in the motor cortex (B), lateral posterior thalamic nucleus (C), and dorsomedial hypothalamic nucleus (D) of GFAP-ChR2-EYFP rats. Right, higher magnification images. (E) Current responses of an astrocyte evoked by clamping voltage (400 ms, 40 mV) ranging from –140 mV to +140 mV. (F) Voltage responses of a neuron evoked by injection of current steps (800 ms, 40 pA) ranging from –100 pA to +100 pA.
Light stimulation increases Ca2+ levels in astrocytes expressing ChR2.
Example video of Ca2+ imaging from a slice expressing ChR2-EYFP.
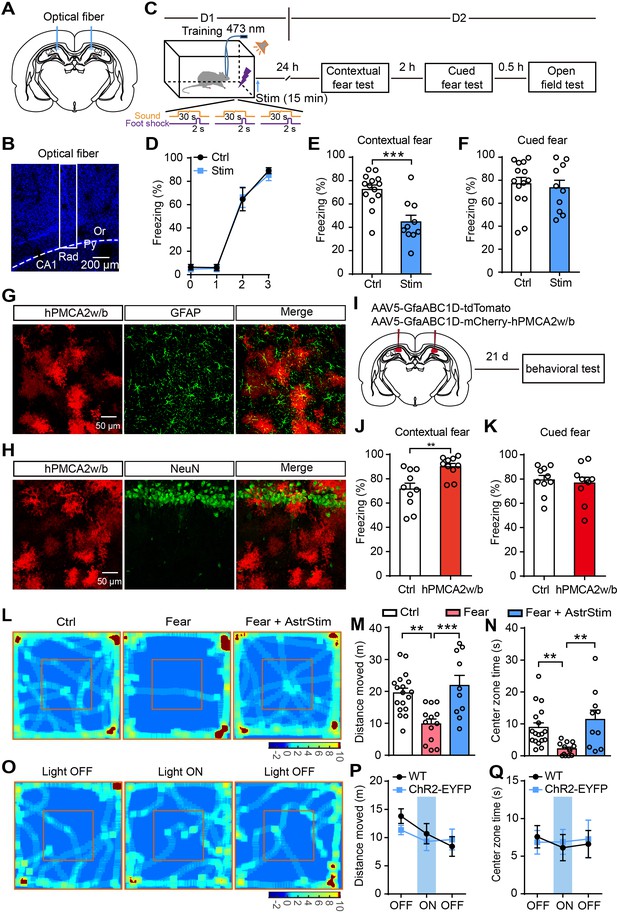
Optogenetic activation of astrocytes reduces fear memory and fear-related anxiety.
(A and B) Schematic and confocal images of coronal sections showing the placement of optical fibers. (C) Schematic of the experimental design of fear conditioning, photostimulation, and the subsequent test protocols. (D) Freezing levels of control (sham operation, n = 14) and photostimulated GFAP-ChR2-EYFP rats (n = 10) during fear conditioning. (E and F) Freezing levels of control and photostimulated rats during contextual (E, p=0.0004, Student’s unpaired two-tailed t-test) and cued fear tests (F, p=0.4377) on day 2. (G and H) IHC confocal images showing co-localization of mCherry labeling with the specific astrocytic marker GFAP, but not with the neuronal marker NeuN. (I) AAV5 microinjection for expressing tdTomato and human PMCA2w/b (hPMCA2w/b) in hippocampal astrocytes. (J and K) Freezing levels of control (n = 10) and hPMCA2w/b (n = 10) rats during contextual fear memory (J, p=0.0044, Student’s unpaired two-tailed t-test) and cued fear memory tests (K, p=0.6784). (L) Representative heatmaps of movement in the open field. Left, Ctrl, rats without fear conditioning; middle, Fear, rats with sham operation and fear conditioning; right, Fear+AstrStim, rats with photostimulation after fear conditioning. (M) Total distance moved of Ctrl (n = 18), Fear (n = 13), and Fear+AstrStim (n = 10) rats in the OFT (p=0.0018, p=0.0008, one-way ANOVA and Tukey's post-hoc test). (N) Center zone exploration time of Ctrl, Fear, and Fear+AstrStim rats in the OFT (p=0.0011, p=0.0033, Kruskal-Wallis and Dunn's post-hoc test). (O) Representative heatmaps of movement in the open field during light OFF–ON–OFF. (P) Total distance moved by WT and GFAP-ChR2-EYFP rats in the open field during light OFF-ON-OFF (group effect, F(2, 78)=2.423, p=0.0953; epoch effect, F(1, 78)=0.406, p=0.5259; interaction, F(2, 78)=0.652, p=0.5238, two-way repeated-measures ANOVA and Bonferroni post-hoc test). (Q) Center zone exploration time of WT and GFAP-ChR2-EYFP rats in the open field during light OFF–ON–OFF (group effect, F(2, 78)=0.05796, p=0.9437; epoch effect, F(1, 78)=0.01926, p=0.89; interaction, F(2, 78)=0.08292, p=0.9205, two-way repeated-measures ANOVA and Bonferroni post-hoc test). Error bars show the mean ± SEM. **p<0.01, ***p<0.001. This figure has four figure supplements.
-
Figure 2—source data 1
Data for Figure 2.
Values for freezing levels during fear-conditioning test; values for moving distance and center zone exploration time in the open-field test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig2-data1-v2.xlsx
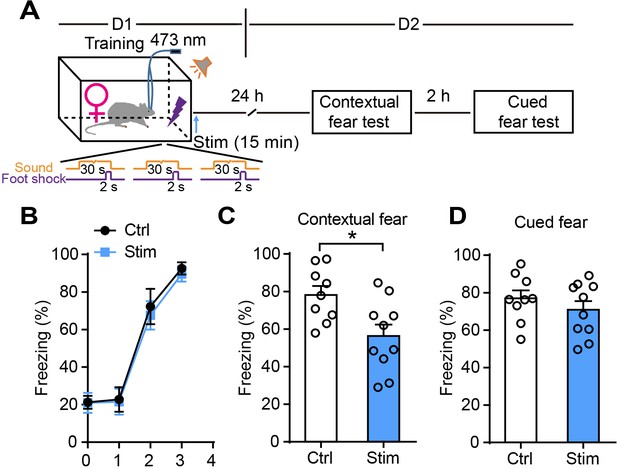
Optogenetic activation of astrocytes in female rats reduces fear memory.
(A) Schematic of the experimental design of fear conditioning, photostimulation, and the subsequent test protocols. (B) Freezing levels of control (sham operation, n = 9) and photostimulated GFAP-ChR2-EYFP rats (n = 10) during fear conditioning. (C and D) Freezing levels of control and photostimulated rats during contextual (C, p=0.0125, Student’s unpaired two-tailed t-test) and cued fear tests (D, p=0.3424) on day 2. Error bars show the mean ± SEM. *p<0.05.
-
Figure 2—figure supplement 1—source data 1
Data for Figure 2—figure supplement 1.
Values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig2-figsupp1-data1-v2.xlsx
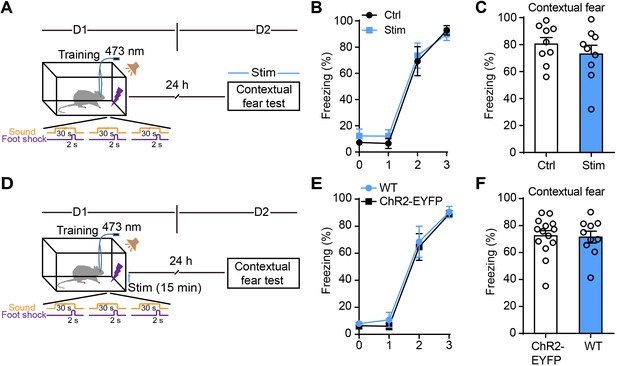
Photostimulation of GFAP-ChR2-EYFP rats during the recall test or photostimulation of WT rats after fear conditioning have no effect on contextual fear memory.
(A, D) Schematic of the experimental design of fear conditioning, photostimulation, and the subsequent test protocols. (B) Freezing levels of control (sham operation, n = 9) and photostimulated GFAP-ChR2-EYFP rats (n = 9) during fear conditioning. (C) Freezing levels tested on day 2 in rats with or without photostimulation during recall (p=0.3758, Student’s unpaired two-tailed t-test). (E) Freezing levels of GFAP-ChR2-EYFP rats (sham operation, n = 14) and photostimulated littermate WT rats (n = 10) during fear conditioning. (F) Freezing levels tested on day 2 of GFAP-ChR2-EYFP and littermate WT rats (p=0.8755, Student’s unpaired two-tailed t-test). Error bars show the mean ± SEM.
-
Figure 2—figure supplement 2—source data 1
Data for Figure 2—figure supplement 2.
Values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig2-figsupp2-data1-v2.xlsx
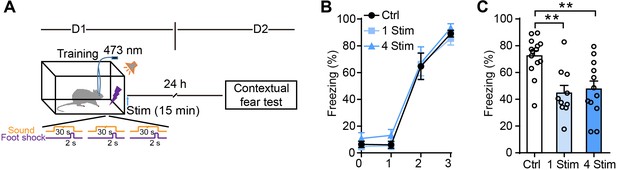
Longer duration photostimulation induces a similar degree of fear memory attenuation.
(A) Schematic of the experimental design of fear conditioning, photostimulation, and the subsequent test protocols. Fifteen-minute photostimulation was given once (1 Stim) or four times at 15-min intervals (4 Stim). (B) Freezing levels of control (sham operation without photostimulation, n = 14) and photostimulated GFAP-ChR2-EYFP rats (1 Stim, n = 10; 4 Stim, n = 12) during fear conditioning. (C) Freezing levels tested on day 2 of control and photostimulated rats (p=0.0019, p=0.0035, one-way ANOVA and Tukey's post-hoc test). Error bars show the means ± SEM. **p<0.01.
-
Figure 2—figure supplement 3—source data 1
Data for Figure 2—figure supplement 3.
Values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig2-figsupp3-data1-v2.xlsx
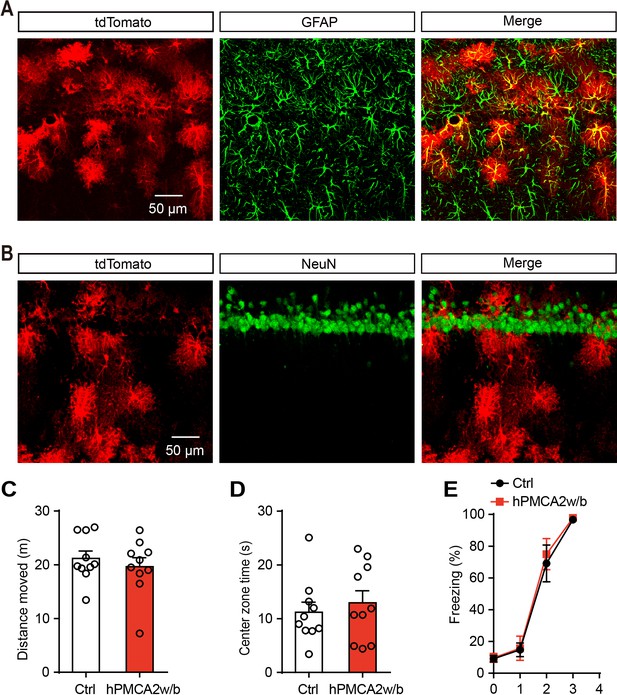
Specific tdTomato expression of control virus in hippocampal astrocytes and effects of hPMCA2w/b on behavioral tests.
(A and B) IHC confocal images showing co-localization of tdTomato labeling with the specific astrocytic marker GFAP, but not with the neuronal marker NeuN. (C and D) Total distance moved (n = 10 ctrl, n = 10 hPMCA2w/b, p=0.4825, Student’s unpaired two-tailed t-test) and center zone exploration time (p=0.5572, Student’s unpaired two-tailed t-test) in the open field following tdTomato and hPMCA2w/b expression in hippocampal astrocytes. (E) Freezing levels of control and hPMCA2w/b rats during fear conditioning. Error bars show the means ± SEM.
-
Figure 2—figure supplement 4—source data 1
Data for Figure 2—figure supplement 4.
Values for moving distance and center zone exploration time in the open-field test; values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig2-figsupp4-data1-v2.xlsx
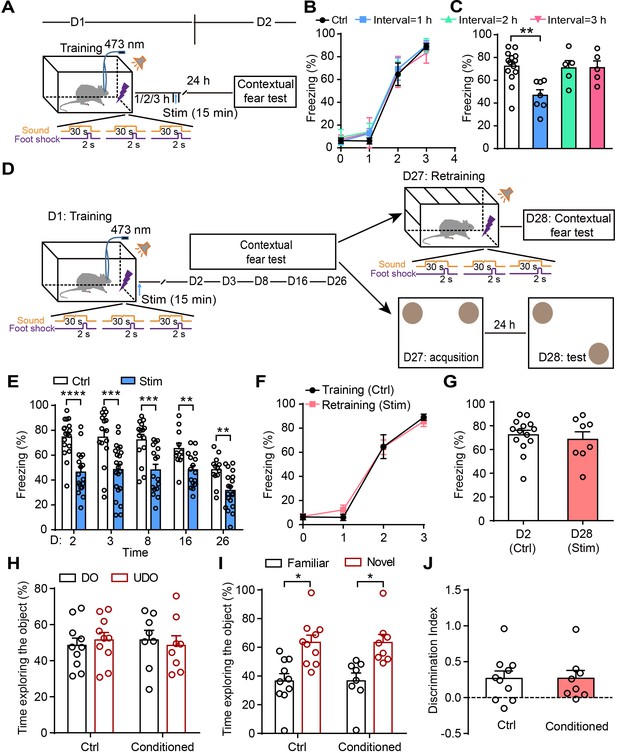
Astrocyte activation disrupts consolidation within a critical time-window and induces long-lasting contextual fear memory attenuation.
(A and D) Schematic of the experimental design of fear conditioning, photostimulation, and the subsequent test protocols. (B) Freezing levels of control (sham operation without photostimulation, n = 14) and photostimulated (Interval = 1 hr, n = 7; Interval = 2 hr, n = 5; Interval = 3 hr, n = 5) GFAP-ChR2-EYFP rats during fear conditioning. (C) Freezing levels of control and photostimulated GFAP-ChR2-EYFP rats during contextual fear tests on day 2 (p=0.0027, one-way ANOVA Tukey's post hoc test). (E) Freezing levels of control and photostimulated GFAP-ChR2-EYFP rats during contextual fear tests on day 2 (n = 18 ctrl, n = 18 stim; p < 0.0001, Student’s unpaired two-tailed t-test), day 3 (n = 16 ctrl, n = 22 stim; p=0.0006), day 8 (n = 15 ctrl, n = 17 stim; p=0.0006), day 16 (n = 11 ctrl, n = 16 stim; p=0.0051), and day 26 (n = 13 ctrl, n = 19 stim, p=0.0017). (F) Freezing levels of control rats conditioned on day 1 and retrained rats conditioned on day 27 (n = 8). (G) Freezing levels of control rats tested on day 2 and retrained rats tested on day 28 (p=0.5929, Student’s unpaired two-tailed t-test). (H) Time spent exploring the displaced object (DO) and undisplaced object (UDO) by control (n = 10) and conditioned rats (n = 8) during the sample phase (ctrl, p=0.7170; conditioned, p=0.7871, Student’s paired two-tailed t-test). (I) Time spent exploring the objects in familiar and novel locations by control and conditioned rats during the test phase (ctrl, p=0.0271; conditioned, p=0.0468, Student’s paired two-tailed t-test). (J) The discrimination index of control and conditioned rats measured during the test phase (p=0.9961, Student’s unpaired two-tailed t-test). Error bars show the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 3—source data 1
Data for Figure 3.
Values for freezing levels during fear conditioning test; values for time spent exploring the object during the OLR task.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig3-data1-v2.xlsx
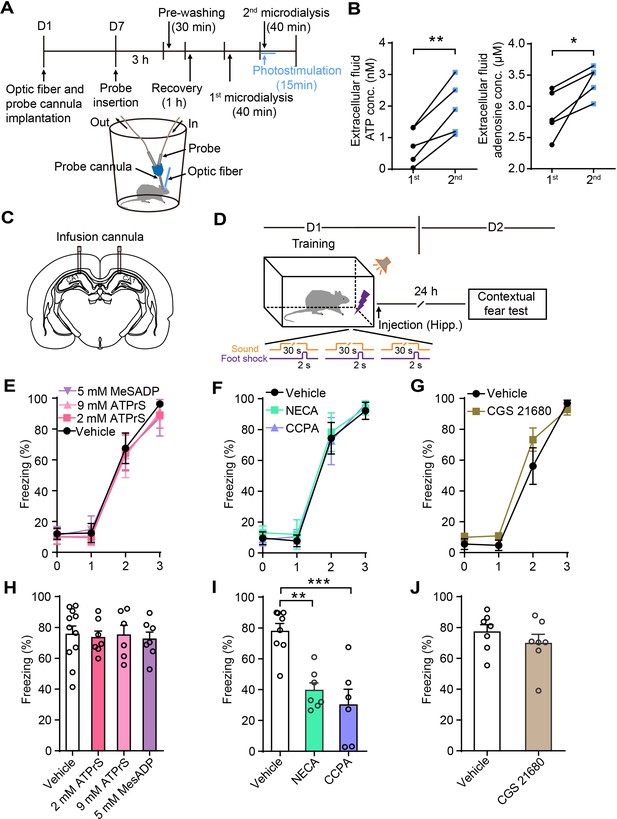
Astrocyte activation in CA1 induces ATP release and A1Rs mediate contextual fear memory attenuation.
(A) Schematic of the experimental design of microdialysis in vivo. (B) Extracellular ATP and adenosine concentrations in the dialysate prior to and following photostimulation (n = 5; p=0.0058, p=0.0028, Student’s paired two-tailed t-test). (C) Schematic showing the placement of implanted cannulae. (D) Schematic of the experimental design of fear conditioning, drug administration, and the subsequent test protocols. (E–G) Freezing levels of vehicle and drug-treated rats during fear conditioning (E, n = 11 Vehicle, n = 7 ATP-γ-S (2 mM), n = 6 ATP-γ-S (9 mM), n = 7 MesADP (5 mM); F, n = 8 Vehicle, n = 7 NECA, n = 6 CCPA; G, n = 7 Vehicle, n = 7 CGS 21680). (H) Freezing levels of vehicle, ATP-γ-S, and MesADP-treated rats during contextual fear tests on day 2 (p>0.05, one-way ANOVA Tukey's post-hoc test). (I) Freezing levels of rats treated with vehicle, NECA (2 mM), and CCPA (5 mM) during contextual fear tests on day 2 (p=0.0015, p=0.0002, one-way ANOVA and Tukey's post hoc test). (J) Freezing levels of vehicle and CGS 21680 (5 mM)-treated rats during contextual fear tests on day 2 (p=0.3481, Student’s unpaired two-tailed t-test). Error bars show the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. This figure has one figure supplement.
-
Figure 4—source data 1
Data for Figure 4.
Values for extracellular ATP and adenosine concentrations prior to and following photostimulation; values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig4-data1-v2.xlsx
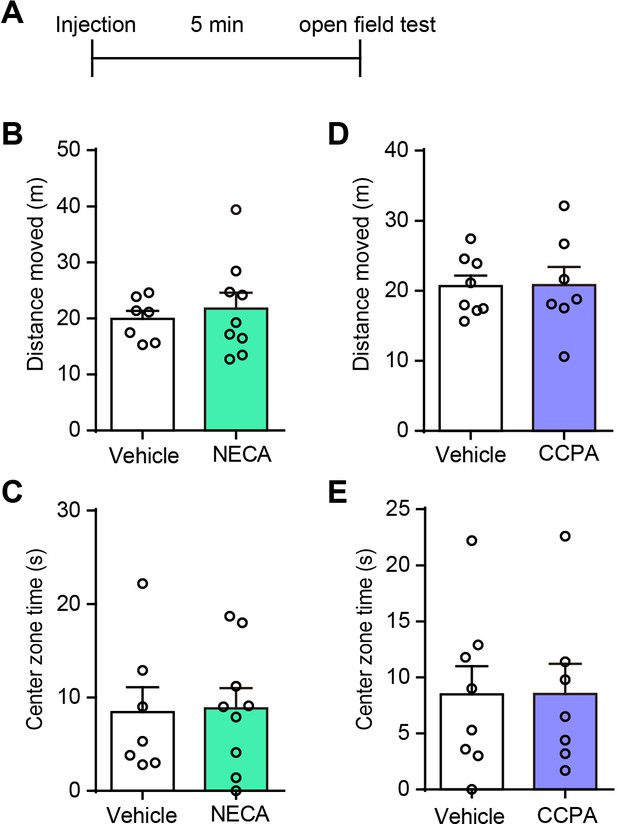
NECA and CCPA in the hippocampus do not affect spontaneous locomotor activity and center zone exploration time of the OFT.
(A) Schematic of the experimental design. (B and D) Total distance rats moved in the open field after NECA and CCPA delivery into the dorsal CA1 (B, n = 7 Vehicle, n = 9 NECA, p=0.6034, Student’s unpaired two-tailed t-test; D, n = 8 Vehicle, n = 7 CCPA, p=0.966). (C and E) Center zone time of exploration in the open field after NECA and CCPA delivery into the dorsal CA1 (p=0.9101, p=0.9917, Student’s unpaired two-tailed t-test). Error bars show the means ± SEM.
-
Figure 4—figure supplement 1—source data 1
Data for Figure 4—figure supplement 1.
Values for moving distance and center zone exploration time in the open-field test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig4-figsupp1-data1-v2.xlsx
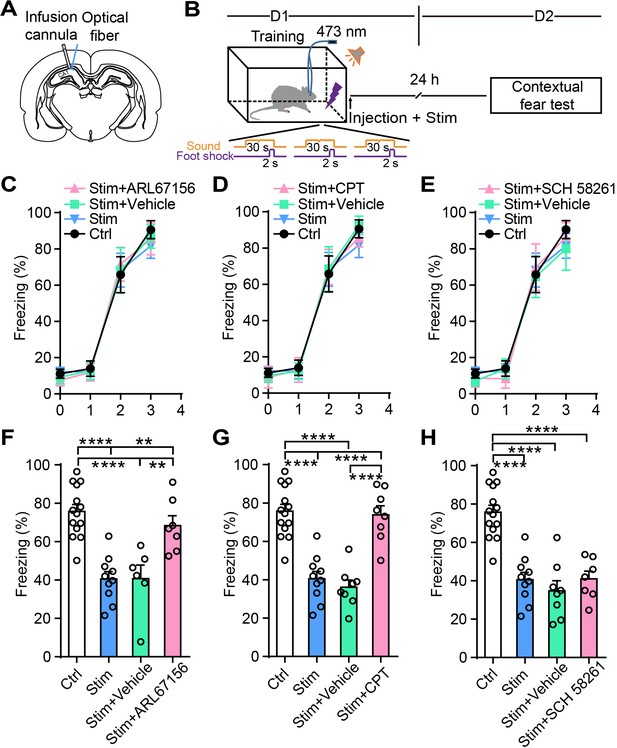
Ectonucleotidase inhibitor and A1R antagonist block the attenuation of the fear memory induced by astrocyte activation.
(A) Schematic showing the placement of the optical fiber and cannula implant. (B) Schematic of the experimental design of fear conditioning, photostimulation, drug administration, and the subsequent test protocols. (C–E) Freezing levels of control (no photostimulation or pharmacological treatment), Stim (photostimulation), Stim+Vehicle (photostimulation paired with vehicle treatment), and Stim+drug (photostimulation paired with ARL67156, CPT, or SCH 58261) rats during fear conditioning. (F) Freezing levels of control (n = 14), Stim (n = 10), Stim+Vehicle (n = 6), and Stim+ARL67156 (30 mM, n = 7) rats during contextual fear tests on day 2 (p<0.0001, p=0.0015, p=0.0055, one-way ANOVA and Tukey's post-hoc test). (G) Freezing levels of control, Stim, Stim+Vehicle (n = 8), and Stim+CPT (n = 8, 1 mM) rats during contextual fear tests on day 2 (p<0.0001, one-way ANOVA and Tukey's post hoc test). (H) Freezing levels of control, Stim, Stim+Vehicle (n = 8), and Stim+SCH 58261 (n = 7, 1 mM) rats during contextual fear tests on day 2 (p<0.0001, one-way ANOVA and Tukey's post-hoc test). Error bars show the mean ± SEM. **p<0.01, ****p<0.0001. This figure has one figure supplement.
-
Figure 5—source data 1
Data for Figure 5.
Values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig5-data1-v2.xlsx
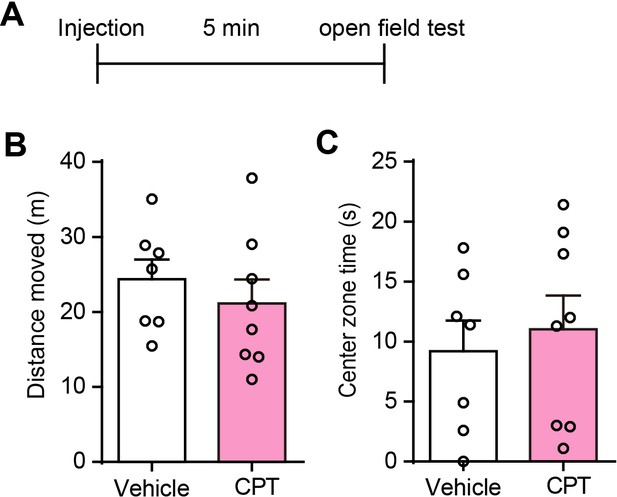
CPT in the hippocampus do not affect spontaneous locomotor activity and center zone exploration time of the OFT.
(A) Schematic of the experimental design. (B) Total distance rats moved in the open field after CPT delivery into the dorsal CA1 (n = 7 Vehicle, n = 8 CPT, p=0.4567, Student’s unpaired two-tailed t-test). (C) Center zone time of exploration in the open field after CPT delivery into the dorsal CA1 (p=0.6451, Student’s unpaired two-tailed t-test). Error bars show the means ± SEM.
-
Figure 5—figure supplement 1—source data 1
Data for Figure 5—figure supplement 1.
Values for moving distance and center zone exploration time in the open-field test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig5-figsupp1-data1-v2.xlsx
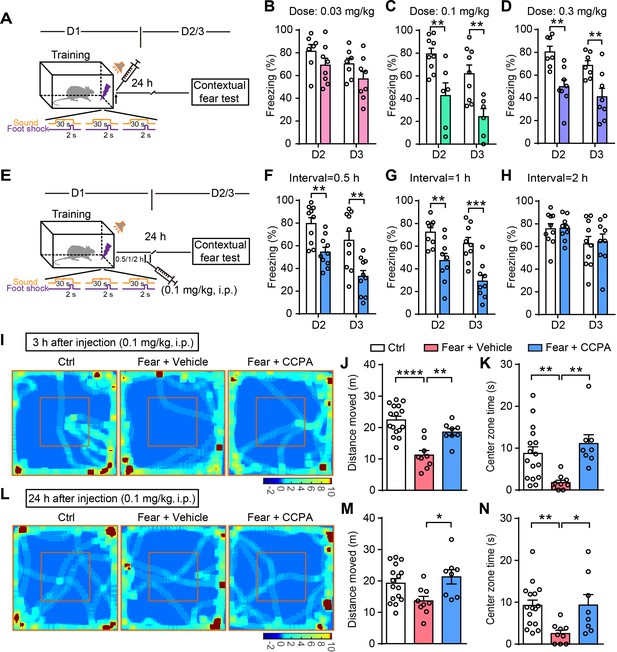
Administration of CCPA (i.p.) within a defined time window reduces contextual fear memory and fear-related anxiety.
(A and E) Schematic of the experimental design of fear conditioning, drug administration, and the subsequent test protocols. (B–D) Freezing levels of rats with different doses of vehicle and CCPA treatment during contextual fear tests on day 2 (B, n = 7 Vehicle, n = 8 CCPA, p=0.1765; C, n = 9 Vehicle, n = 6 CCPA, p=0.0053; D, n = 7 Vehicle, n = 8 CCPA, p=0.0021, Student’s unpaired two-tailed t-test) and day 3 (B, p=0.1409; C, p=0.0048; D, p=0.0086). (F–H) Freezing levels of rats with vehicle and CCPA treatment at different time tested on day 2 (F, n = 10 Vehicle, n = 10 CCPA, p=0.0016; G, n = 9 Vehicle, n = 9 CCPA, p=0.0041; H, n = 10 Vehicle, n = 9 CCPA, p=0.9417, Student’s unpaired two-tailed t-test) and day 3 (F, p=0.0033; G, p=0.0004; H, p=0.8366). (I and L) Representative heatmaps of movement in the open field 3 hr and 24 hr after CCPA injection (0.1 mg/kg). Left, Ctrl, rats without fear conditioning; middle, Fear+Vehicle, fear-conditioned rats with vehicle injection; right, Fear+CCPA, fear-conditioned rats with CCPA injection. (J and M) Total distance moved by Control (n = 16), Fear+Vehicle (n = 9) and Fear+CCPA (n = 8) rats in the OFT 3 hr and 24 hr after injection (J, 3 hr, p<0.0001, p=0.0031, one-way ANOVA and Tukey's post hoc test; M, 24 hr, p=0.022). (K and N) Center zone exploration time of Control, Fear+Vehicle, and Fear+CCPA rats in the OFT 3 hr and 24 hr after injection (K, 3 hr, p=0.0069, p=0.0014, Kruskal-Wallis and Dunn's post hoc test; N, 24 hr, p=0.0025, p=0.041). Error bars show the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. This figure has two figure supplements.
-
Figure 6—source data 1
Data for Figure 6.
Values for freezing levels during fear-conditioning test; values for moving distance and center zone exploration time in the open-field test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig6-data1-v2.xlsx
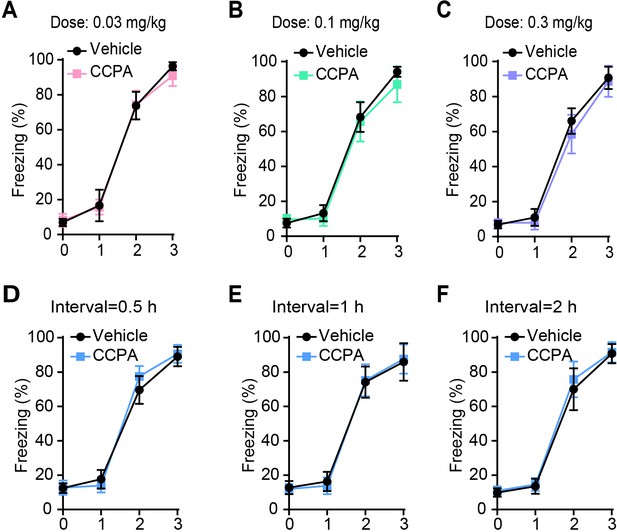
Rats with vehicle and CCPA injection have comparable learning curves for fear conditioning.
(A–C) Freezing levels of rats with different doses of vehicle and CCPA treatment after fear conditioning (A, n = 7 Vehicle, n = 8 CCPA; B, n = 9 Vehicle, n = 6 CCPA; C, n = 7 Vehicle, n = 8 CCPA). (D–F) Freezing levels of rats with vehicle and CCPA treatment (0.1 mg/kg, i.p.) at different time after fear conditioning (D, n = 10 Vehicle, n = 10 CCPA; E, n = 9 Vehicle, n = 9 CCPA; F, n = 10 Vehicle, n = 9 CCPA). Error bars show the means ± SEM.
-
Figure 6—figure supplement 1—source data 1
Data for Figure 6—figure supplement 1.
Values for freezing levels during fear-conditioning test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig6-figsupp1-data1-v2.xlsx
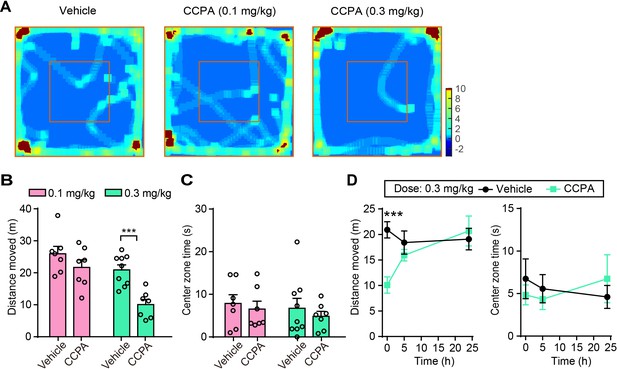
Intraperitoneal injection of CCPA at a lower effective dose (0.1 mg/kg) does not affect locomotor and center zone exploration time in the OFT.
(A) Representative heatmaps of movement in the open field after vehicle or CCPA injection at 0.1 and 0.3 mg/kg. (B) Histograms showing moving distance in the open field after vehicle and CCPA injection at 0.1 mg/kg (n = 7 vehicle, n = 7 CCPA; p=0.2277, Student’s unpaired two-tailed t-test) and 0.3 mg/kg (n = 9 vehicle, n = 7 CCPA, p=0.0003). (C) Histograms showing center zone exploration time in the open field after vehicle and CCPA injection at 0.1 mg/kg (p=0.8770, Mann Whitney test) and 0.3 mg/kg (p=0.7365). (D) Graphs showing moving distance (5 hr, p=0.3877; 24 hr, p=0.6568, Student’s unpaired two-tailed t-test) and center zone exploration time (5 hr, p=0.5812; 24 hr, p=0.4727) at different time points in the open field after vehicle and CCPA injection at 0.3 mg/kg. Error bars show the means ± SEM. ***p<0.001.
-
Figure 6—figure supplement 2—source data 1
Data for Figure 6—figure supplement 2.
Values for moving distance and center zone exploration time in the open-field test.
- https://cdn.elifesciences.org/articles/57155/elife-57155-fig6-figsupp2-data1-v2.xlsx
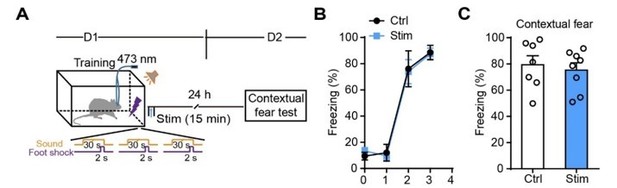
Photostimulation in the motor cortex of GFAP-ChR2-EYFP rats after fear conditioning has no effect on contextual fear memory.
(A) Schematic of the experimental design for fear conditioning, photostimulation, and the subsequent test protocols. (B) Freezing levels of control (sham operation, n = 7) and photostimulated GFAP-ChR2-EYFP rats (n = 8) during fear conditioning. (C) Freezing levels tested on day 2 of control and photostimulated rats (p = 0.6548, Student’s unpaired two-tailed t-test).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Rattus norvegicus, male/female) | Sprague-Dawley rats | Shanghai SLAC Laboratory Animal C.,Ltd | N/A | |
| Strain, strain background (R. norvegicus, male/female) | GFAP-ChR2-EYFP rats | Institute of Neuroscience, Chinese Academy of Sciences | N/A | |
| Genetic reagent (virus) | pZac2.1 GfaABC1D mCherry-hPMCA2w/b | Addgene | Cat# 111568 | |
| Genetic reagent (virus) | AAV5 GfaABC1D mCherry-hPMCA2w/b | Vigene Biosciences | N/A | |
| Genetic reagent (virus) | AAV5 GfaABC1D tdTomato | Vigene Biosciences | N/A | |
| Antibody | Anti-GFAP, rabbit polyclonal | Millipore | Cat# AB5804 | (1:500) |
| Antibody | Anti-NeuN, mouse monoclonal | Millipore | Cat# MAB377 | (1:400) |
| Antibody | Anti-DsRed, rabbit polyclonal | Clontech | Cat# 632496 | (1:500) |
| Antibody | Alexa Fluor 568 donkey anti-rabbit IgG (H+L) | Invitrogen | Cat# A10042 | (1:1000) |
| Antibody | Alexa Fluor 647 donkey anti-mouse IgG (H+L) | Invitrogen | Cat# A31571 | (1:1000) |
| Antibody | Alexa Fluor 488 donkey anti-mouse IgG (H+L) | Invitrogen | Cat# A21202 | (1:1000) |
| Commercial assay or kit | ATP Assay Kit | Sigma-Aldrich | Cat# FLAA | |
| Commercial assay or kit | Adenosine Assay Kit | BioVision | Cat# K327-100 | |
| Chemical compound, drug | ATP-γ-S | Sigma-Aldrich | Cat# A1388; CAS: 93839-89-5 | |
| Chemical compound, drug | MesADP | Tocris | Cat# 1624; CAS: 475193-31-8 | |
| Chemical compound, drug | NECA | Sigma-Aldrich | Cat# E2387; CAS: 35920-39-9 | |
| Chemical compound, drug | CCPA | Sigma-Aldrich | Cat# C7938; CAS: 37739-05-2 | |
| Chemical compound, drug | CPT | Sigma-Aldrich | Cat# C102; CAS: 35873-49-5 | |
| Chemical compound, drug | SCH58261 | Tocris | Cat# 2270; CAS: 160098-96-4 | |
| Chemical compound, drug | ARL67156 trisodium salt hydrate | Sigma-Aldrich | Cat# A265; CAS: 160928-38-1 | |
| Chemical compound, drug | CGS 21680 hydrochloride | Tocris | Cat# 1063; CAS: 124431-80-7 | |
| Chemical compound, drug | Rhod-2 AM | Invitrogen | Cat# R1245MP | |
| Software, algorithm | GraphPad Prism (6.01) | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ | |
| Software, algorithm | ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/ | |
| Software, algorithm | MATLAB R2017b | MathWorks | https://se.mathworks.com/products/matlab.html | |
| Software, algorithm | ANY-maze tracking software | Stoelting Co | https://www.stoeltingco.com/anymaze.html | |
| Software, algorithm | FREEZING (2.0.05) | Panlab Harvard Apparatus | https://www.panlab.com |





