Feed-forward recruitment of electrical synapses enhances synchronous spiking in the mouse cerebellar cortex
Figures
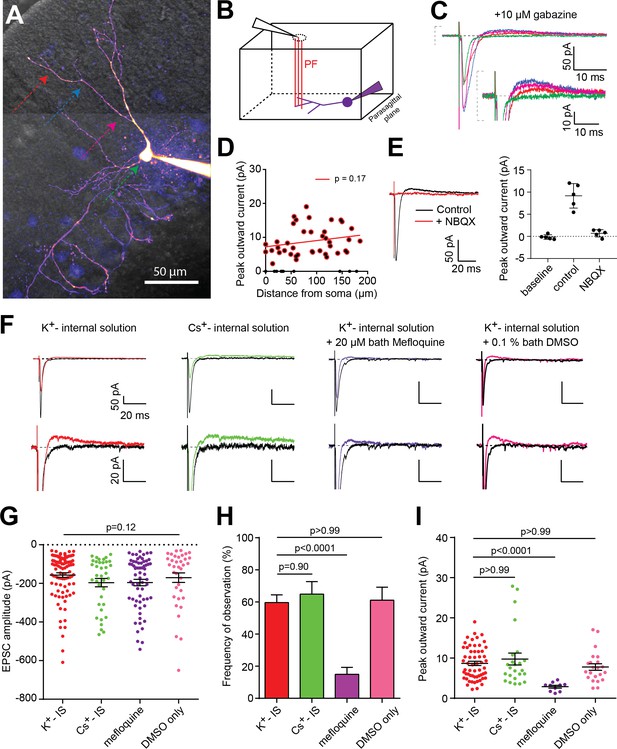
PF-basket cell (BC) synapse-evoked outward currents that are insensititive to GABAergic receptor antagonists are sensitive to gap junction antagonists.
(A) 2-photon laser scanning microscopy image (2PLSM - maximal intensity projection) of a cerebellar BC patch-loaded with 20 µM Alexa-594. Color arrows indicate the positions of the stimulating pipette along the somato-dendritic axis. (B) Schematic diagram showing the stimulation pipette (black triangle) and granule cell axons or parallel fibers (PFs - red lines) projecting perpendicularly to, and synapsing onto, the dendritic tree of the patch-clamped BC (purple) in a parasagittal cerebellar slice configuration. (C) Averaged excitatory postsynaptic currents (EPSCs) were recorded from the cell in A and in the presence of 10 µM gabazine, following a single brief (50 µs) extracellular voltage stimulation of a PF beam using a monopolar glass electrode placed at different locations in the dendritic tree (same color code as in A). (D) Average peak amplitude of the outward current versus distance between synaptic current entry and somatic compartment. Linear regression analysis reveals a non-significant relationship between the two parameters (p=0.17, n = 52 stimulation sites over 13 cells; n = 40 stimulation sites with significant outward currents). (E) Representative experiment showing 10 µM NBQX block of both inward and outward currents. Right, summary results from 5 cells. Error bars are standard deviation. (F) Representative traces of PF-mediated synaptic responses recorded in four different pharmacological conditions. Two traces recorded when the stimulus electrode was placed at a site with (color) or without synapse-evoked outward currents (black). (G) EPSC amplitude following PF stimulation is not significantly different between the four groups (Kruskal-Wallis test, p-value=0.12 K+-IS: n = 96 stimulation sites over 26 cells; Cs+-IS: n = 37 sites over 10 cells; Mefloquine: n = 64 sites over 16 cells; DMSO only: n = 36 sites over 11 cells). (H) The frequency of observing an outward current is significantly smaller only in the mefloquine-treated group (Brown-Forsythe, F = 57.13, p-value<0.0001; followed by Dunnett's multiple comparisons tests; K+-IS: n = 104 stimulation sites; Cs+-IS: n = 37 sites; Mefloquine: n = 67 sites; DMSO only: n = 36 sites). Error bars are SEM and calculated assuming binomial statistics (see Materials and methods). (I) Outward current peak amplitude (in cases where it can be detected) is significantly smaller only in the mefloquine-treated group (Kruskal-Wallis test followed by Dunn's multiple comparison tests, p<0.0001; K+-IS: n = 58 stimulation sites over 20 cells; Cs+-IS: n = 24 sites over 10 cells; Mefloquine: n = 10 sites over 8 cells; DMSO only: n = 22 sites over 10 cells). See also Figure 1—source data 1.
-
Figure 1—source data 1
PF-basket cell (BC) synapse-evoked outward currents that are insensititive to GABAergic receptor antagonists are sensitive to gap junction antagonists.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig1-data1-v1.xlsx
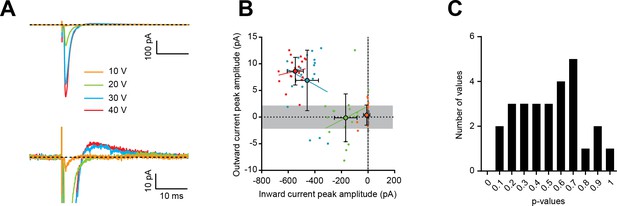
The amplitude of direct excitatory postsynaptic currents (EPSCs) and outward currents are not correlated.
(A) Average compound synaptic responses recorded at a single site, and at different stimulation intensities, reveal an increase in the amplitude of both the direct EPSC (upper panel) and the secondary outward current (lower panel). (B) Outward current peak amplitude versus inward current peak amplitude of single sweeps (small dots) and their corresponding average responses (large dots) for the cell shown in A. Linear regression analysis for each stimulus condition (color-coded according to panel A) reveals that for each stimulation intensity between 20 and 40 V where a significant EPSC was detected, no correlation is observed between the peak amplitude of both components of the compound synaptic response for single sweeps (linear least squares regression analysis, 20 V: p=0.31, 30 V: p=0.38, 40 V: p=0.57). Error bars are standard deviation. (C) P-value distribution of the analysis shown in (B) for n = 27 trials (from 9 cells, one site per cell, 2 to 4 stimulation intensities per site). All p-values are above 0.05, showing no significant correlation was observed between EPSC and outward current peak amplitudes. See also Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
The amplitude of direct EPSCs and outward currents are not correlated.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig1-figsupp1-data1-v1.xlsx
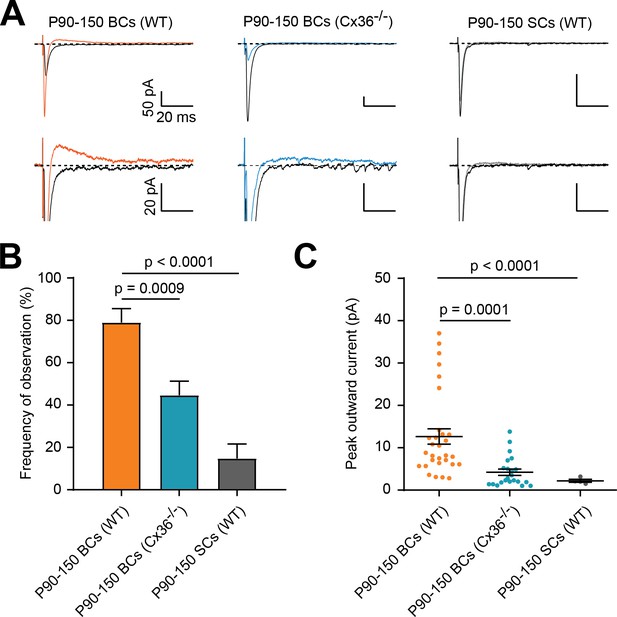
Outward currents are reduced in Cx36KO mice and stellate cells (SCs).
(A) Representative traces of PF-mediated synaptic responses recorded in three different conditions (WT P90-150 BCs, P90-150 BCs in Cx36KO mice, and WT P90-150 SCs). Black and colored trace for each group are averages of 20–30 sweeps at a location without and with a detectable outward current, respectively. Bottom row is an expanded amplitude scale to better visualize outward current. (B) The frequency of observing the outward current is significantly smaller in P90-P150 SCs (WT) and BCs (Cx-36 KO), as compared to P90-P150 BCs (WT) (Brown-Forsythe with Dunnetts multiple comparisons test, F = 17.09, p<0.0001; P90-P150 BCs (WT): n = 38 stimulation sites over 11 cells, P90-P150 BCs (Cx36-/-): n = 56 sites over 14 cells, P90-P150 SCs (WT): n = 27 sites over 8 cells). (C) Outward current peak amplitude is significantly smaller in P90-P150 SCs (WT) and BCs (Cx-36 KO), as compared to P90-P150 BCs (WT) (Kruskal-Wallis test with Dunn’s multiple comparisons test, p<0.0001; P90-P150 BCs (WT): n = 30 sites over 10 cells, P90-P150 BCs (Cx36-/-): n = 22 sites over 8 cells, P90-P150 SCs (WT): n = 4 sites over 2 cells). See also Figure 2—source data 1.
-
Figure 2—source data 1
Outward currents are reduced in Cx36KO mice andstellate cells(SCs).
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig2-data1-v1.xlsx
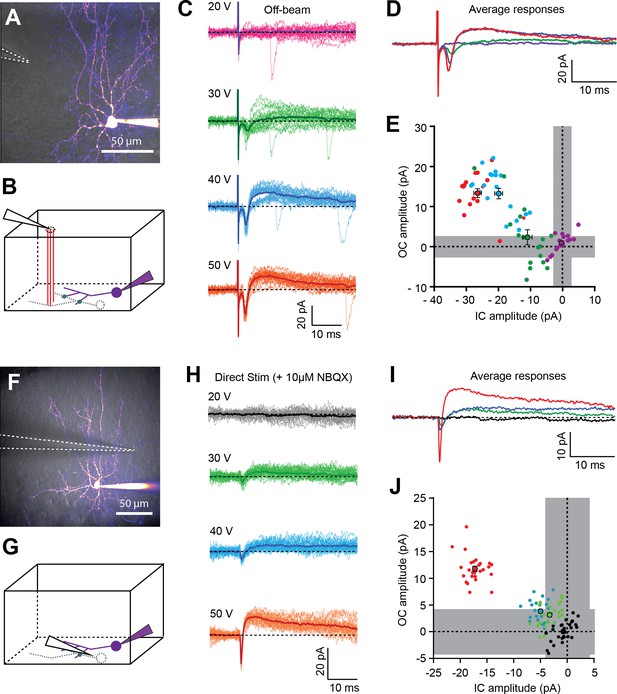
In the absence of direct excitatory transmission from PFs, GABAergic-independent outward currents are preceded by a short-lasting inward current.
(A) 2PLSM image (maximal intensity projection) of a BC loaded with 20 µM Alexa-594. Dashed white lines indicate the position of the extracellular stimulation electrode on the surface of the slice (off-beam stimulation). (B) Schematic diagram of a parasagittal slice indicating off-beam stimulation of parallel fibers (PFs - red lines) synapsing onto the dendritic tree of an unpatched neighboring MLI (dashed gray lines), which forms putative electrical synapse(s) (green dots) with the patched cell (purple). (C) Postsynaptic current responses recorded in the patched cell in response to a single 50 µs voltage pulse at different stimulation intensities (20–50V). Dark lines represent averages of 15–30 single trials. (D) Superimposition of average responses from C, shown for visual comparison of the differences in mean peak amplitude of inward and outward currents. (E) Summary plot of peak amplitudes of inward and outward currents from individual trials shown in C, with corresponding averages +/- SEM represented by larger dots. gray regions represent 2*SD of baseline values (averaged over all traces). (F-J) Similar as in A-E, but spikelets were elicited by direct extracellular stimulation of a neighboring putative BC in the presence of 10 µM NBQX in the bathing solution to block AMPAR-mediated EPSCs. See also Figure 3—source data 1.
-
Figure 3—source data 1
In the absence of direct excitatory transmission from PFs, GABAergic-independent outward currents are preceded by a short-lasting inward current.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig3-data1-v1.xlsx
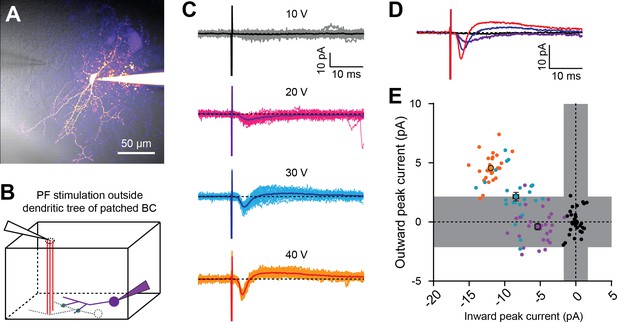
Evidence for subthreshold excitatory postsynaptic current (EPSC) transmission across electrical synapses.
(A) 2PLSM image (maximal intensity projection) of a BC loaded with 20 µM Alexa-594. Dashed white lines indicate the position of the extracellular stimulation electrode on top of the slice (off-beam stimulation). (B) Schematic diagram of a parasagittal slice indicating off-beam stimulation of parallel fibers (PFs - red lines) synapsing onto the dendritic tree of an unpatched neighboring MLI (dashed gray lines), which forms putative electrical synapse(s) (green dots) with the patched BC (purple). (C) Postsynaptic current responses recorded in the patched cell in response to a single 50 µs extracellular voltage pulse delivered off-beam and at different intensities. Dark lines represent averages of 15–30 single trials. (D) Superimposition of average responses, shown for visual comparison of the differences in mean peak amplitude of inward and outward currents. Note the responses recorded at 20 V of stimulation intensity, which do not display outward currents, but only inward currents. (E) Summary plot of peak amplitudes of inward and outward currents shown in C, with corresponding averages +/- SEM represented by larger dots. Gray regions represent 2*SD of baseline values, averaged over all traces. See also Figure 3—figure supplement 1—source data 1.
-
Figure 3—figure supplement 1—source data 1
Evidence for subthreshold excitatory postsynaptic currents (EPSC) transmission across electrical synapses.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig3-figsupp1-data1-v1.xlsx
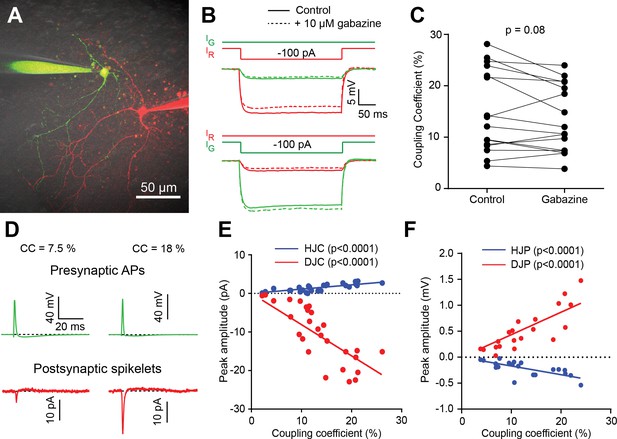
Electrical coupling strength between BCs correlates with spikelet amplitude.
(A) 2PLSM image (maximal intensity projection) of two BCs loaded with 20 µM Alexa 594 (red), or 20 µm Alexa 488 (green) using dual whole-cell patch clamp. (B) Membrane potential of both cells (same color code as in A), when the red cell is injected with a hyperpolarizing current pulse (upper panel), or when the green cell is injected with the same current pulse (lower panel). Solid lines indicate responses in control conditions, while dashed lines correspond to responses recorded in 10 µM gabazine. Traces are averages of 50 sweeps. (C) Summary plot of unidirectional coupling coefficients (CCs), showing that they are not significantly altered by gabazine addition (n = 16 cells from 8 BC pairs, Wilcoxon matched-pairs signed rank test, p=0.08). (D) Two representative paired BC recordings, one with an average (left) and one with a high bidirectional CC (right), and their corresponding spikelet currents (red traces, holding membrane potential of −70 mV) resulting from an AP elicited in the presynaptic cells (green traces, RMP of −70 mV). (E) Summary plot of paired recordings showing the relationship between spikelet inward currents (depolarizing junction current, DJC, red), fAHP-mediated outward current (hyperpolarizing junction current, HJC, blue) and CC (n = 20 spikelet recordings). Note that in these recordings, presynaptic (transmitting) cells were maintained at approximately −70 mV, and 10 µM gabazine was present to block GABAergic inputs. Lines are linear regressions (n = 27 cells from 14 pairs; HJC: p<0.0001, DJC: p<0.0001) (F) Similar to B, but for spikelets recorded in current clamp (n = 27) DJP: depolarizing junction potential; HJP: hyperpolarizing junction potential. Lines are linear regressions (n = 20 cells from 10 pairs; HJP: p<0.0001, DJP: p<0.0001). Holding potentials were ~−70 mV. See also Figure 4—source data 1.
-
Figure 4—source data 1
Electrical coupling strength between BCs correlates with spikelet amplitude.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig4-data1-v1.xlsx
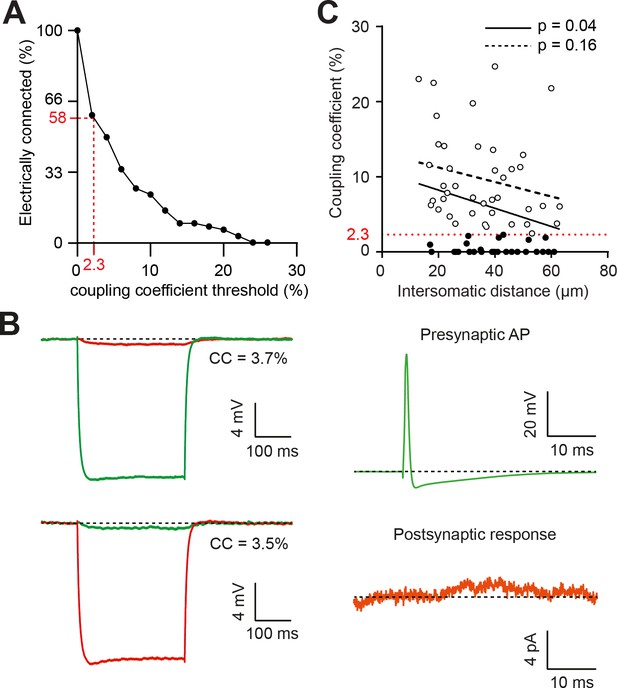
Relationship between coupling coefficients and spikelet transmission, and distance-independence of electrical coupling.
(A) Fraction of BC pairs that are considered ‘connected’ according to different coupling coefficient (CC) thresholds. The dotted red lines indicate the observed frequency of detectable spikelet transmission, which corresponds to a CC of 2.3%. (B) Paired recordings of long current pulses injected into one cell (green, top panel; red, bottom panel), with the corresponding hyperpolarization in the second cell of the pair (red, top panel; green, bottom panel). Calculated CCs in either direction give 3.7% and 3.5%, respectively. In this pair, AP firing in the green cell does not cause detectable postsynaptic currents (either inward or outward; traces are averages of 30 sweeps). The presynaptic cell holding potential was −70 mV and the postsynaptic cell holding potential was −60 mV. This may result from electrical coupling via a third MLI. (C) CC versus intersomatic distance between simultaneously patched BCs. Linear regression analysis reveals that for strongly coupled BC-BC pairs (CC >2.3%, white dots) CCs do not significantly decrease with intersomatic distance (within 70 µm; p=0.16, dotted line). However, if all pairs are considered (black and white dots), CCs do significantly decrease with intersomatic distance (p=0.04, black line). See also Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
Relationship between coupling coefficients and spikelet transmission, and distance-independence of electrical coupling.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig4-figsupp1-data1-v1.xlsx
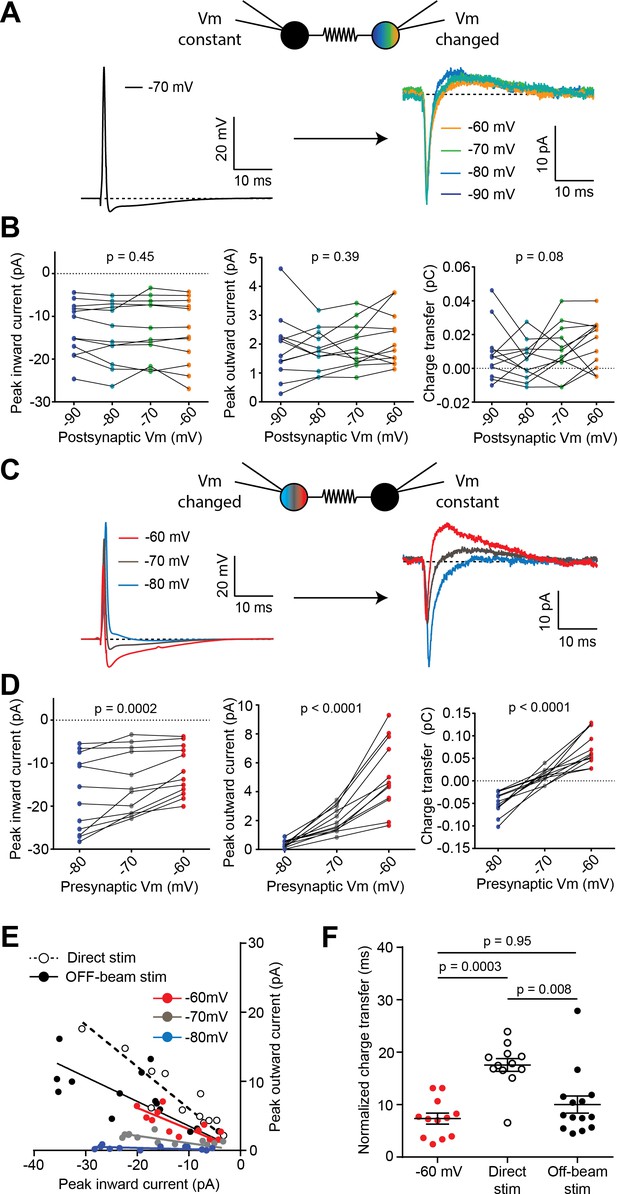
Presynaptic resting potential influences polarity of spikelets.
(A) Action potential from one cell of a paired BC recording (left; holding potential = −70 mV) and corresponding spikelets current recorded in a voltage-clamped postsynaptic cell at different holding membrane potentials (Vm, right). (B) Inward current peak amplitude (left panel), outward current peak amplitude (middle panel) and charge transfer (right panel) are not significantly altered by changing postsynaptic Vm (n = 12 spikelets from 6 BC pairs, three independent Friedman tests, p-values from left to right: p=0.45, p=0.39, p=0.08). (C) Action potential waveforms from the same cell recorded at three different resting Vm (left), with corresponding spikelets recorded in the postsynaptic cell, held at −70 mV in voltage-clamp (right). (D) Inward current peak amplitude (left panel), outward current peak amplitude (middle panel) and charge transfer (right panel) are all significantly altered by changing the resting membrane potential of the presynaptic cell (n = 12 spikelets from six pairs, three independent Friedman tests). (E) Plot of outward versus inward current peak amplitudes of spikelets recorded under five conditions: either from pairs of connected BCs (Presynaptic Vm = −60,–70, −80 mV), from extracellular direct stimulation (dotted) or off-beam stimulation (black). Regression lines were performed on the individual groups. Slopes were significantly different between the −60, –70, and −80 mV groups (F-test, p-value=0.0003), significantly different between the direct stimulation and the −60 mV groups (p-value=0.013), but not different between the off-beam stimulation and −60 mV groups (p-value=0.93). (F) Summary plot of peak-normalized inward charge transfer of spikelets recorded in voltage clamp and elicited from a transmitting BC pair whose holding potential (Vm) was −60 mV, or from extracellular direct and off-beam stimulation (Kruskal-Wallis test with Dunn’s multiple comparisons; −60 mV (Pair): n = 12 cells from six pairs, Direct stim.: n = 12 stimulation sites over 10 cells, off-beam stim.: n = 14 sites over 11 cells). See also Figure 5—source data 1.
-
Figure 5—source data 1
Presynaptic resting potential influences polarity of spikelets.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig5-data1-v1.xlsx
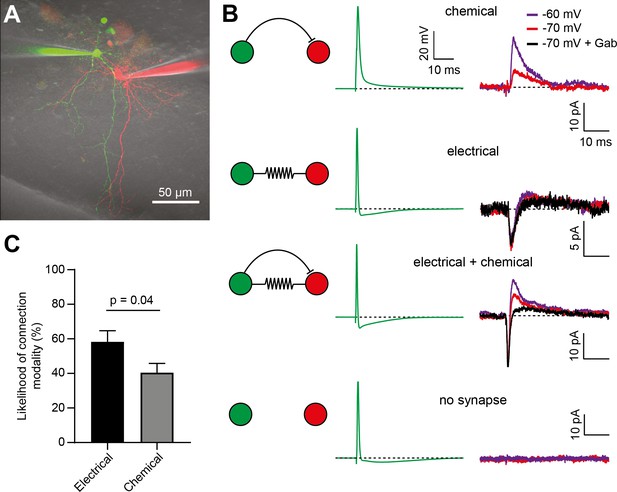
Electrical synapses are more frequent than chemical inhibitory synapses between BCs.
(A) 2P-LSM image (maximal intensity projection) of two BCs loaded with 20 µM Alexa 594 (red) or 20 µm Alexa 488 (green). (B) Representative examples of each of the 3 types of unidirectional connections. Presynaptic AP waveforms are shown in green, with the corresponding responses observed in the postsynaptic cells shown on their right (black, purple or red). Chemical synapses were identified by the presence of a postsynaptic Vm-sensitive or gabazine-sensitive outward current (Gab). Electrical synapses were identified by the presence of a spikelet-mediated inward current. The fourth condition includes those paired recordings with no evidence of electrical or chemical synapses (no synapse). (C) Bar graph showing that electrical synapses are significantly more frequent than chemical synapses in the BC network. 35 out of 60 pairs showed bidirectional spikelets (electrical synapses), whereas 35 out of 85 unidirectional connections showed evidence of chemical synapses (unpaired t-test assuming binomial distributions, p=0.04). See also Figure 6—source data 1.
-
Figure 6—source data 1
Electrical synapses are more frequent than chemical inhibitory synapses between BCs.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig6-data1-v1.xlsx
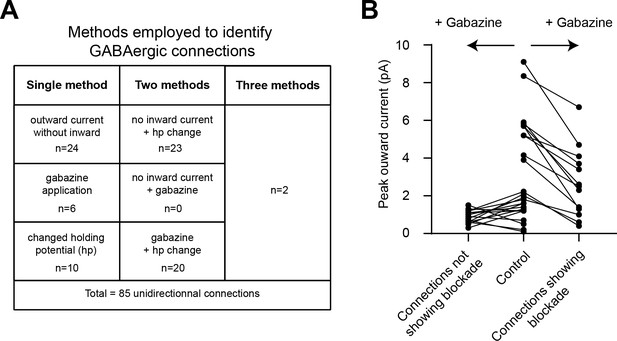
Identification of GABAergic connections in BC paired recordings.
(A) Table summarizing how often each of three methods were used to detect chemical inhibitory synapses in paired BC recordings: (1) presence of outward current without inward component (i.e. spikelet), (2) changes in peak amplitude of outward currents after gabazine addition, or (3) changes in peak amplitude of outward currents when driving force was changed from −70 mV (control for all cases) to −60 mV. (B) Summary of changes in peak outward currents (n = 28 connections) for those connections tested with 10 μM gabazine: 15 connections from BC paired recordings did not show detectable blockade by gabazine using a within-connection statistical test (Wilcoxon matched pairs signed rank test, p>0.05), whereas 13 connections did show detectable blockade by gabazine (average 50 ± 6% SEM, n = 13). See also Figure 6—figure supplement 1—source data 1.
-
Figure 6—figure supplement 1—source data 1
Identification of GABAergic connections in BC paired recordings.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig6-figsupp1-data1-v1.xlsx
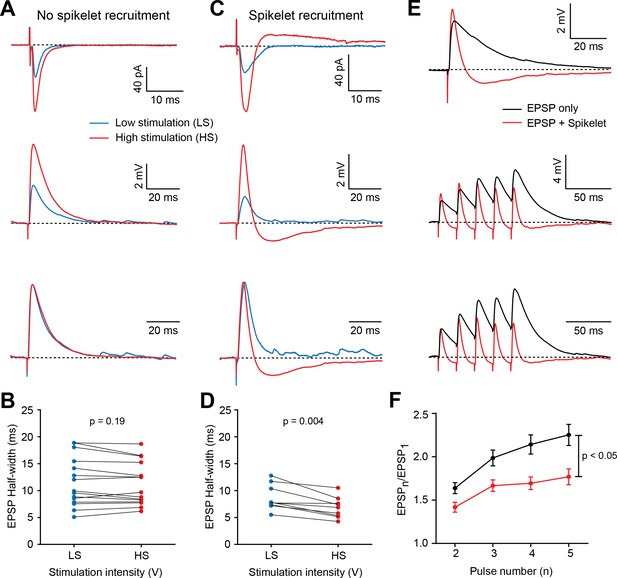
Feed-forward (FF) recruitment of spikelets narrows excitatory postsynaptic potential (EPSP) width and dampens temporal summation.
(A) Top, voltage-clamp recordings of PF-mediated synaptic responses in a representative BC in response to low stimulation intensity (LS – blue traces) and high stimulation intensity (HS – red traces). Increasing stimulation intensity leads to increased peak amplitude of excitatory postsynaptic currents (EPSCs), with no additional recruitment of spikelets. Middle, current-clamp recording of the same cell, showing that increasing stimulation intensity increased the amplitude but not the half-width of the normalized EPSPs (bottom). Traces are averages of 20 to 30 trials. (B) Summary plot showing no increase in half-width of EPSPs if no outward current was observed in voltage clamp (p=0.19, Wilcoxon matched-pairs signed rank test, n = 15 stimulation sites over 12 cells). (C) Example cell in which increasing the stimulation intensity recruits an additional spikelet, observed in voltage clamp (top) and current clamp (middle). The half-width of normalized EPSPs was shortened. (D) Summary plot showing a decrease in half-width of EPSPs if outward currents were observed in voltage clamp (p=0.004, Wilcoxon matched-pairs signed rank test, n = 9 sites from 8 cells). (E) Top, superimposed averaged postsynaptic responses (n = 30 trials) from two different cells: one recorded in response to LS, and another in which the direct EPSP is followed by a spikelet-induced hyperpolarization (red) and the other is not (black). Middle, EPSPs evoked by a 50 Hz stimulus train in the same cells as above. Note the decreased temporal summation in the group where spikelets are present. (F) Summary plot showing relative EPSP amplitudes in response to all five stimuli for stimulation sites that did (red) or did not (black) evoke spikelets (Two-way repeated measures ANOVA, column factor (with versus without spikelet): p=0.004; interaction factor (difference in curve shape with stimulus number): p=0.001; EPSP only: n = 27 stimulation sites over 16 cells, EPSP with spikelet: n = 22 sites over 17 cells). See also Figure 7—source data 1.
-
Figure 7—source data 1
Feed-forward(FF)recruitment of spikelets narrowsexcitatory postsynaptic potential (EPSP)width and dampens temporal summation.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig7-data1-v1.xlsx
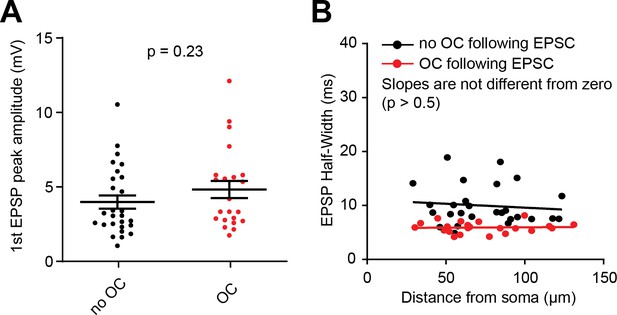
Control experiments for spikelet effect on excitatory postsynaptic potentials (EPSPs).
(A) The amplitude of the first EPSP is not significantly different between those extracellular stimulation sites where an outward current could be detected (OC, red group, n = 22 sites) and those lacking a detectable outward current after the initial excitatory postsynaptic current (EPSC) (no OC, black group, n = 27 sites - Mann-Whitney test, p=0.23). (B) Relationship between EPSP half-width and distance between stimulation electrode tip location and patched somata (same color code as in A). In both groups, no significant relationship is observed (No OC: p=0.61, OC: p=0.87). See also Figure 7—figure supplement 1—source data 1.
-
Figure 7—figure supplement 1—source data 1
Control experiments for spikelet effect onexcitatory postsynaptic potentials(EPSPs).
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig7-figsupp1-data1-v1.xlsx
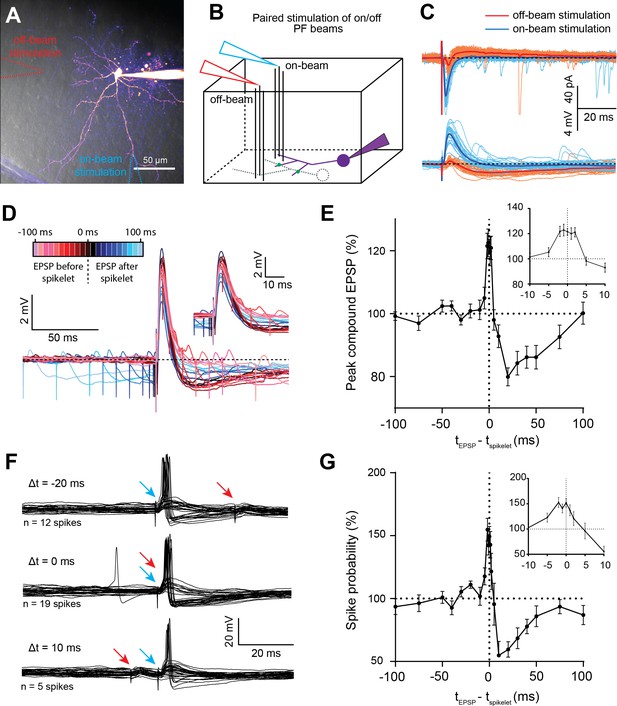
Spikelet transmission enables temporal contrast enhancement of basket cell (BC) excitation.
(A) 2PLSM image (maximal intensity projection) of a BC loaded with 20 µM Alexa 594. Dashed lines indicate the position of the stimulating pipettes on top of the slice (off-beam stimulation in red, on-beam stimulation in blue). (B) Parasagittal block diagram showing on- (blue) and off-beam (red) extracellular electrode stimulation. (C) Superimposed voltage-clamp recordings (upper panel) and corresponding current-clamp recordings (lower panel) of postsynaptic responses to on-beam stimulation (light blue), or off-beam stimulation to elicit a spikelet (orange). Dark blue and red traces represent the averages of 30 trials for on and off-beam stimulation, respectively. (D) Superimposed compound excitatory postsynaptic potentials (EPSPs) recorded in current clamp and for different time delays between on- and off- beam stimulation (each trace is an average of 25 to 30 trials). Time delays (Δt) are relative to on-beam stimulation times. Positive time delays (i.e., off-beam stimulation before on-beam stimulation) are shown in blue shades, coincident stimulations are shown in black, and negative time delays are shown in red shades. Inset is an expanded time scale. (E) Peak amplitude of the compound synaptic responses (normalized to the mean amplitude of the single EPSPs alone) versus the time delay between EPSP and spikelet recruitment (n = 12 cells). Inset is an expanded timescale around the peak and in units of milliseconds. (F) Single trial AP responses to different delays between on- and off-beam stimulation. Shown here are three representative cases: spikelet (red arrows) recruited 20 ms after EPSPs (blue arrows) (top panel), coincidentally with EPSPs (middle panel), or 10 ms before the EPSP (lower panel). In each instance, 30 sweeps are shown. (G) Summary plot of the probability of eliciting an AP by on-beam stimulation (normalized to the mean probability of AP firing by on-beam stimulation alone) versus the time delay between EPSP (on-beam stimulation) and spikelet recruitment (off-beam stimulation; n = 11 cells). Inset is an expanded timescale around the peak and in units of milliseconds. See also Figure 8—source data 1.
-
Figure 8—source data 1
Spikelet signaling transmission enables temporal contrast enhancement of basket cell (BC) excitation.
- https://cdn.elifesciences.org/articles/57344/elife-57344-fig8-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus – male and female) | CB6F1 | Mouse Genome Informatics | RRID:MGI:5649749 | |
| Strain, strain background (Mus musculus – male and female) | CX36 KO, 129 brown mice (129S1/SvImJ) | David Paul, Harvard University | Deans et al. Neuron 2001. | The Cx36 coding sequence is replaced by a LacZ-IRES-PLAP reporter cassette |
| Chemical compound, drug | D-AP5 | Abcam | Cat#:ab120003 | |
| Chemical compound, drug | Gabazine (SR-95531) | Abcam | Cat#:ab120042 | |
| Chemical compound, drug | QX-314 | Abcam | Cat#:ab120118 | |
| Chemical compound, drug | NBQX | Tocris | Cat#:1044 | |
| Chemical compound, drug | Alexa Fluor 488 | ThermoFisher Scientific | Cat#:A10436 | |
| Chemical compound, drug | Alexa Fluor 594 | ThermoFisher Scientific | Cat#:A10438 | |
| Chemical compound, drug | Mefloquine hydrochloride | Sigma-Aldrich | Cat#:M2319 | |
| Chemical compound, drug | DMSO | Sigma-Aldrich | Cat#:D2650 | |
| Software, algorithm | Igor Pro | Wavemetrics | RRID:SCR_000325 | |
| Software, algorithm | Neuromatic | Rothman and Silver, 2018; DOI:10.3389 | RRID:SCR_004186 | |
| Software, algorithm | ImageJ | National Institutes of Health | RRID:SCR_003070 | |
| Software, algorithm | GraphPad Prism 6 | GraphPad Software | RRID:SCR_002798 |





