Curvature-processing domains in primate V4
Figures
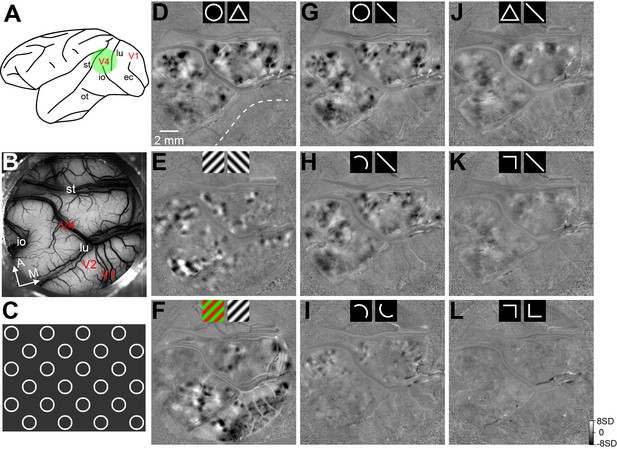
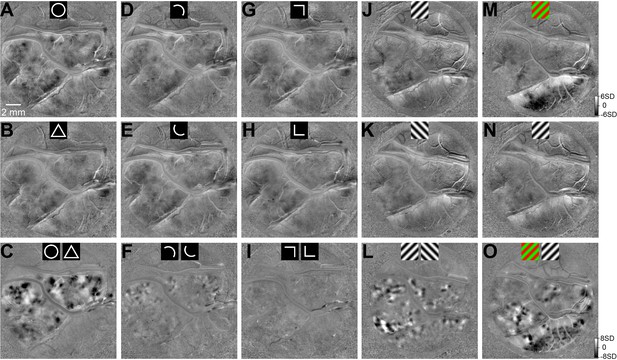
Intrinsic signal optical imaging (ISOI) of curvature domains in area V4.
(A) A schematic representation of the macaque brain showing the imaging region (green circle) and sulci locations. lu, lunate sulcus; st, superior temporal sulcus; and io, inferior occipital sulcus. (B) In vivo image of the blood vessel pattern in the 16 mm diameter imaging region in Case 1, which included parts of V1, V2, and V4. The exposed V4 region was between the st and lu sulci. A, anterior; M, medial. (C) An illustration of the full-screen stimulus pattern used for ISOI imaging. Circle diameter: 2.5°. Drifting speed: 4°/s. (D–L) Functional maps (SVM maps) from Case 1. The icons shown at the top represent the stimulus conditions being compared. In the ISOI maps, dark and white regions were preferentially activated by the stimulus icons shown on the left and right, respectively. For the maps shown in G, H, J, and K, data from different stimulus orientations were pooled for lines, curves, and angles. Other maps were obtained with the exact stimuli as shown on the maps. (D) The circle vs. triangle map shows clear patches in V4 (dark regions preferred circles and white regions preferred triangles), which were absent in V1 and V2. The dotted line represents the border between V1 and V2. (E) The orientation preference map shows the 45° (dark) and 135° (white) orientation domains in V2 and V4. The lack of orientation domains in V1 could have been resulted from the low SF of the stimulus gratings (0.25 c/degrees). (F) The color preference map shows the color domains in V1, V2, and V4. (G) The circle vs. straight line map shows a preference pattern similar to that in the circle vs. triangle map (D). (H) The curve vs. straight line map shows patterns similar to those in D and G. The curve was a half-circle. (I) The curve-orientation map shows smaller and weaker patches preferring different curve orientations. (J) The triangle vs. straight line map shows weaker and larger patches than those in the circle vs. straight line map in G. The dark domains preferred triangles over lines and occupied the same regions as the white patches in D. (K) The angle vs. straight line map shows a very weak pattern similar to that in J. (L) There was no clear pattern in the angle-orientation map.
Comparison of single-condition maps and difference maps (Case 1).
(A) A single-condition SVM map obtained by comparing circle response and blank response from Case 1. (B) As in A, but for triangle response. (C) The difference map comparing circle and triangle responses (same as shown in Figure 1D). (D–O) As in A–C, single-condition maps (top two rows) and their corresponding difference maps (bottom row). The single-conditions maps contained stimulus-non-specific hemodynamic signals, which were diffuse and low contrast. Difference maps removed these common responses and revealed stimulus-specific responses. Maps A–I and J–O were obtained from the same chamber in two separate experiments.
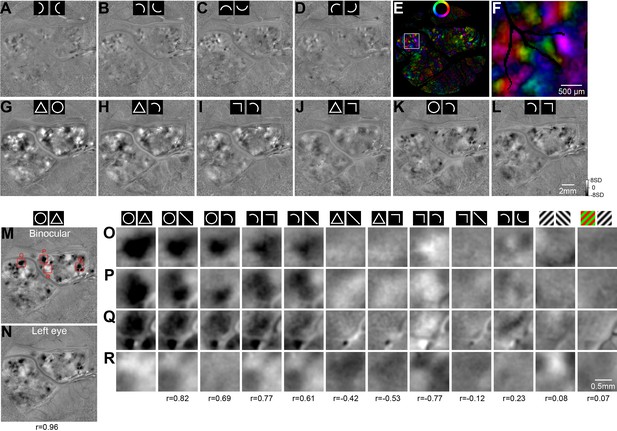
Additional functional maps of Case 1.
(A–D) All four curve-orientation maps of Case 1 comparing opposite curve orientations. B is the same map as shown in Figure 1I. (E) The vector-summation map for the curve-orientation preferences calculated based on the maps in A-D. (F) An enlarged view of the vector-summation map showed in E. (G–L) Functional maps of Case 1 obtained with the same method as those in Figure 1. (M and N) Circle vs. triangle maps obtained with binocular stimulation (M, same as in Figure 1D) or monocular left-eye stimulation (N). The red-framed regions in M are enlarged in O-R. The value at bottom of N is the correlation between V4 regions in the two maps. (O–R) Zoom-in views of 4 regions (locations are indicated in M) from 12 functional maps (columns) for detailed comparisons. The stimulus conditions are illustrated on the top of each column. Values at the bottom are correlation values between the whole V4 region in the corresponding map (not only the enlarged regions) and the V4 region in the circle vs. triangle map (M).
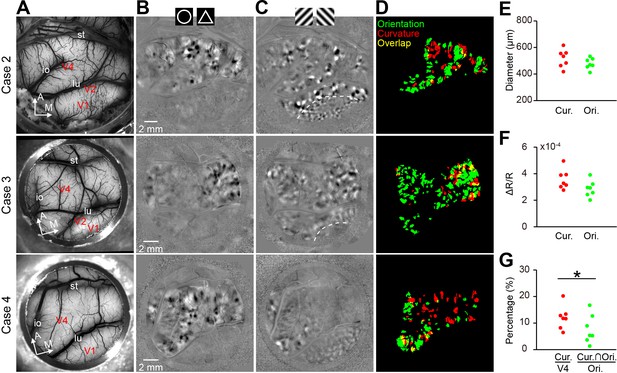
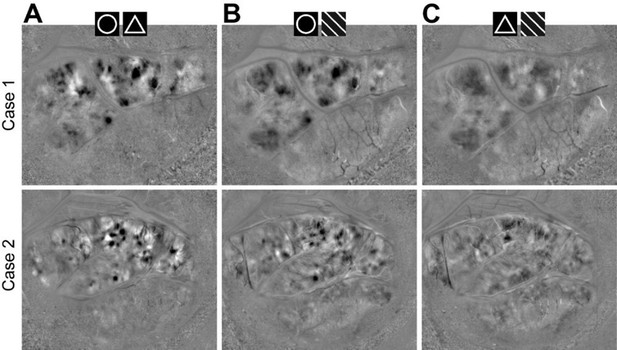
Comparison of curvature and orientation maps.
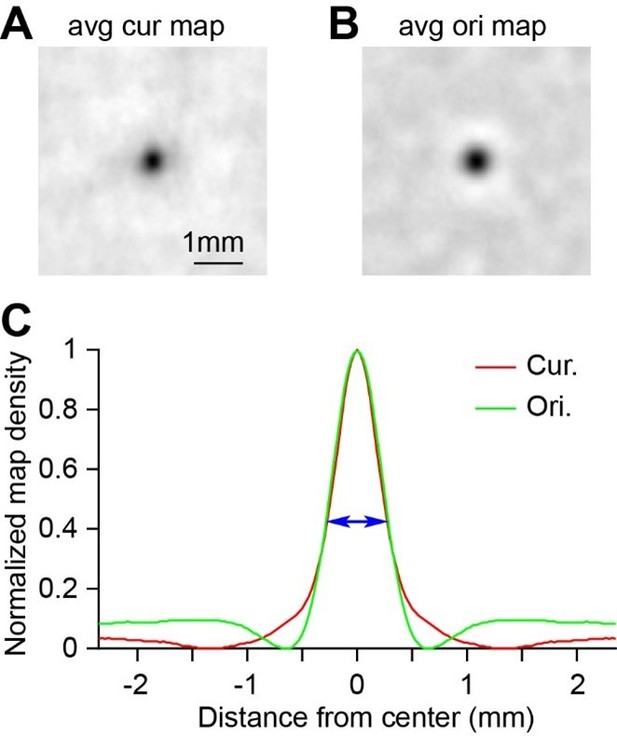
(A–D) Maps from Cases 2–4 are shown in rows 1–3. (A) The blood vessel patterns of the imaging regions. (B) The circle vs. triangle maps obtained using the same stimuli as those for Figure 1D. (C) Orientation preference maps showing the 45° vs. 135° orientation patterns in V1, V2, and V4. The dotted lines represent the borders between V1 and V2. Case 4 did not have V2 exposed on the surface. (D) The spatial relationship between the curvature and orientation domains (0°, 45°, 90°, 135°) in each case. (E) The curvature and orientation domains had similar domain sizes. (F) The curvature and orientation domains had similar response amplitudes. (G) The size of the overlap regions between the curvature and orientation domains was smaller than that of the random prediction (p=0.030; paired t-test, n = 7), which indicates a tendency of separation of these two types of domains.
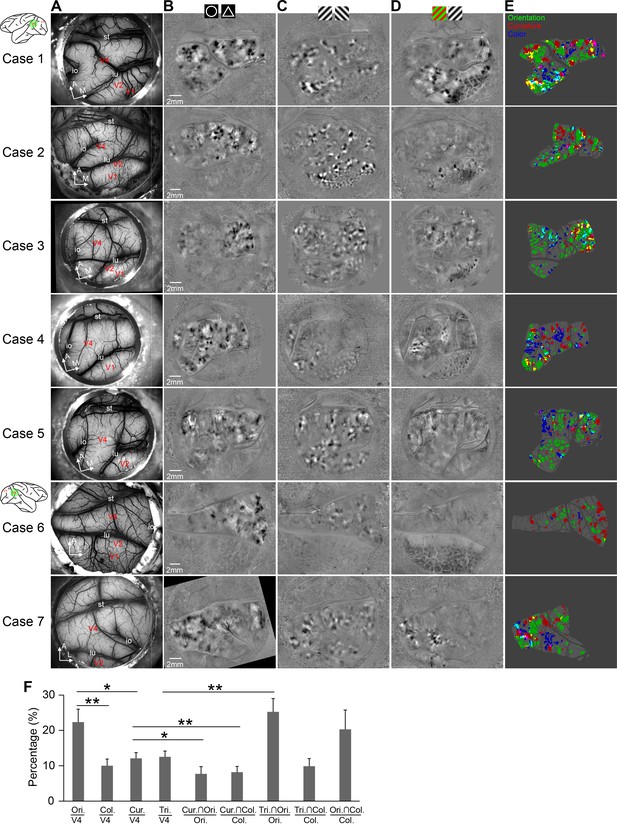
Three types of maps from all seven cases.
(A) Blood-vessel patterns of the seven chambers used in ISOI imaging, plotted in the same convention as that in Figure 1B. Cases 1–5 were from left hemispheres, and Cases 6 and 7 were from right hemispheres. A, anterior; M, medial; lu, lunate sulcus; st, superior temporal sulcus; and io, inferior occipital sulcus. (B) Circle vs. triangle maps. (C) Orientation preference maps. (D) Color preference maps. (E) The overlap of three types of domains. (F) Percentages of areas occupied by four types of domains in V4 (first four columns), which serve as a random distribution control for the proportion of overlapped region by another type of domain (five columns on the right). One or two asterisks indicates p<0.05 or p<0.01, respectively (paired t-test, n = 7). Error bars: s.e.m.
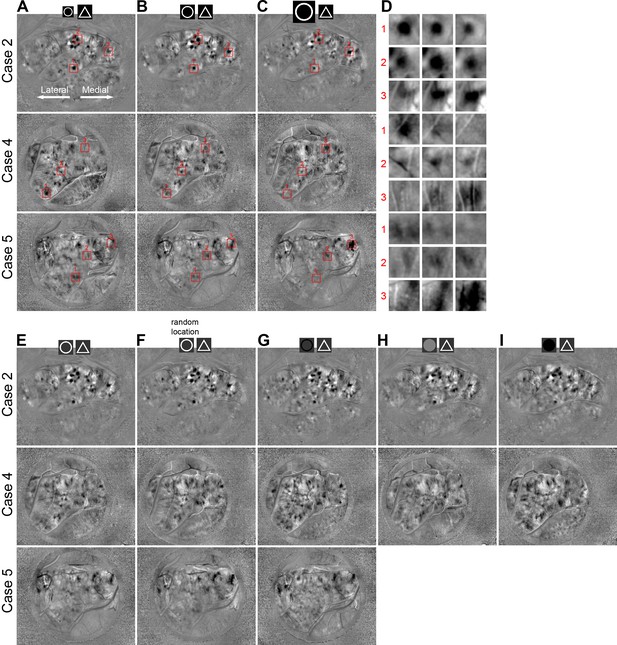
Circle vs. triangle maps obtained with different stimulus parameters.
(A–C) Circle vs. triangle maps obtained with three different circle sizes (A: 1.25°; B: 2.5°; C: 5°). The size of the triangle was kept at 2.5°. Three rows are maps from three different cases. Red frames in the maps are enlarged in D for detailed comparison. The lateral V4 represents the foveal visual field (lower visual eccentricity), and the medial V4 represents peripheral (higher visual eccentricity). When the circle size increased, stronger activation domains in V4 shifted from lateral to medial. (D) Zoom-in views of the regions indicated in A–C. Maps in the three columns correspond to the three columns in A–C. When circle size increased, the activation of the lateral domains (labeled 1) decreased (from the left column to the right column), while medial domains (labeled 3) increased their activity. Domain in the middle (labeled 2) were more activated by mid-sized circles. (E) Standard circle vs. triangle maps of Cases 2, 4, and 5. These maps are the same as those shown in Figure 2—figure supplement 1B and were obtained with a regular stimulus protocol. (F–I) Circle vs. triangle maps obtained with different types of circle stimuli, while triangle stimuli were kept the same as in E, including: circles randomly positioned as contrast to orderly positioned ones (e.g. Figure 1C) (F); Black circles (G), Filled gray disks (H), and Filled dark disks (I).
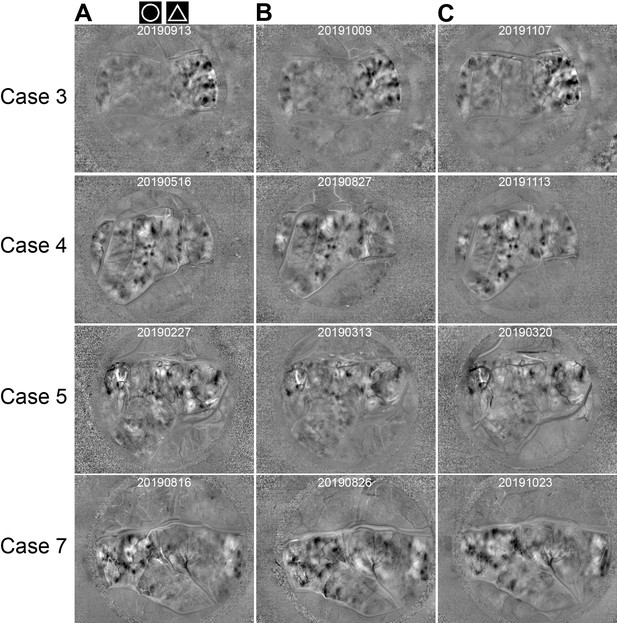
Repeated imaging of the maps on different days.
(A–C) Circle vs. triangle maps obtained from four cases (four rows) on different days (three columns). The stimulus protocols were the same as the example shown in Figure 1D. Imaging dates are indicated on the top of each map.
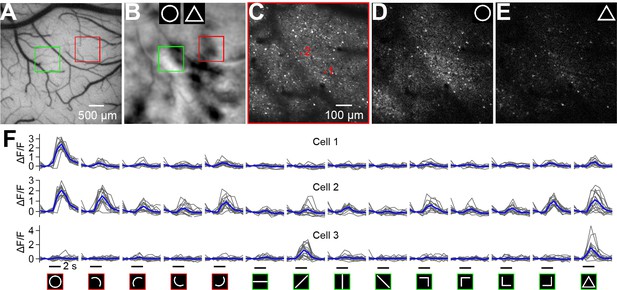
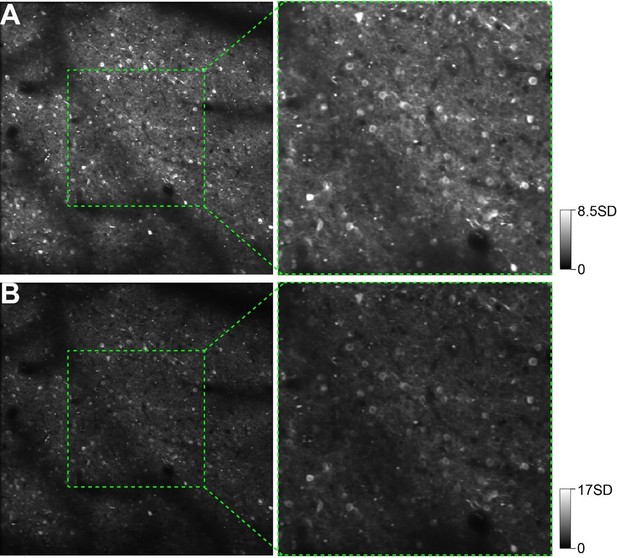
Two-photon calcium imaging of neuronal shape responses.
(A) An image of the cortical region obtained from Case 3 in which AAV-GCaMP6s was injected for calcium imaging. The red and green frames indicate two regions examined using two-photon imaging. (B) Circle vs. triangle map of the region shown in A. (C) Neurons in the red-frame region shown in A and B that were imaged using a 16× objective at a 210 μm depth from the cortical surface. Two neurons marked in red are examined in detail in panel F. Scale bar applies to C–E. (D and E) Single-condition response maps (ΔF) for the circle (D), and triangle (E) stimuli. Each map was obtained after averaging 15 repeats and pooling of 4 orientations. (F) Responses (ΔF/F) of 3 neurons to 14 typical contour stimuli (showed at the bottom, red: curvature stimuli; green: rectilinear stimuli). The responses to circle and triangle were the best-orientation ones. Cells 1 and 2 were selected from the curvature domain shown in C. Although both neurons preferred circle stimuli, they exhibited different response levels to other stimuli types. Cell 3 was selected from a rectilinear region (marked in Figure 4F) and was strongly activated by the triangle stimulus and one of its line segment. The gray and blue lines represent individual trials and the average, respectively. The stimulus duration (2 s) is labeled at the bottom of the last row.
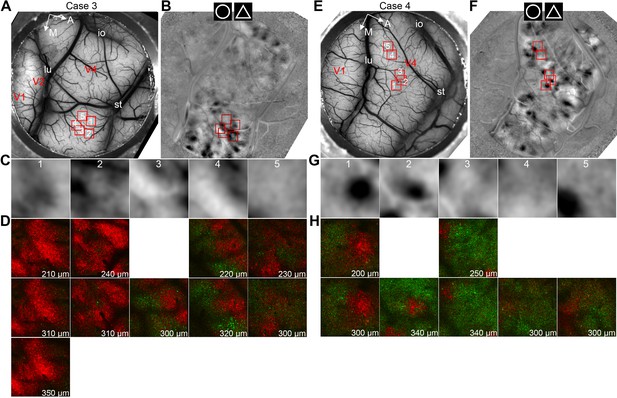
All the 10 two-photon-imaged V4 regions in this study.
(A) The blood vessel map of Case 3, in which five V4 regions studied with the two-photon imaging are indicated (red squares). A, anterior; M, medial; lu, lunate sulcus; st, superior temporal sulcus; and io, inferior occipital sulcus. (B) The circle vs. triangle map of Case 3. The two-photon imaged regions (red squares) are further enlarged in C. (C) The zoom-in views of the five regions indicated in the circle vs. triangle map in B. Site one was the same as shown in Figure 4A. Site four was the same site as shown in Figure 4D. (D) Curvature (red) vs. rectilinear (green) preference maps of the five regions (five columns), plotted with the same convention as those in Figure 4B and C. The different maps in each column were from different imaging depths (indicated at the bottom right of each map). A consistency among different depths, as well as with the ISOI maps in C, can be observed. (E–H) Similar to A–D, the maps from Case 4 show the same trend. Site one was the same as shown in Figure 4G.
Fluorescent responses of V4 neurons to contour shapes.
Single-trial two-photon images from one experiment session were extracted from the raw data and displayed in a real-time speed. This imaging site is also shown in Figure 3C. For each stimulus condition, seven consecutive frames after the stimulus onset were extracted and displayed. This include three frames collected during the stimulus presentation and four frames collected during interstimulus interval. The top-down refreshing display mimics the scanning sequence of the Galvo mode imaging (1.3 Hz) used in the experiments. Each frame was clipped to the range of 0 and mean+7SD, where ‘mean’ is the average of pixel values in all the frames in this video and ‘SD’ is the average of standard deviations of all the frames. Images were aligned to correct motion noises. Frame size was resized from 512 × 512 pixels to 410 × 410 pixels to reduce file size. Stimuli elicited the fluorescent responses were illustrated on the bottom right. Each stimulus was ~1.6°, moved across a range of 4° in one of eight directions. The size of the population RF for this imaged region is approximately four degrees.
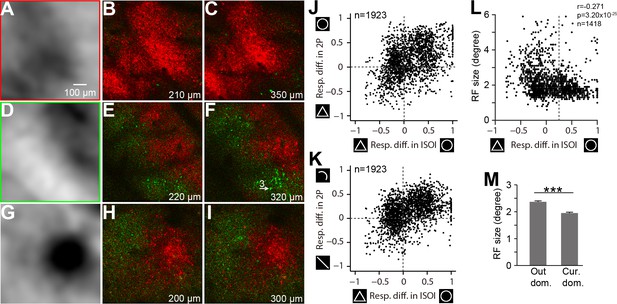
Consistency between ISOI and two-photon imaging results.
(A) An enlarged view of a curvature domain in an ISOI circle-triangle map in Case 3 (the red-framed region in Figure 3B). (B) A curvature (red) vs. rectilinear (green) preference map for the same image used in Figure 3C. Each pixel is color coded for its preferred responses to the curvature (red-framed icons in Figure 3F) or rectilinear stimuli (green-framed icons in Figure 3F). The brightness of the pixels is proportional to the fluorescence strength and shape preference (see Materials and methods). (C) Preference maps for the same location shown in B but obtained from a deeper layer (350 μm from the surface). Contour-type preferences are similar as shallower depths (B). (D–F) As in A-C, but for another location in Case 3 (green-framed regions in Figure 3A and B). This region contained a subregion preferring curved stimuli (top right) and a subregion preferring rectilinear stimuli (lower left). Two-photon preference maps show consistent contour-type preferences with the ISOI map, as well as consistency between different depths. Cell 3 with an arrow was also used in Figure 3F. (G–I) As in above two sites, a third example obtained from Case 4, which contained a small curvature domain. Consistency between ISOI imaging and two-photon imaging, as well as consistency between different depths of two-photon images are evident. (J) Comparison of the ISOI and two-photon responses. The Y-axis represents differences in the fluorescent responses (optimal orientation) to circles and triangles in all neurons. The X-axis represents differences in hemodynamic responses for corresponding neuron pixels in the ISOI circle vs. triangle maps. There was a significant correlation between the two types of responses (Pearson r = 0.42, p=9.45×10−83, n = 1923). (K) Similar to J, there was a significant correlation between the fluorescent responses (optimal orientation) to curves vs. lines and ISOI responses to circles vs. triangles (Pearson r = 0.43, p=9.35×10−88, n = 1923). (L) The RF sizes of all neurons measured (Y-axis) had a negative correlation with the circle-triangle preferential responses of the corresponding pixels in the ISOI imaging (X-axis, same as the X-axis values in J and K). The dotted vertical line represents the threshold chosen (2SD) in determining whether a pixel is inside the curvature domains (the right side of the line) or outside (the left side of the line). (M) The average RF size was smaller for neurons inside the curvature domains (1.95 ± 0.032°, n = 583) than those outside (2.37 ± 0.038°, n = 835, p=1.15×10−14, t-test). Error bar: s.e.m.
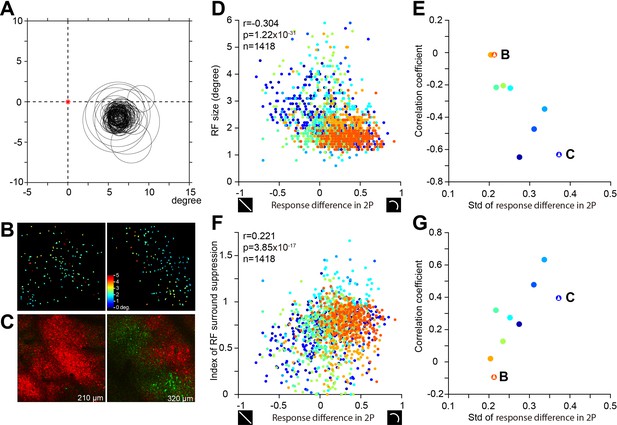
RF sizes of the sampled V4 neurons.
(A) The RF plot for the imaged neurons in Figure 3C. The red asterisk indicates the fovea location estimated from a back-projected plot of the blind spot. The center locations of the RFs were calculated from the responses to the 5 × 5 grid RF mapping stimuli, and the RF sizes were calculated from the size-tuning fitting functions. (B) The RF size of two example sites which shows progressive change in the right image, but not in the left image. (C) The curvature (red) vs. rectilinear (green) preference maps corresponding to the site shown on the top. (D) The RF sizes of all neurons measured (Y-axis) had a negative correlation with the curve-line preferential responses (optimal orientation) measured in the two-photon imaging (X-axis). Different colors indicate different two-photon sites (n = 9, different depths of the same site were pooled). (E) The distribution of the correlation values between RF sizes and curve-line preferential responses for 9 two-photon sites, measured from panel D. The X axis represents the standard deviation of curve-line preferential responses among the neurons in each two-photon site. (F and G) As in D and E, but for neurons’ surround suppression, which was positively correlated with their curvature preference, and such correlation was also influenced by the homogeneity of neurons in the imaging site (G). Note: A total of 1493 neurons had significant RF size tuning. The RF sizes were calculated from the size-tuning fitting functions. In these neurons, 13 had RF sizes smaller than 0.5°, and 62 had RF sizes larger than 12°. These 75 neurons were not included in the above plot or the analysis in panels D–G. In addition, 18 neurons had RF sizes too large (6–12°) to fit in the above plots, but were included in the population analysis.
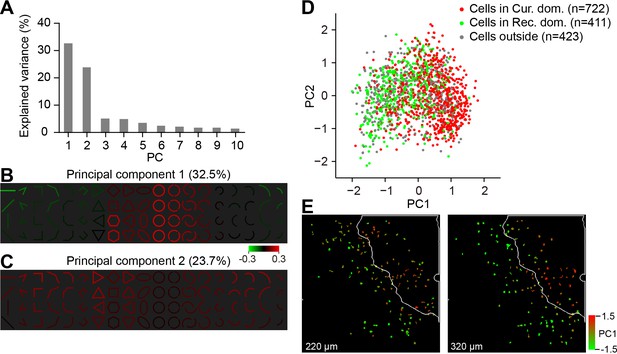
PCA results show different response patterns for neurons inside and outside the curvature domains.
(A) Percentages of the response variance accounted for by the top 10 principle components obtained from the PCA analysis on neurons’ response matrices (n = 1556). Stimulus orientations are sorted according to the responses before the PCA analysis. (B) PC1 shows a negative response relationship between cirlces and rectilinear lines, comfirming the ISOI findings. The color of each stimulus represents the sign and strength of the population response to that stimulus correlated to this principle component. PC1 explained 32.5% of the response variance. (C) As in B, for PC2, which shows positive contribution from many stimuli except for the circles, PC2 explained 23.7% of the variance. (D) All neurons (n = 1556) plotted according to their PC1 and PC2 coordinates. Although had a large overlap, curvature neurons (red) tended to separate from the rectilinear neurons (green). Neurons in gray were those located outside of these two types of domains. (E) An example two-photon site in which neurons were labeled according to their PC1 coordinates. Neurons with positive PC1 coordinates (red) were mostly found inside the curvature domain on the top right cornor (with white outline). Neurons with negative PC1 coordinates (yellow and green) were mostly located outside the curvature domain.
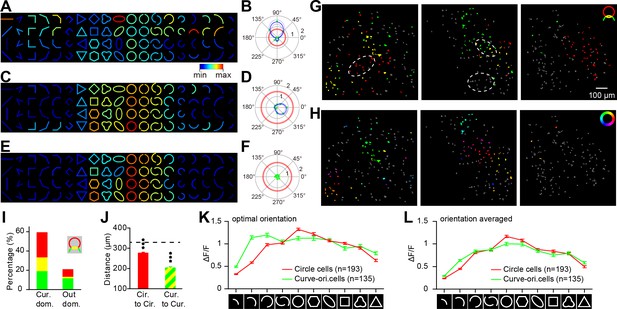
Microarchitectures inside the curvature domains.
(A) Response matrix of an example ‘curve-orientation-preferring neuron’, which showed strong preference for a half circle oriented at 90° and an oval contained a similar curve fragment. (B) Orientation tuning curve for curves (half circles). Green dots represent response values to the curves at eight different orientations. Blue line represents fitting curve for the responses. Red circle represents the neurons’ average response level (a single value) for the circle stimuli. Pale-blue and red lines represent ± s.e.m of the responses. (C, D) As in A and B, but for a ‘dual-preference neuron’. This neuron showed significant orientation tuning to curves, along with a maximal response for the circle. (E, F) As in A-D, but for a ‘circle-preferring neuron’. This neuron responded best to the circles, but did not exhibit curve-orientation tuning. There was no fitting curve for the neuron shown in F due to its weak responses. (G) Neurons tended to cluster according to their preferences for circles or curves. Three maps are from three different two-photon imaged locations, and neurons are color coded as shown in the icon: red: circle-preferring neurons; green: curve-orientation-preferring neurons; yellow: dual-preference neurons. Neurons in gray did not pass the circle or curve-orientation preference tests. The neurons in the dotted ovals are further examined in Figure 7. (H) Neurons showed curve-orientation tuning (including curve-orientation-preferring and dual-preference neurons) tended to cluster according to their preferred orientations. The color of the neurons represents their preferred orientation (0–360°). (I) The percentages of the three neuron types inside and outside the curvature domains. The color code is the same as that in G. (J) The average cell-to-cell distances for circle-preferring neurons (red) and neurons showing curve-orientation tuning with similar preferred orientations (differences < 45°, green and yellow) were shorter than the overall average distance (dotted line) (p<0.001, t-test). Error bar: s.e.m. (K) Population averaged fluorescent responses to different contours. For each contour, the response to the optimal orientation was used. The circle-preferring neurons (red) showed gradual increase of response with the length or completeness of the circle, while the curve-orientation-preferring neurons (green) did not. Error bar: s.e.m. (L) As in K, but used orientation-averaged responses.
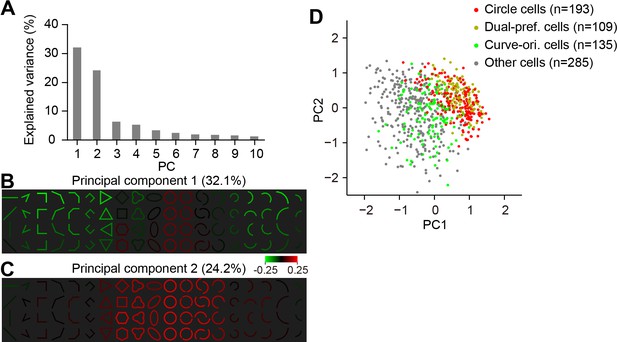
PCA results for neurons inside curvature domains.
(A) Percentages of the response variance accounted for by the top 10 principle components obtained from the PCA analysis of neurons inside curvature domains (n = 722). (B) PC1 shows a negative response relationship between cirlces and most of the other stimuli that unlike circles. PC1 explained 32.1% of the response variance. (C) As in B, for PC2, which shows a positive contribution from circles and other closed shapes, PC2 explained 23.7% of the variance. (D) All neuron in curvature domains are plotted according to their PC1 and PC2 coordinates. Circle-preferring neurons (red) tended to separate from curve-orientation-preferring neruons (green). Dual-preference neurons (yellow) tend to co-located with circle neurons. Neurons in gray were those do not belong to the above three groups.
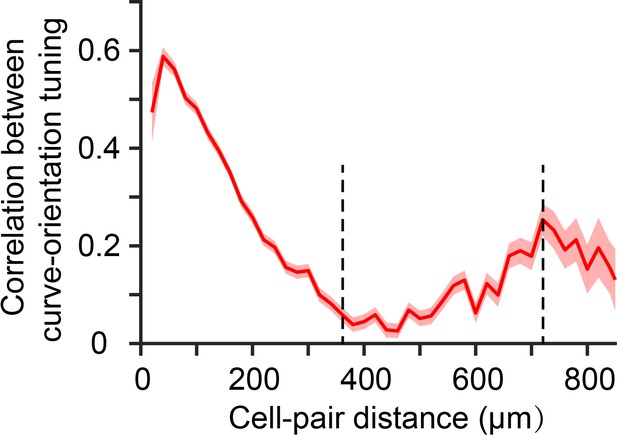
Spatial periodicity of curve-orientation subdomain.
The correlation coefficients of curve-orientation tuning of cell pairs inside the curvature domains, based on their responses to the eight half-circle curves, were calculated, and then grouped based on the distance of the cell pairs at a step of 20 μm. The relationship between the average correlation coefficients of curve-orientation tuning and the distances of the cell pairs were plotted, and shown a trough around 360 μm and a second peak around 720 μm. Red line and shadow represent mean ± s.e.m of the correlation coefficients.
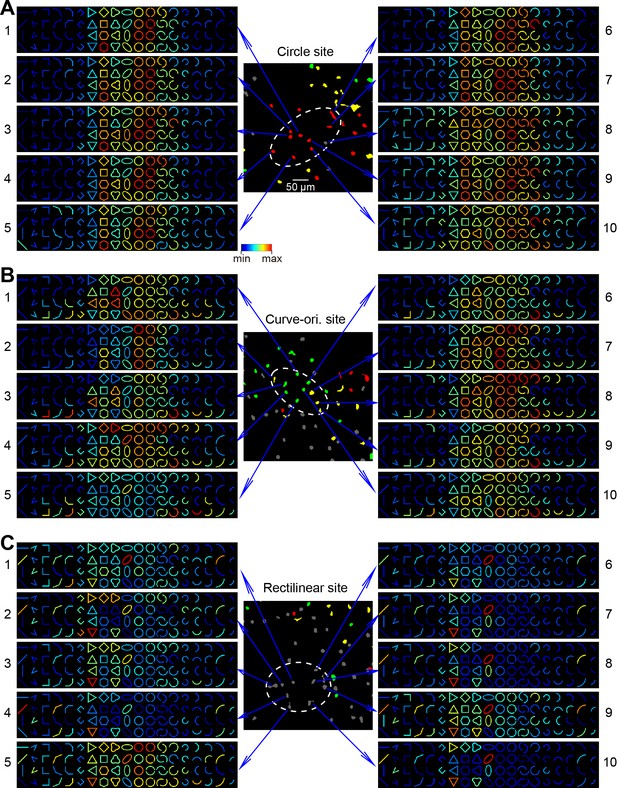
Single-neuron responses in three types of cortical sites.
(A) Response matrices of 10 neighboring neurons in a circle-preferring two--photon site (also shown in Figure 6G). Each matrix is normalized to its maximal and minimal responses. (B) As in A, but for a curve-orientation-preferring site. (C) As in A and B, but for a rectilinear site.
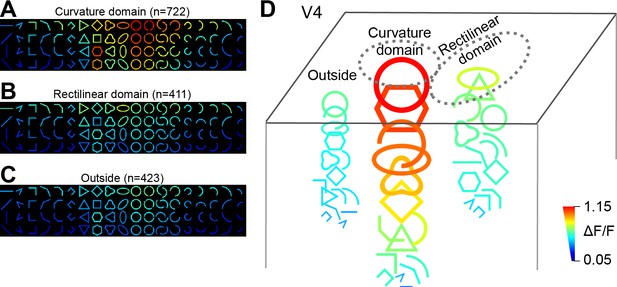
A summary of neurons’ response patterns in different V4 regions.
(A–C) Average response matrices (without normalization) from neurons in the curvature domains (A), rectilinear domains (B) and those outside of these two types of domains (C). Responses are orientation-sorted before averaging. (D) An illustration of the contour-preferences for neurons in the three regions, calculated based on the optimal-orientation responses in A–C (the top rows). The size and color of the shapes represent the strength of the corresponding responses. For clarity, not all stimulus icons were drawn.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Macaque, male) | Macaca mulatta | Suzhou Xishan Zhongke animal Company, Ltd Hubei Topgene Biotechnology Co.,Ltd | http://xsdw.bioon.com.cn/ http://topgenebio.com/ | |
| Strain, strain background (Macaque, male) | Macaca fascicularis | Beijing Inst. of Xieerxin Bology Resource | http://www.xexbio.com/ | |
| Recombinant DNA reagent | AAV1.Syn.GCaMP6S.WPRE.SV40 | Addgene | v25497 | |
| Recombinant DNA reagent | AAV9.Syn.GCaMP6S.WPRE.SV40 | Addgene | CS1282 | |
| Software, algorithm | MATALAB R2017b | MathWorks | https://www.mathworks.com | |
| Software, algorithm | Codes for ISOI data analysis | This paper | https://osf.io/qydj5/ | |
| Software, algorithm | Codes for 2P data analysis | This paper | https://osf.io/qydj5/ |