EphrinB2-EphB4 signalling provides Rho-mediated homeostatic control of lymphatic endothelial cell junction integrity
Figures
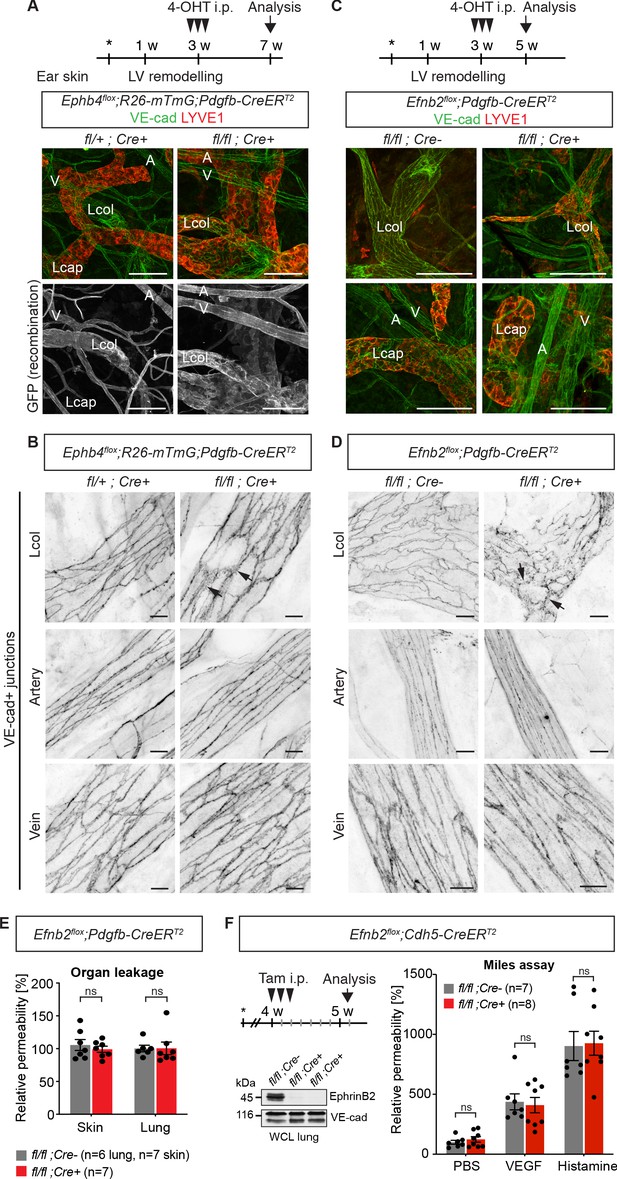
Endothelial deletion of Ephb4 or Efnb2 selectively disrupts dermal collecting lymphatic vessels.
(A) Experimental scheme for Ephb4 deletion in the mature vasculature by three consecutive intraperitoneal (i.p.) 4-OHT injections (arrowheads) into 3-week-old Ephb4flox;R26-mTmG;Pdgfb-CreERT2 mice. Ear skin whole-mount immunofluorescence from 7-week-old Ephb4 mice using antibodies against VE-cadherin (green) and LYVE1 (red) or GFP (single channel images). GFP expression demonstrates Cre recombination in arteries (A), veins (V) and LYVE1- collecting lymphatic vessels (Lcol), but not LYVE1+ lymphatic capillaries (Lcap) in control ear. Mutant collecting vessels show abnormal LYVE1 expression. (B) Ear skin whole-mount immunofluorescence of 7-week-old Ephb4 mice using an antibody against VE-cadherin. Note altered morphology of collecting lymphatic vessel junctions (arrow) in Ephb4 mutant compared to heterozygous littermates. (C) Experimental scheme for Efnb2 deletion in the mature vasculature by 3 consecutive 4-OHT injections (arrowheads) into 3-week-old Efnb2flox;Pdgfb-CreERT2 mice. Ear skin whole-mount immunofluorescence of 5-week-old Efnb2 mice using antibodies against VE-cadherin (green) and LYVE1 (red). (D) Ear skin whole-mount immunofluorescence of 5-week-old Efnb2 mice using an antibody against VE-cadherin. Note altered morphology of collecting lymphatic vessel junctions (arrow) in Efnb2 mutant compared to Cre negative littermates already after 2 weeks of Cre induction. (E) In vivo basal permeability assay in skin and lung of 5-week-old Efnb2 mutants and Cre negative littermates. Data represent mean ± s.e.m. (n = 6–7 mice from two independent experiments). Efnb2 deletion does not impact on basal barrier function of skin and lung blood vasculature. (F) Experimental scheme for Efnb2 deletion using the Cdh5-CreERT2 line and three consecutive tamoxifen injections (arrowheads). Vascular leakage in the skin of 5-week-old Efnb2 mutants and Cre negative littermates was induced with VEGF or histamine. Note, endothelial deletion of Efnb2 does not impact on junctional regulation in leakage-induced dermal blood vasculature. Data represent mean ± s.e.m. (n = 7-8 mice from two independent experiments). Western blot from total lung lysates 8 days after the first tamoxifen administration showing depletion of EphrinB2 in Cre+ mice. VE-cadherin was used as a loading control. Source data for panels (E,F) are provided. Scale bars: 100 μm (A,C), 10 μm (B,D).
-
Figure 1—source data 1
Flow cytometric analysis of endothelial cell proliferation in postnatal mouse ear skin.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig1-data1-v1.xlsx
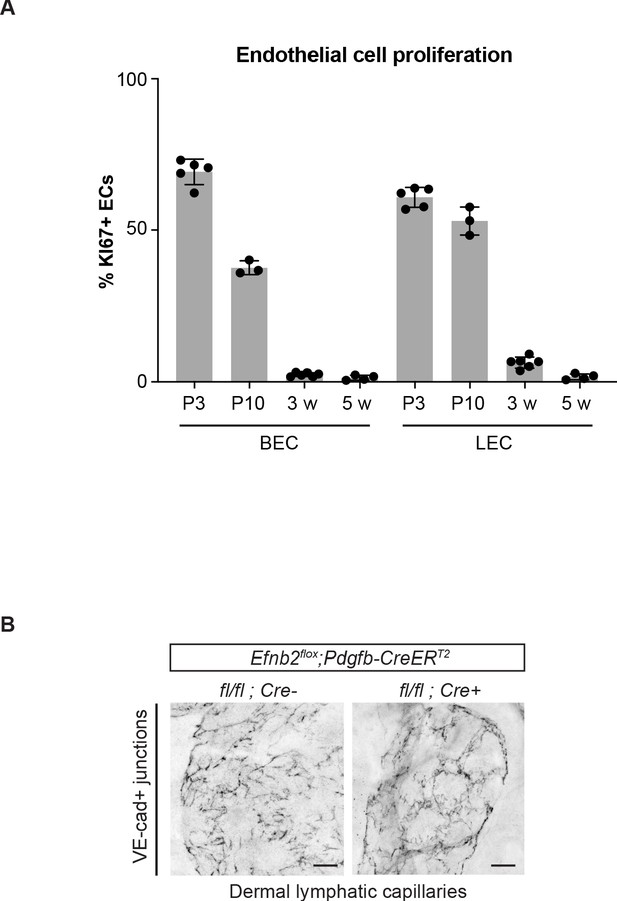
Pdgfb-CreERT2-mediated deletion of Efnb2 in mature collecting vessels leads to defective cell-cell junctions in lymphatic capillaries.
(A) Flow cytometric analysis of dermal BEC (PECAM1+PDPN-) and LEC (PECAM1+PDPN+) proliferation in the ear skin of 3 day (n = 5), 10 day (n = 3), 3-week-old (n = 6) and 5-week-old (n = 4) mice. Data represent mean % of KI67+ ECs ± s.d. (B) Ear skin whole-mount immunofluorescence of lymphatic capillary junctions from 5-week-old Efnb2flox/flox;Pdgfb-CreERT2 and Cre- littermate mice using an antibody against VE-cadherin. Mice were administered with three consecutive injections of tamoxifen at 3 weeks of age. Note disruption of button-like junctions of lymphatic capillaries, not targeted by the Pdgfb-CreERT2 transgene, in the mutant. Source data for panel (A) is provided. Scale bar: 100 μm.
-
Figure 1—figure supplement 1—source data 1
Measurement of blood vessel permeability in Efnb2 mutants and control littermates.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig1-figsupp1-data1-v1.xlsx
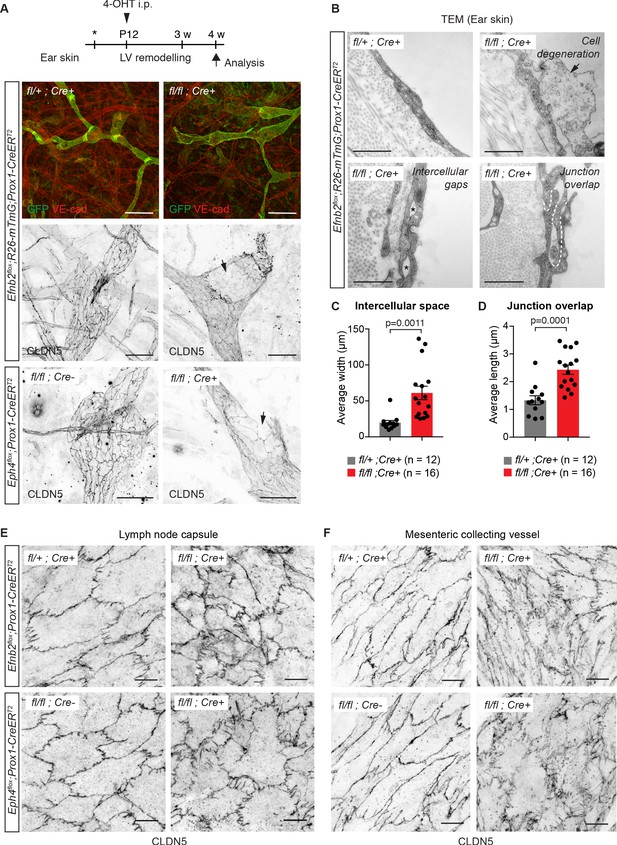
Lymphatic endothelial specific deletion of Efnb2 or Ephb4 disrupts endothelial cell-cell junctions in several organs.
(A) Experimental scheme for LEC-specific deletion of Efnb2 and Ephb4 in the remodeling lymphatic vasculature (LV) using the Prox1-CreERT2 line and a single 4-OHT treatment at P12 (arrowhead). Ear skin whole-mount immunofluorescence of 4-week-old Efnb2 and Ephb4 mice using antibodies against VE-cadherin (red) and GFP (green) (shown for Efnb2 mice only) or CLDN5 alone (single channel images). GFP expression demonstrates Cre recombination in lymphatic endothelia. Note disintegration of collecting lymphatic vessels in Efnb2 and Ephb4 mutant mice (arrows). (B) Ultrastructural analysis of the ear vasculature using transmission electron microscopy showing abnormal features of LEC junctions in Efnb2 mutant mice compared to heterozygous littermate: intercellular gaps (asterisks), cell degeneration (arrow) and increased junction overlap (white dotted line). (C,D) Quantification of intercellular gaps and junctional overlap. Data represent mean ± s.e.m., p-value, Two-tailed unpaired Student’s t-test (n = 12 vessels/194 junctions/3 mice (fl/+) or n = 16 vessels/250 junctions/2 mice (fl/fl)). (E) Whole-mount immunofluorescence of the outer surface of the subcapsular sinus (SCS) of the inguinal lymph node of 4-week-old Efnb2 and Ephb4 mice using an antibody against CLDN5. (F) Whole-mount immunofluorescence of P11 mesenteric collecting vessels in Efnb2 and Ephb4 mice using an antibody against CLDN5. Source data for panels (C,D) are provided. Scale bars: 500 μm (A), 100 μm (A single channel images), 1 µm (B), 10 μm (E,F).
-
Figure 2—source data 1
Quantification of LEC junction parameters in the skin.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig2-data1-v1.xlsx
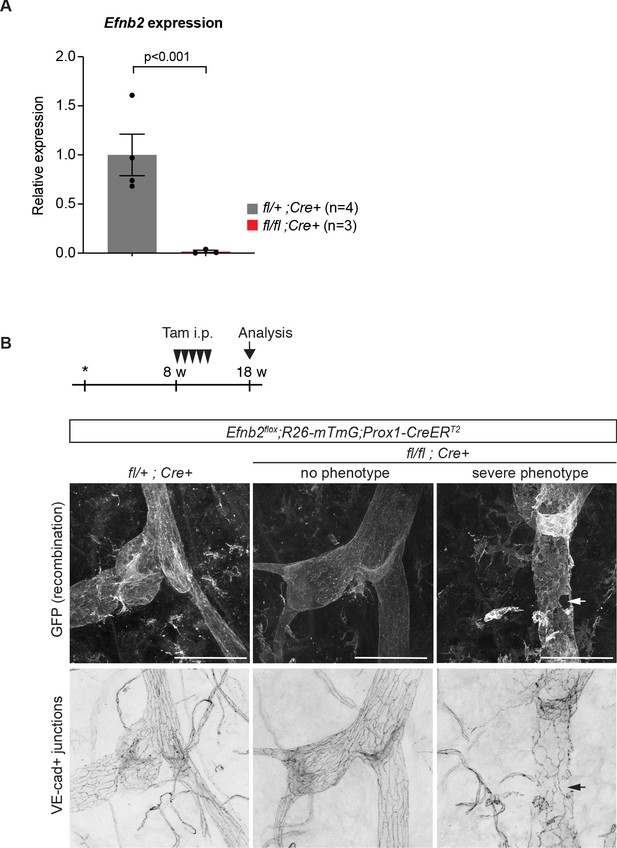
Efnb2 deletion in 4-week-old and 18-week-old mice.
(A) Relative mRNA expression levels of Efnb2 in freshly isolated LECs from 4-week-old mutant (n = 3) and heterozygote/wildtype control (n = 4) ear skin. (B) Experimental scheme for LEC-specific deletion of Efnb2 in adult mice. 8-week-old Efnb2flox;Prox1-CreERT2 mice were administered with five consecutive intraperitoneal injections of tamoxifen (arrowheads) and ear skin was analysed at 18 weeks by whole-mount immunofluorescence using antibodies against GFP (upper panel) and VE-cadherin (lower panel). GFP expression demonstrates Cre-mediated recombination in lymphatic endothelial cells. Efnb2 mutants showed variable phenotype, some vessels with apparently normal LEC junctions (no phenotype) and others showing severe disintegration of junctions (severe phenotype). Source data for panel (A) is provided. Scale bar: 100 μm.
-
Figure 2—figure supplement 1—source data 1
qRT-PCR analysis of Efnb2 expression in FACS sorted dermal LECs.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig2-figsupp1-data1-v1.xlsx
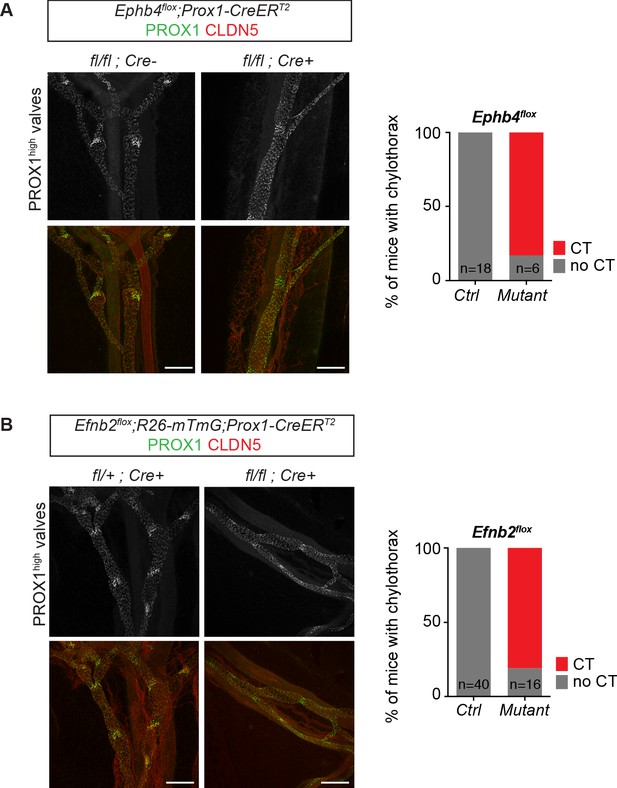
Dysfunctional lymphatic valves and chylothorax in Efnb2 and Ephb4 mutant mice.
Whole-mount immunofluorescence of P11 mesenteric collecting vessels (on the left) and incidence of chylotorax (on the right) in Ephb4flox;Prox1-CreERT2 (A) and Efnb2flox;Prox1-CreERT2 (B) mice neonatally (P4) treated with 4-OHT. Lymphatic valves show high PROX1 expression and are malformed in the mutant vessels. n = 18 (control) and n = 6 (mutant) neonatal Ephb4 mice were analyzed from five independent litters. n = 40 (control) and n = 16 (mutant) neonatal Efnb2 mice were analyzed from eight independent litters. Source data for panels (A,B) are provided. Scale bar: 200 μm.
-
Figure 2—figure supplement 2—source data 1
Incidence of chylothorax in Ephb4 and Efnb2 mutant mice.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig2-figsupp2-data1-v1.xlsx
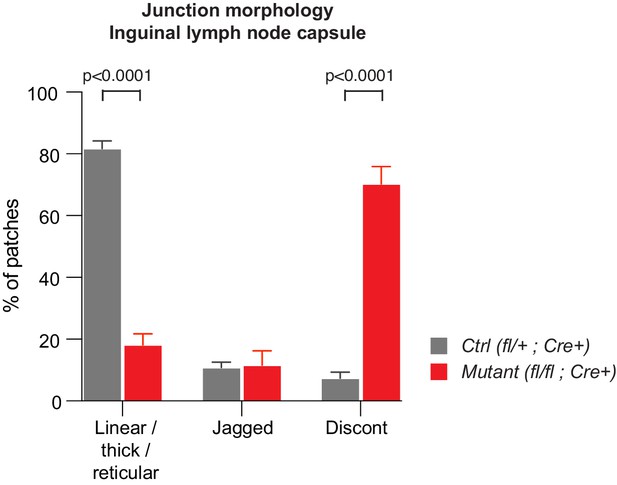
Abnormal morphology of LEC junctions in the inguinal lymph node capsule of Efnb2 mutant mice.
Quantification of lymphatic junctional morphology in CLDN5-stained lymph nodes upon Efnb2 deletion. Junctional categories are defined as 1. linear/thick/reticular, 2. jagged and 3. discontinuous junctions. Data represent mean ± s.e.m. adjusted p-value, Multiple t-tests, (n = 150 patches/2 mice per genotype). Source data is provided.
-
Figure 2—figure supplement 3—source data 1
Quantification of LEC junction morphology in the inguinal lymph node capsule.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig2-figsupp3-data1-v1.xlsx
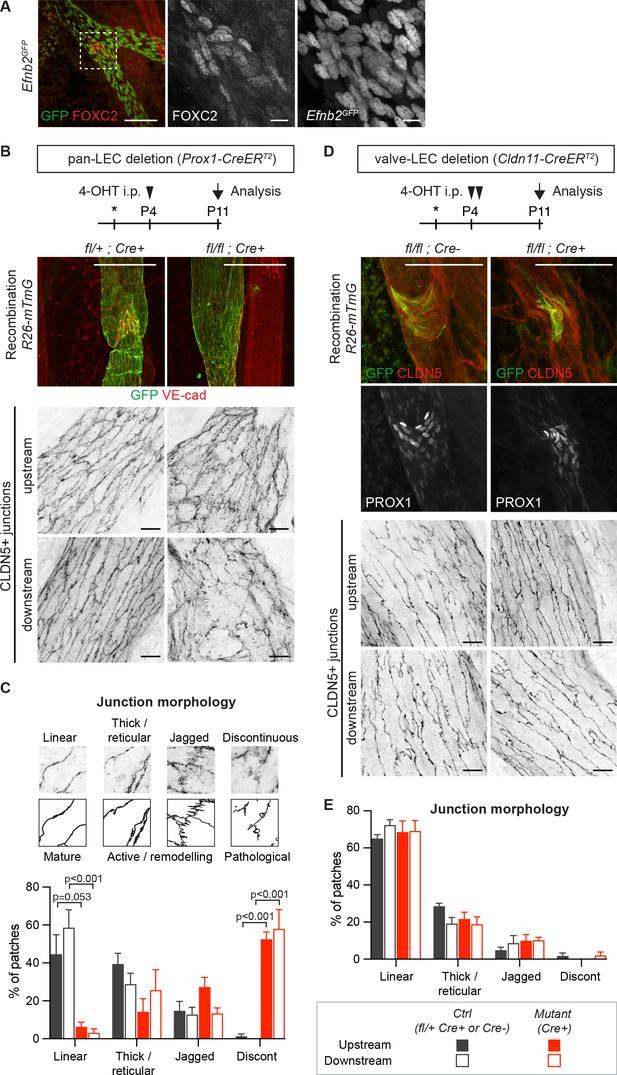
Lymphatic valve-specific deletion of Efnb2 leads to valve defects but unaltered LEC junctions on the collecting vessel wall.
(A) Whole-mount immunofluorescence of mesenteric lymphatic vessels of P11 Efnb2GFP mice using antibodies against GFP (green) and FOXC2 (red). Enlarged view of the boxed area with single channel images show valve-restricted FOXC2 expression but widespread GFP (reporting Efnb2) expression. (B) Experimental scheme for neonatal Efnb2 deletion in all LECs (pan-LEC) using the Prox1-CreERT2 mice. Top panels: Whole-mount immunofluorescence of P11 mesenteric vessels of Efnb2flox;R26-mTmG;Prox1-CreERT2 mice using antibodies against VE-cadherin (red) and GFP (green). Note pan-LEC Cre deletion (GFP expression), and valve defect in the Efnb2 mutant. Lower panels: Visualization of LEC junctions in vessels regions downstream or upstream of the valve using CLDN5 antibodies. (C) Quantification of junction morphology in CLDN5-stained mesenteric vessels upon pan-LEC Efnb2 deletion. The defined junctional categories are illustrated above: linear (mature), thick/reticular and jagged (active), and discontinuous (pathological) junctions. Data represent mean ± s.e.m. adjusted p-value, Multiple t-tests, (n = 64–96 patches/3 mice per genotype and region). (D) Experimental scheme for neonatal Efnb2 deletion in valve LECs using the Cldn11-CreERT2 mice. Top panels: Whole-mount immunofluorescence of P11 mesenteric vessels of Efnb2flox;R26-mTmG;Cldn11-CreERT2 mice using antibodies against CLDN5 (red), GFP (green) and PROX1 (single channel images). Lower panels: Visualization of LEC junctions in vessels regions downstream or upstream of the valve using CLDN5 antibodies. Note valve-restricted Cre deletion (GFP expression), and misshaped valves but unaffected LEC junctions in the Efnb2 mutant. (E) Quantification of junction morphology in CLDN5-stained mesenteric vessels upon valve-LEC specific Efnb2 deletion. Data represent mean ± s.e.m., (n = 64 patches/2 mice per genotype and region). Source data for panels (C,E) are provided. Scale bars: 100 μm (A, B, D merge images), 10 μm (A, B, D single channel images).
-
Figure 3—source data 1
Quantification of LEC junction morphology in the mesentery.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig3-data1-v1.xlsx
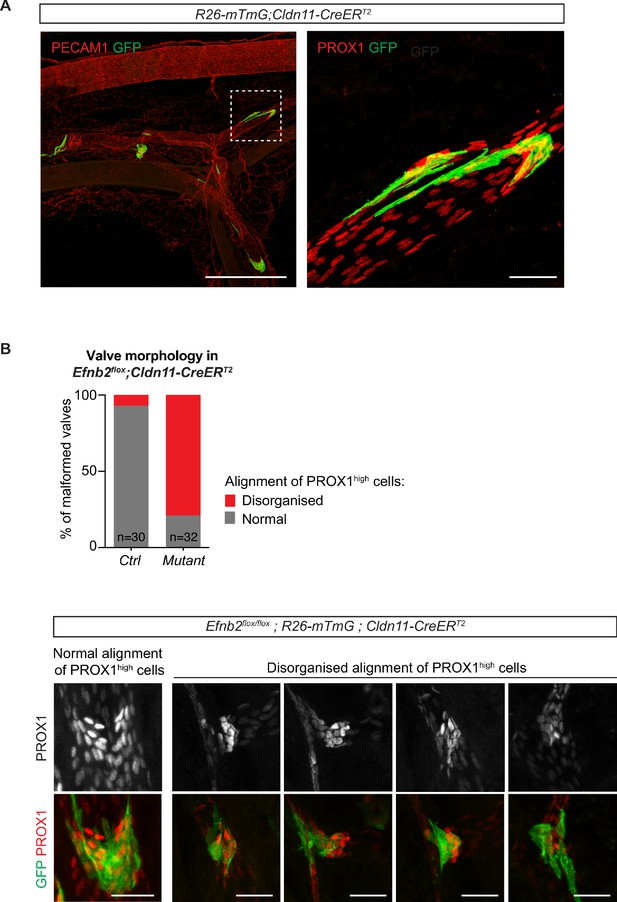
Valve-specific deletion of Efnb2 using the Cldn11-CreERT2 line.
(A) Whole mount immunofluorescence of mesentery of a R26-mTmG;Cldn11-CreERT2 mesentery showing GFP expression (Cre-mediated recombination) in lymphatic valves. Boxed area of the PECAM1-stained mesentery is magnified on the right and shows PROX1 staining to highlight the valve region. 4-OHT was administered at P5 and mesentery was analysed at P12. (B) Quantification of alignment of PROX1high nuclei in whole-mount stained Cre-targeted (GFP positive) valve regions in Efnb2flox;R26-mTmG;Cldn11-CreERT2 mesenteries. Two categories (normal and disorganized) were defined and are exemplarily shown below. 4-OHT was administered at P4 and P5 and mesentery was analysed at P11. n = 30 (control) and n = 32 (mutant) valves from three mice per genotype. Source data for panel (B) is provided. Scale bar: 500 µm (A, overview), 50 µm (A, magnification), 50 µm (B).
-
Figure 3—figure supplement 1—source data 1
Quantification of valve phenotype upon valve-specific deletion of Efnb2.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig3-figsupp1-data1-v1.xlsx
Loss of junctional CLDN5 occurs after EphrinB2 blockade in vitro but does not lead to breakdown of LEC junctions in vivo.
(A) Top: Western blot of EphrinB2-Fc-precipitated EphB4 HDLECs for phosphotyrosine (4G10) and EphB4. Cells were treated with control (siCTRL) or EphrinB2 siRNA (siEFNB2), and stimulated with pre-clustered EphrinB2-Fc or control Fc. Total cell lysates (TCL) were immunoblotted for EphB4 and actin. Bottom: Quantification of relative EphB4 phosphorylation (adjusted to total precipitated EphB4 protein) from four independent experiments, showing EphrinB2-dependent basal EphB4 activity in unstimulated LECs. (B) Immunofluorescence of HDLECs treated with EphrinB2 blocking antibody (B11) for 3 hr using antibodies against VE-cadherin (green) and CLDN5 (red). Single channel images are depicted in grey. EphrinB2 blockade induces junction remodeling and removal of junctional CLDN5. (C) Quantification of HDLEC monolayer permeability to 40 kDa FITC-dextran showing increase upon B11 treatment for 3 hr (n = 10 replicates per condition from three independent experiments). (D) Quantification of junctional CLDN5 immunofluorescence in VE-cadherin+ junctions (n = 6–7 images from three independent experiments). (E) Immunofluorescence of HDLECs transfected with CTRL, CLDN5 or CDH5 (VE-cadherin) siRNA for 48 hr. Staining for CLDN5 (green) and VE-cadherin (grey) show disruption of VE-cadherin+ junctions upon CLDN5 siRNA transfection. Note, CLDN5+ junctions are not affected upon VE-cadherin silencing. (F) Quantification of HDLEC monolayer permeability to 40 kDa FITC-dextran showing minor and moderate increase upon CDH5 or CLDN5 silencing, respectively (n = 7–8 replicates per condition from two independent experiments). (G) Whole-mount immunofluorescence of mesenteric collecting vessels and valves in P11 Cldn5flox;Prox1-CreERT2 mice using antibodies against CLDN5 and VE-cadherin. Note efficient CLDN5 depletion apart from a few CLDN5 hot spots and altered morphology but no disintegration of the junctions. Data in A represent mean ± s.e.m. p value, One-sample t-test. Data in C, D, F represent mean ± s.e.m. p value, Two-tailed unpaired Student’s t-test. Source data for panels (A,C,D,F) are provided. Scale bars: 100 μm (B, E, G mesenteric valves), 50 μm (G mesenteric collecting vessel).
-
Figure 4—source data 1
Quantification of the effects of EphrinB2 inhibition on EphB4 phosphorylation, endothelial monolayer permeability and junctional CLDN5.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig4-data1-v1.xlsx
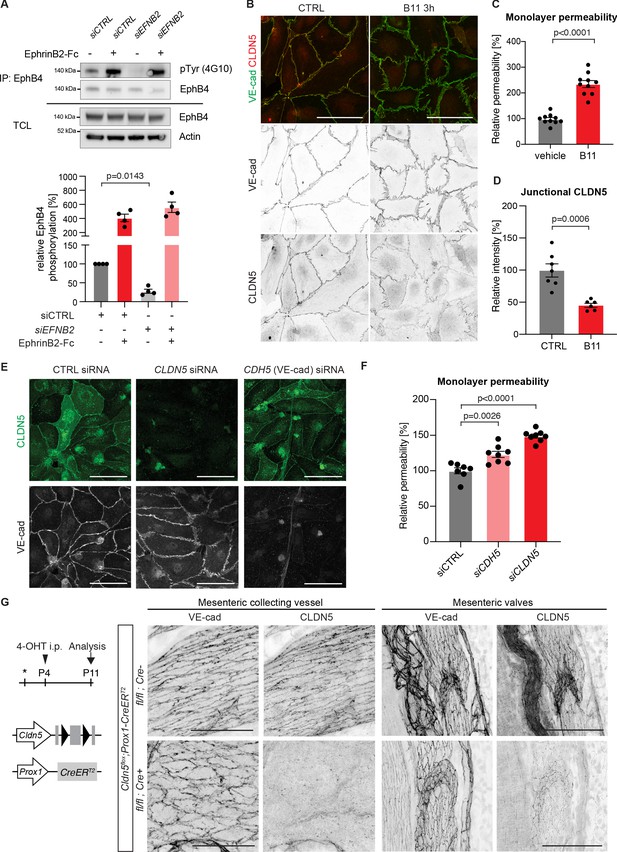
Loss of junctional CLDN5 after long-term EphrinB2 inhibition in primary LECs.
Immunofluorescence of HDLECs treated with EphrinB2 blocking antibody (B11) for 16 hr using antibodies against VE-cadherin (red) and CLDN5 (green). Single channel images are depicted in grey. Note almost complete removal of junctional CLDN5 and disruption of VE-cadherin+ junctions (arrows) in B11-treated cells. Scale bar: 50 µm.
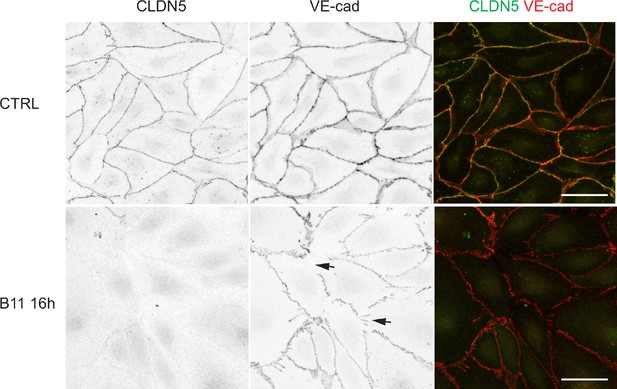
The effect EFNB2 silencing on CLDN5 and VE-cadherin.
(A) Western blot analysis of total cell lysates (TCL) of HDLECs treated for 48 hr with control (siCTRL) or EFNB2 siRNA (siEFNB2). Note reduced CLDN5 but unchanged VE-cadherin levels in EFNB2-silenced cells. Actin was used as a loading control. Western blot is shown as representative of 3 independent experiments. (B) Immunofluorescence of HDLECs transfected with CTRL or EFNB2 siRNA for 48 hr. Staining for CLDN5 and VE-cadherin shows removal of junctional CLDN5 and disruption of VE-cadherin+ junctions (arrows) upon EFNB2 silencing. Scale bar: 50 μm.
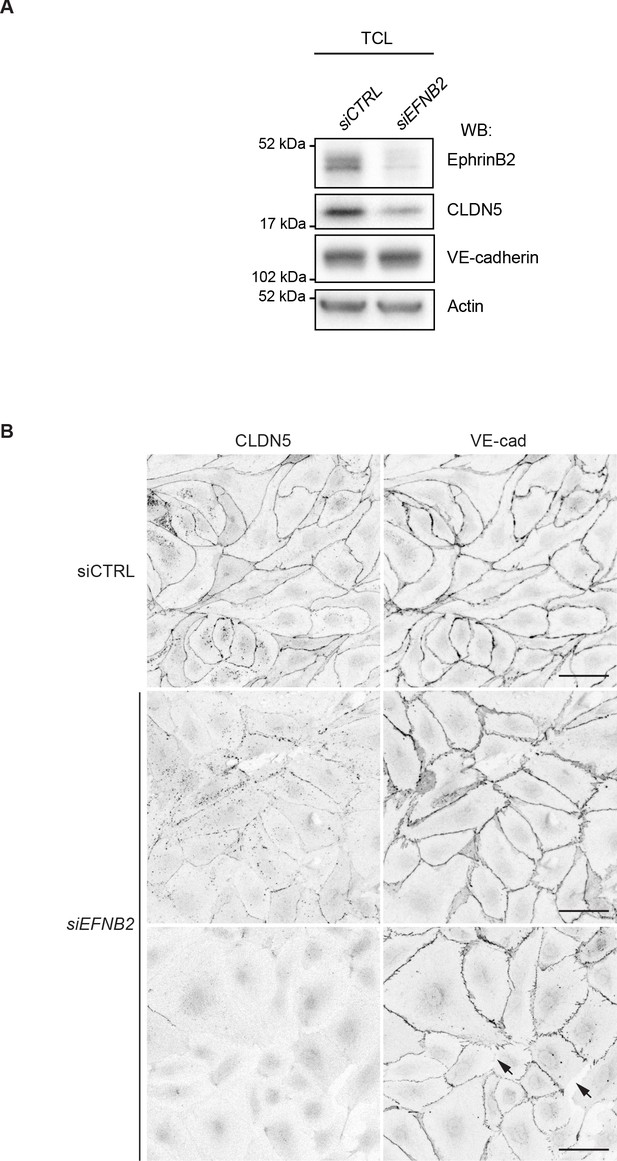
Silencing of CDH5 and CLDN5 in HDLECs.
Western blot analysis of total cell lysates (TCL) of HDLECs treated for 72 hr with control (siCTRL), CDH5 siRNA (siCDH5) and CLDN5 siRNA (siCLDN5). Western blot is shown as representative of 2 independent experiments.
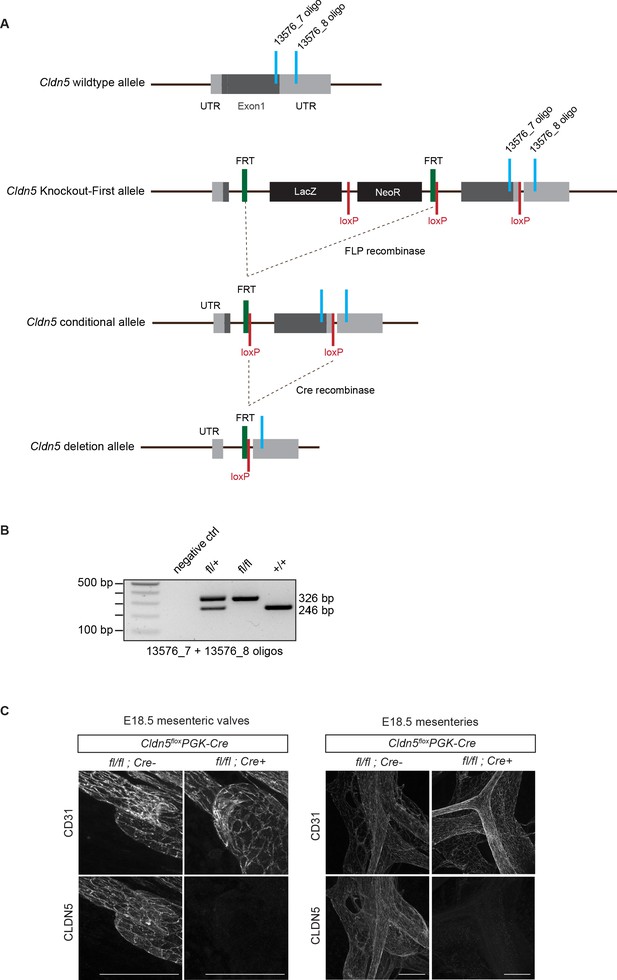
Generation of conditional Cldn5 knock-out mice.
(A) Schematic of the Cldn5 wild type allele, targeted ‘Knockout-First’ allele, conditional allele (floxed) and deletion allele. The ‘Knockout-First’ allele Cldn5tm1a(EUCOMM)Wtsi contains an IRES:lacZ trapping cassette and a floxed promoter-driven neo cassette inserted into the (single) coding exon of the Cldn5 gene, disrupting Cldn5 gene function. Flp convert the ‘Knockout-First’ allele to a conditional allele. Cre deletes the floxed 3’ sequence of the coding exon generating a null allele. The locations of PCR genotyping primers are indicated in light blue. (B) PCR genotyping of wild type and conditional floxed Cldn5 mice. (C) Whole-mount immunofluorescence of mesenteries of E18.5 Cldn5flox/flox;PGK-Cre (i.e. germline Cldn5 homozygous) and Cre negative littermate embryos showing lack of CLDN5 expression in the mutant. Scale bar: 100 μm.
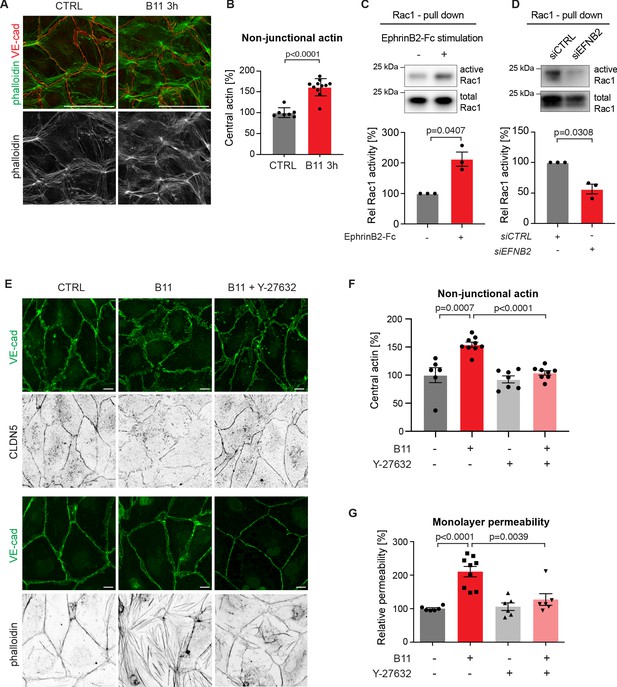
Basal EphrinB2/EphB4 signalling controls the stability of LEC junctions through regulation of Rho-mediated cytoskeletal contractility.
Immunofluorescence of HDLECs for VE-cadherin (red) and phalloidin (green), showing increase in actin stress fibres after 3 h-treatment with the EphrinB2 blocking antibody (B11) compared to untreated controls (CTRL). Phalloidin single channel images are depicted in grey. (B) Quantification of relative central actin (total actin – junctional actin) from n = 7–11 images from three independent experiments shown in A. (C, D) Assessment of Rac1 activity in HDLECs after EphrinB2-Fc stimulation (30 min) (C) or siRNA-mediated EFNB2 silencing (48 hr) (D) Rac1-GTP-pull down and total cell lysates were immunoblotted using Rac1 antibodies. Representative Western blots are shown. Quantification was done from three independent experiments. (E) Immunofluorescence of HDLECs incubated with EphrinB2 blocking antibody (B11) for 3 hr with and without pre-incubation of the ROCK inhibitor Y-27632 using antibodies against VE-cadherin (green) and CLDN5 (grey, upper panel) or phalloidin (grey, bottom panel). Note inhibition of EphrinB2 blockade (B11)-induced junctional and cytoskeletal effects by Y-27632 treatment. (F) Quantification of relative central actin (total actin – junctional actin) from n = 6–9 images from two independent experiments shown in E. (G) Quantification of HDLEC monolayer permeability to 40 kDa FITC-dextran. Note inhibition of EphrinB2 blockade (B11)-induced increase in permeability by Y-27632 treatment. (n = 6–9 replicates per condition from three independent experiments). Data in B, F, G represent mean ± s.e.m. p value, Two-tailed unpaired Student’s t-test. Data in C, D represent mean ± s.e.m. p value, One-sample t-test. Source data for panels (C-D) are provided. Scale bars: 100 μm (A), 10 μm (E).
-
Figure 5—source data 1
Quantification of the effect of EphrinB2 blockade on the actin cytoskeleton and Rac1 activity.
- https://cdn.elifesciences.org/articles/57732/elife-57732-fig5-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | Efnb2flox | Grunwald et al., 2004 | Efnb2tm4Kln; RRDI: MGI:2182626 | |
| Genetic reagent (Mus musculus) | Ephb4flox | Martin-Almedina et al., 2016 | ||
| Genetic reagent (Mus musculus) | Pdgfb-iCreERT2iresGFP | Claxton et al., 2008 | Tg(Pdgfb-icre/ERT2)1Frut; RRDI: MGI:3793852 | |
| Genetic reagent (Mus musculus) | R26-mTmG | Muzumdar et al., 2007 | Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo; RRDI: MGI:3716464 | |
| Genetic reagent (Mus musculus) | Prox1-CreERT2 | Bazigou et al., 2011 | Tg(Prox1-cre/ERT2)1Tmak; RRDI: MGI:5617984 | |
| Genetic reagent (Mus musculus) | Efnb2GFP | Davy and Soriano, 2007 | Efnb2tm2Sor; RRDI: MGI:3526818 | |
| Genetic reagent (Mus musculus) | Cldn11-CreERT2 | This paper | H Ortsäter and T Mäkinen, manuscript in preparation. | |
| Genetic reagent (Mus musculus) | Cldn5flox | This paper | Cldn5tm1a(EUCOMM)Wtsi; MGI:5473167 | Obtained from The European Conditional Mouse Mutagenesis Program (EUCOMM). |
| Cell line (Homo sapiens) | Dermal lymphatic endothelial cell (normal, juvenile, male) | PromoCell | Cat# C12216 | Primary cell line isolated from foreskin, tested negative for mycoplasma contamination |
| Antibody | anti-EphrinB2 (Human single chain variable fragment) | Abéngozar et al., 2012 | B11 | (80 μg/ml) |
| Antibody | anti-human IgG (Goat polyclonal) | Jackson ImmunoResearch | Cat# 109-005-098 | (5 μg/ml) |
| Antibody | anti-human/mouse/rat EphrinB2 (Goat polyclonal) | R and D Systems | Cat# AF496 | (0.5 μg/ml) |
| Antibody | anti-human EphB4 (Goat polyclonal) | R and D Systems | Cat# AF3038 | (0.5 μg/ml) |
| Antibody | anti-human VE-cadherin (Goat polyclonal) | Santa Cruz Biotechnology | C19, Cat# sc-6458 | (IF: 0.2 μg/ml; WB 2 μg/ml) |
| Antibody | anti-human/mouse CLDN5 (Rabbit polyclonal) | Invitrogen | Cat# 34–1600 | (0.5 μg/ml) |
| Antibody | anti-phosphotyrosine 4G10 (Mouse monoclonal) | Merck | Cat# 05–321 | (0.5 μg/ml) |
| Antibody | anti-β-actin (Rabbit polyclonal) | Cell Signalling Technologies | Cat# 4967 | (0.1 μg/ml) |
| Antibody | anti-mouse Podoplanin (Syrian hamster monoclonal) | eBioscience | eBio8.1.1, PE; Cat# 12-5381-82; RRDI:AB_1907439 | (2 µg/ml) |
| Antibody | anti-mouse CD31 (Rat monoclonal) | eBioscience | 390, PE-Cy7, Cat# A14715; RRDI:AB_2534231 | (0.7 µg/ml) |
| Antibody | anti-mouse CD45 (Rat monoclonal) | eBioscience | 30-F11, PerCP-Cyanine5.5; Cat# 5-0451-82, RRDI:AB_1107002 or eFluor450; Cat# 48-0451-82; RRDI:AB_1518806 | (4 µg/ml) |
| Antibody | anti-mouse CD11b (Rat monoclonal) | eBioscience | M1/70, PerCP-Cyanine5.5; Cat# 45-0112-82; RRDI:AB_953558 or eFluor450; Cat# 48-0112-82; RRDI:AB_1582236 | (4 µg/ml) |
| Antibody | anti-TER-119 (Rat monoclonal) | eBioscience | TER-119, eFluor450; Cat# 48-5921-82; RRDI:AB_1518808 | (4 µg/ml) |
| Antibody | anti-Ki-67 (Rat monoclonal) | eBioscience | SolA15, eFluor 660; Cat# 50-5698-82; RRDI:AB_2574235 | (1:100) |
| Antibody | anti-human VE-cadherin (Mouse monoclonal) | Santa Cruz Biotechnology | F-8, Cat# sc-9989 | (2 μg/ml) |
| Antibody | anti-mouse LYVE1 (Rat monoclonal) | R and D Systems | Cat# MAB2125 | (1 μg/ml) |
| Antibody | anti-GFP (Rabbit polyclonal) | Abcam | Cat# ab290 | (1 μg/ml) |
| Antibody | anti-mouse FoxC2 (Sheep polyclonal) | R and D Systems | Cat# AF6989 | (2 μg/ml) |
| Sequence-based reagent | TaqMan probe: Gapdh | ThermoFisher Scientific | Cat# Mm_03302249_g1 | |
| Sequence-based reagent | TaqMan probe: Efnb2 | ThermoFisher Scientific | Cat# Mm00438670_m1 | |
| Sequence-based reagent | siRNA: human CLDN5 | Dharmacon | Cat# D-011409-03-0010 | |
| Sequence-based reagent | siRNA: human CDH5 | Dharmacon | Cat# D-003641-03-0010 | |
| Sequence-based reagent | siRNA: human EFNB2 | Dharmacon | Cat# L-003659-00-0005 | |
| Sequence-based reagent | siRNA: negative control, human | Qiagen | Cat# 1027281 | |
| Peptide, recombinant protein | Human EphrinB2-Fc | R and D Systems | Cat# 7397-EB | (0.5 μg/ml: activation assay, 3 μg: IP) |
| Chemical compound, drug | ROCK inhibitor (Y-27632) | Sigma | Cat# SCM075 | (10 µM) |
| Commercial assay or kit | Rac1-GTP pulldown assay | Cytoskeleton Inc | Cat# BK030 |





