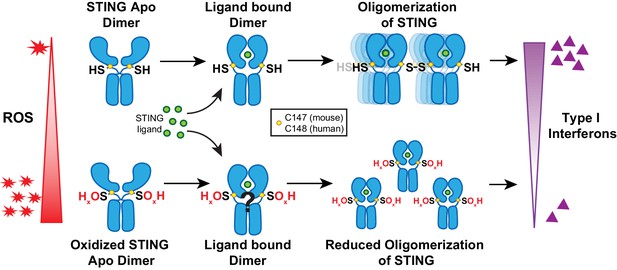
Reactive oxygen species oxidize STING and suppress interferon production
Figures
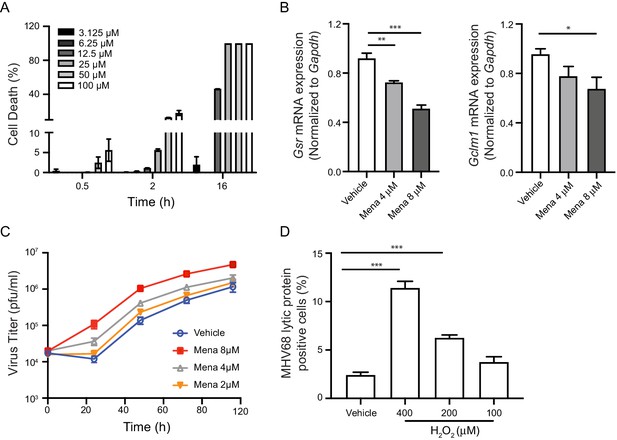
ROS promote herpesvirus replication in macrophages.
(A) Fully differentiated bone marrow-derived macrophages (BMDMs) were treated with vehicle or different concentrations of menadione as indicated. Cell viability was determined at 0.5 hr, 2 hr and 16 hr after treatment. n = 2 with two technical repeats each time. (B) BMDMs were treated with vehicle, 4 μM or 8 μM menadione for 16 hr. Transcripts of Gsr and Gclm1 were determined using qRT-PCR. n = 6. (C) BMDMs were treated with vehicle or different concentrations of menadione (mena) for 16 hr and then infected with MHV68 at multiplicity of infection (MOI) = 5. Virus titer was determined by plaque assay at 0 hr, 24 hr, 48 hr, 72 hr and 96 hr after infection. n = 3 with three technical repeats each time. (D) BMDMs were treated with vehicle or different concentrations of H2O2 for 16 hr in culture medium containing 10% fetal bovine serum, then infected with MHV68 at MOI = 5. Twenty-four hours after infection, cells were fixed and cells expressing virus lytic proteins were determined by flow cytometry. n = 3 with two technical repeats each time. Data are shown as mean ± SE, an ordinary one-way ANOVA was performed followed by Dunnett’s multiple comparison test, only the p value for the most relevant comparisons are shown for simplicity. *, p<0.05, **, p<0.01, ***, p<0.001.
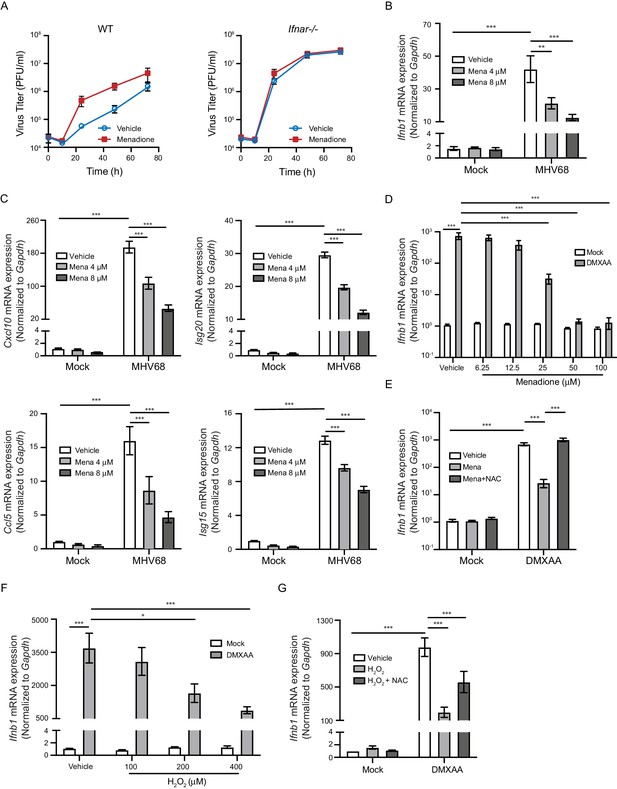
ROS inhibit interferon response upon STING activation.
(A) BMDMs isolated from WT or Ifnar-/- mice were treated with vehicle or 8 μM menadione for 16 hr, then infected with MHV68 at MOI = 5. Virus titer was determined by plaque assay at 0 hr, 10 hr, 24 hr, 48 hr and 72 hr after infection. n = 1 with three technical repeats. (B, C) BMDMs were treated with vehicle, 4 μM or 8 μM menadione for 16 hr, then infected with MHV68 at MOI = 5. Transcripts of Ifnb (B) or ISGs (Cxcl10, Isg20, Ccl5, Isg15) (C) were determined at 6 hr after infection. n = 6. (D) BMDMs were treated with vehicle or different concentrations of menadione as indicated for 30 mins, then stimulated with DMXAA at 1 μg/ml. Transcripts of Ifnb were determined at 2 hr after stimulation. n = 3. (E) BMDMs were treated with vehicle, 25 μM menadione or 25 μM menadione and 2 mM NAC for 30 mins, then stimulated with 1 μg/ml DMXAA. Transcripts of Ifnb were determined at 2 hr after stimulation. n = 3. (F) BMDMs were treated with vehicle or different concentrations of H2O2 in serum free medium for 10 mins, then stimulated with 1 μg/ml DMXAA. Transcripts of Ifnb were determined 2 hr after stimulation. n = 3. (G) BMDMs were treated with vehicle, 200 μM H2O2 for 10 mins or 200 μM H2O2 for 10 mins followed by 5 mM NAC for 30 mins, then stimulated with 1 μg/ml DMXAA. Transcripts of Ifnb were determined 2 hr after stimulation. n = 4. Data are shown as mean ± SE, statistical analysis was conducted using two-way ANOVA followed by Tukey’s multiple comparison test, only the p value for the most relevant comparisons are shown for simplicity. *, p<0.05, **, p<0.01, ***, p<0.001.
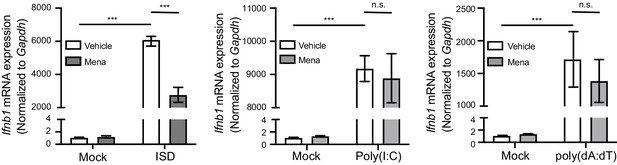
ROS regulate interferon response in macrophages upon STING activation.
BMDMs were treated with 20 μM menadione for 30 min, then stimulated with 10 μg/ml ISD, 1 μg/ml poly(I:C), or 1 μg/ml poly(dA:dT). Ifnb transcripts were determined 4 hr after stimulation. n = 4. Bars represent mean ± SE. p value was calculated using two-way ANOVA followed by Tukey’s multiple comparison test, only the p value for the most relevant comparisons are shown for simplicity. ***, p<0.001, n.s., p>0.05.
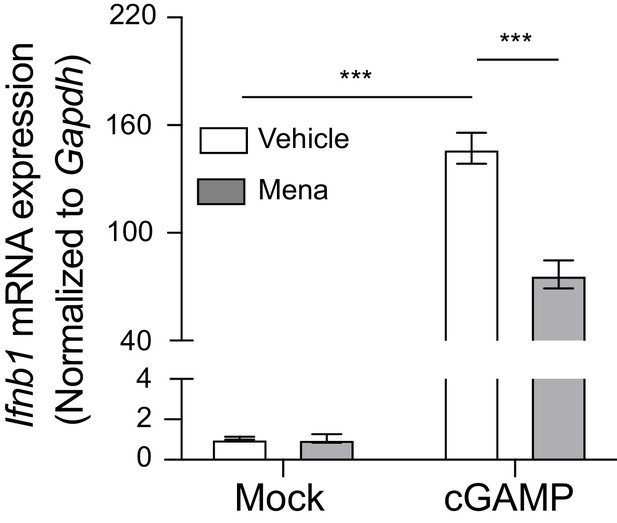
ROS regulate interferon response in macrophages upon STING activation with 2’,3’-cGAMP.
BMDMs were treated with 20 μM menadione for 30 min, then stimulated with 10 μg/ml 2’,3’-cGAMP. Ifnb transcripts were determined 4 hr after stimulation. n = 4. Bars represent mean ± SE. p value was calculated using two-way ANOVA followed by Tukey’s multiple comparison test, only the p value for the most relevant comparisons are shown for simplicity. ***, p<0.001, n.s., p>0.05.
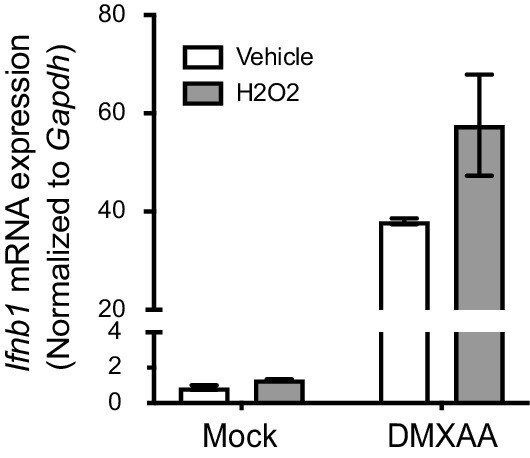
ROS do not suppress interferon in primary fibroblasts.
Primary fibroblast cells were treated with 200 μM H2O2 in serum free medium, then stimulated with 2 μg/ml DMXAA. Ifnb transcript levels were determined 2 hr after stimulation. Bars represent mean ± SE. n = 2. Statistical analysis was not performed due to limited repeats.
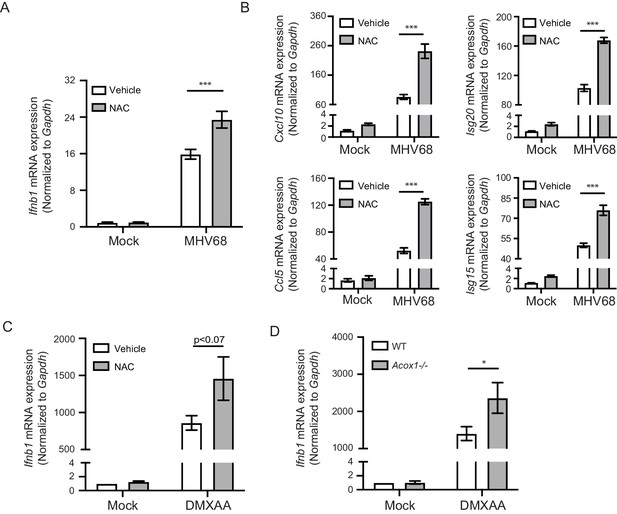
Endogenous ROS regulate interferon response upon STING activation.
(A, B) BMDMs were treated with 2 mM NAC for 30 min, then infected with MHV68 at MOI = 5. Transcripts of Ifnb (A) or ISGs (Cxcl10, Isg20, Ccl5, Isg15) (B) were determined 6 hr after infection. n = 6. (C) BMDMs were treated with 2 mM NAC for 30 min, then stimulated with 1 μg/ml DMXAA. Transcripts of Ifnb were determined 2 hr after stimulation. n = 4. (D) BMDMs isolated from Acox1-/- or WT littermate control were stimulated with 1 μg/ml DMXAA. Transcripts of Ifnb were determined 2 hr after stimulation. n = 4. Data are shown as mean ± SE, statistical analysis was conducted using two-way ANOVA followed by Tukey’s multiple comparison test, only the p value for the most relevant comparisons are shown for simplicity. *, p<0.05, **, p<0.01, ***, p<0.001.
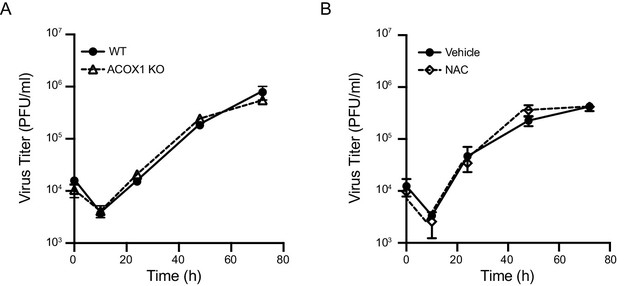
Inhibition of endogenous ROS has no effect on virus replication.
(A) BMDMs isolated from Acox1-/- or WT littermate control were infected with MHV68 at MOI = 5. n = 1 with three technical repeats. (B) BMDMs were treated with vehicle or 5 mM NAC for 1 hr, then infected with MHV68 at MOI = 5. Virus titer was determined by plaque assay at 0 hr, 10 hr, 24 hr, 48 hr and 72 hr after infection. n = 1 with three technical repeats.
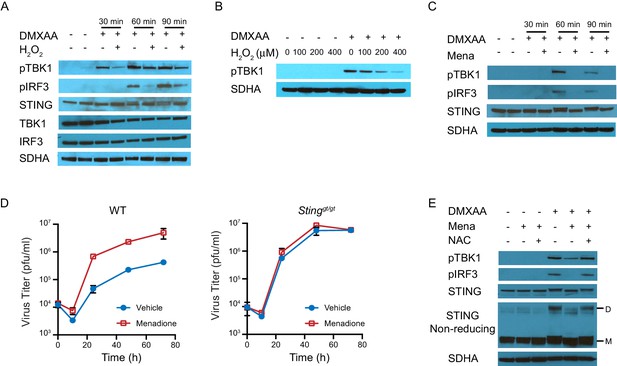
ROS regulate interferon response by inhibiting STING dimerization.
(A) BMDMs were treated with vehicle or 200 μM H2O2 for 10 min in serum free culture medium, then stimulated with 1 μg/ml DMXAA. Western blots of TBK1, IRF3, STING, pTBK1 and pIRF3 were performed at 0 min, 30 min, 60 min and 90 min after stimulation. Data shown are representative of 2 independent experiments. (B) BMDMs were treated with vehicle or different concentrations of H2O2 in serum free culture medium for 10 mins, then stimulated with 1 μg/ml DMXAA. Level of pTBK1 was determined at 60 min after stimulation. n = 1 (C) BMDMs were treated with vehicle or 25 μM menadione for 30 min, then stimulated with 1 μg/ml DMXAA. Western blot of TBK1, IRF3, STING, pTBK1 and pIRF3 was performed at 0 min, 30 min, 60 min and 90 min after stimulation. Data shown are representative results of two independent experiments. (D) BMDMs isolated from WT control or Stinggt/gt mice were treated with vehicle or 8 μM menadione for 16 hr, then infected with MHV68 at MOI = 5. Virus titer was determined by plaque assay at 0 hr, 10 hr, 24 hr, 48 hr and 72 hr after infection. n = 3 with three technical repeats each time. (E) BMDMs were treated with vehicle, 25 μM menadione, 25 μM menadione and 2 mM NAC for 30 min, then stimulated with 1 μg/ml DMXAA. STING polymerization was determined by non-reducing SDS-PAGE. M: STING monomer; D: STING dimer. Data shown are representative of 2 experiments.

Schematic diagrams of STING monomer, dimer and polymer on different electrophoresis gels.
STING is a dimeric protein at resting state, which is formed by non-covalent bonds and can be disrupted by SDS treatment. Ligand binding alters the conformation of STING and triggers its oligomerization through the formation of covalent disulfide bonds. These disulfide bonds are resistant SDS treatment but sensitive to reducing reagents. As such, STING proteins that are manifested as monomer and dimer on non-reducing SDS PAGE gels are actually dimer and oligomer, respectively.
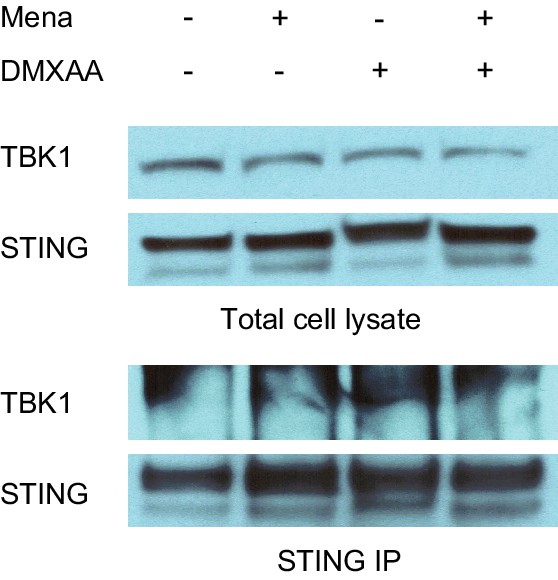
ROS decrease TBK1 recruitment during STING activation.
BMDMs were treated with vehicle or 25 μm menadione for 30 min, then stimulated with 1 μg/ml DMXAA for 1 hr. Cells were lysed and immunoprecipitated with anti-STING antibody, and the protein levels of TBK1 and STING in both total cell lysate and immunoprecipitated samples were determined by western blot.
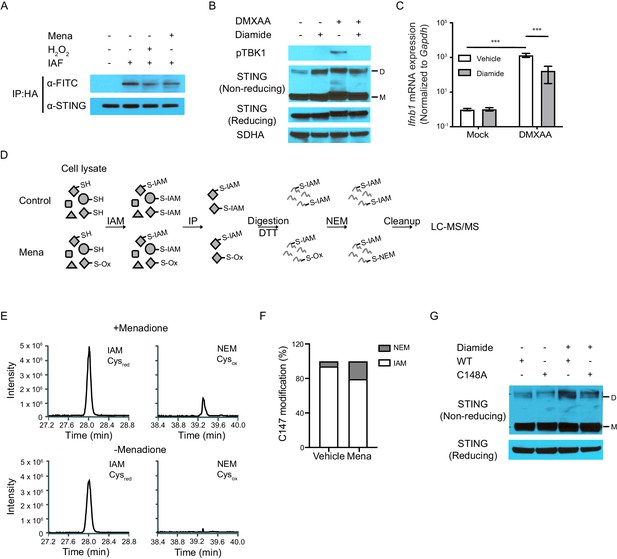
ROS oxidize C148 on STING.
(A) Sting-/- fibroblasts stably expressing HA-tagged human STING were treated with vehicle or 200 μM H2O2 in serum free medium for 10 min. Cell lysates were incubated with 5 μM 5-IAF for 1 hr at room temperature to label free thiols. Protein levels of STING and FITC were detected after immunoprecipitation for HA-tagged protein. Data shown are representative results of two independent experiments. (B, C) BMDMs were treated with 200 μM diamide for 30 mins. STING polymers (B, n = 2) and Ifnb transcripts (C, n = 4) were determined at 1 hr after 1 μg/ml DMXAA stimulation. M: STING monomer; D: STING dimer. Bars represent the mean ± SE, p value was calculated using two-way ANOVA followed by Tukey’s multiple comparison test. Only the p values for the most relevant comparison are shown for clarity purpose. ***, p<0.001. (D) Schematic of differential alkylation (IAM labeling followed by DTT reducing and NEM labeling) of cysteines for mass spectrometry analysis. (E) Mass spectra of IAM- and NEM-modified STING in vehicle and menadione treated samples. n = 1 (F) Quantification of Cysred and Cysox from mass spectrometry analysis. (G) Vectors with WT STING or C148A mutated STING were transfected into HEK293T cells. Twenty-four hours after transfection, cells were treated with vehicle or 200 μM diamide for 30 min. Polymer of STING was determined with non-reducing SDS-PAGE. M: STING monomer; D: STING dimer. n = 1.

Representative MS/MS spectra of SAVC147EEK peptide in STING protein modified by both IAM (CAM) and NEM.
This analysis was performed on the samples shown in Figure 5D.

Sequence alignment of STING from multiple species suggested C148 of STING is highly conserved across mammalian species.
C148 residue is highlighted in yellow.
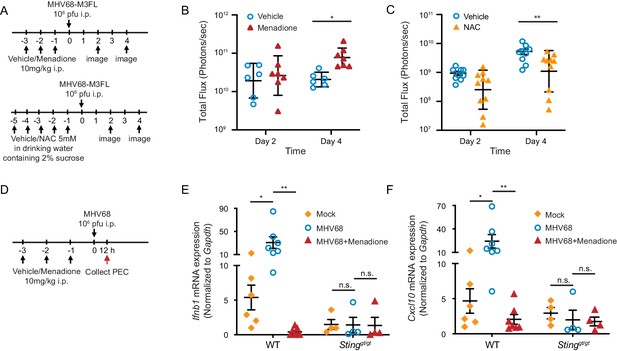
ROS regulate MHV68 replication in vivo.
(A) Schematic of MHV68 replication in mice with menadione treatment or NAC treatment. (B, C) 8–12 weeks old mice were sex-matched and randomly assigned to groups prior to experiment. Mice were treated and infected as shown in (A). Total flux (photons/second) was measured using IVIS bioluminescence imager at day 2 and day 4 after infection to quantitate lytic replication of MHV68. Data shown were the results obtained from a pool of two independent experiments. Bars represent geometric mean ±geometric SD. Each dot represents an individual mouse. (D) Schematic for the quantification of interferon and ISG transcripts in mice with menadione treatment during MHV68 infection. 7–12 weeks old mice were sex-matched and randomly assigned to groups prior to experiment. Mice were treated with either vehicle control (5% DMSO in corn oil) or menadione (10 mg/kg in corn oil) for 3 days, then infected with 106 PFU MHV68 peritoneally. Twelve hours post infection, transcripts of Ifnb and Cxcl10 in peritoneal exudate cells (PECs) were determined from individual mice. (E, F) Transcripts of Ifnb and Cxcl10 in PECs from mock (vehicle treatment, no infection), MHV68 (vehicle treatment, MHV68 infected) and MHV68+Menadione (Menadione treatment, MHV68 infected) mice. Bars represent mean ± SE, each dot represent an individual mouse. Data are collected from one controlled experiment. Statistical analysis was conducted using two-way ANOVA (repeated measures). *p<0.05.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6J | The Jackson Laboratory | Stock No. 000664 | Bred at UTSW facility with IACUC approval |
| Strain, strain background (Mus musculus) | C57BL/6J-Tmem173gt/J | The Jackson Laboratory | Stock No. 017537 | Bred at UTSW facility with IACUC approval |
| Strain, strain background (Mus musculus) | B6.129S2-Ifnar1tm1Agt/J | The Jackson Laboratory | Stock No. 32045 | Bred at UTSW facility with IACUC approval |
| Strain, strain background (Mus musculus) | B6.129P2-Acox1tm1Jkr/J | The Jackson Laboratory | Stock No. 029747 | Bred at UTSW facility with IACUC approval |
| Cell line (Herpesviridae, Rhadinovirus) | Murine gamma herpesvirus 68 (WUSM stain) | ATCC | VR-1465 | |
| Cell line (Herpesviridae, Rhadinovirus) | Murine gamma herpesvirus 68-M3FL | Home made Hwang et al., 2008 | ||
| Cell line (Mus musculus) | 3T12 | ATCC | Cat# ATCC CCL-164; RRID:CVCL_0637 | |
| Cell line (Homo sapiens) | 293T | ATCC | Cat# ATCC CRL-3216; RRID:CVCL_0063 | |
| Transfected construct (Homo sapiens) | STING | GenBank | AVQ94753.1 | Express STING into 293T cells |
| Antibody | Anti-STING (Rabbit polyclonal) | Proteintech | Cat# 19851-1-AP; RRID:AB_10665370 | WB (1:1000); IP (1 ug/ml) |
| Antibody | Anti-STING (Rabbit monoclonal) | Cell Signaling | Cat# 50494S; RRID:AB_2799375 | WB (1:1000) |
| Antibody | Anti-TBK1/NAK (Rabbit monoclonal) | Cell Signaling | Cat# 3504S; RRID:AB_2255663 | WB (1:1000) |
| Antibody | Anti-IRF-3 (Rabbit monoclonal) | Cell Signaling | Cat# 4302S; RRID:AB_1904036 | WB (1:1000) |
| Antibody | Anti-Phospho-TBK1 (Ser172) (Rabbit monoclonal) | Cell Signaling | Cat# 5483S; RRID:AB_10693472 | WB (1:1000) |
| Antibody | Anti-Phospho-IRF-3 (Ser396) (Rabbit monoclonal) | Cell Signaling | Cat# 4947S; RRID:AB_823547 | WB (1:1000) |
| Antibody | Mouse monoclonal anti-SDHA antibody | Abcam | Cat# ab14715; RRID:AB_301433 | WB (1:5000) |
| Antibody | Anti-MHV68 (Rabbit polyclonal) | Home made Weck et al., 1997 | FACs (1:1000) | |
| Antibody | Anti-Rabbit IgG(H+L) secondary antibody, Alexa Fluor 647 (Goat polyclonal) | Thermo Fisher Scientific | Cat# A-21245; RRID:AB_2535813 | FACs (1:4000) |
| Antibody | Anti-Rabbit IgG, Peroxidase (Donkey polyclonal) | Jackson ImmunoResearch Laboratory | Cat# 711-035-152; RRID:AB_10015282 | WB (1:5000) |
| Antibody | Anti-Mouse IgG, Peroxidase (Goat polyclonal) | Jackson ImmunoResearch Laboratory | Cat# 115-035-174; RRID:AB_2338512 | WB (1:5000) |
| Antibody | Anti-FITC (Rabbit polyclonal) | Thermo Fisher Scientific | Cat# 71-1900; RRID:AB_2533978 | WB (1:2000) |
| Recombinant DNA reagent | pcDNA 3.1(+) Mammalian Expression Vector (plasmid) | Thermo Fisher Scientific | Cat# V79020 | Vector for the expression of human STING |
| Sequence-based reagent | Ifnb forward | This paper | qPCR primers | CAGCTCCAAGAAAGGACGAAC |
| Sequence-based reagent | Ifnb reverse | This paper | qPCR primers | GGCAGTGTAACTCTTCTGCAT |
| Sequence-based reagent | Cxcl10 forward | This paper | qPCR primers | TTAACGTCAGGCCAACAGAG |
| Sequence-based reagent | Cxcl10 reverse | This paper | qPCR primers | GAGGGAAACCAGGAAAGATAGG |
| Sequence-based reagent | Isg15 forward | This paper | qPCR primers | CAGGACGGTCTTACCCTTTCC |
| Sequence-based reagent | Isg15 reverse | This paper | qPCR primers | AGGCTCGCTGCAGTTCTGTAC |
| Sequence-based reagent | Isg20 forward | This paper | qPCR primers | CCATGGACTGTGAGATGGTG |
| Sequence-based reagent | Isg20 reverse | This paper | qPCR primers | CTCGGGTCGGATGTACTTGT |
| Sequence-based reagent | Gapdh forward | This paper | qPCR primers | GGGTGTGAACCACGAGAAATA |
| Sequence-based reagent | Gapdh reverse | This paper | qPCR primers | GTCATGAGCCCTTCCACAAT |
| Sequence-based reagent | Gsr forward | This paper | qPCR primers | CACCGAGGAACTGGAGAATG |
| Sequence-based reagent | Gsr reverse | This paper | qPCR primers | ATCTGGAATCATGGTCGTGG |
| Sequence-based reagent | Gclm forward | This paper | qPCR primers | AATCAGCCCCGATTTAGTCAG |
| Sequence-based reagent | Gclm reverse | This paper | qPCR primers | CGATCCTACAATGAACAGTTTTGC |
| Sequence-based reagent | C148 forward | This paper | Site direct mutagenesis PCR primers | CTCTGCAGTGCTGAAAAAGGGAATTTCAACGTGGC |
| Sequence-based reagent | C148A reverse | This paper | Site direct mutagenesis PCR primers | ATCTCAGCTGGGGCCAGG |
| Commercial assay or kit | Lipofectamine 3000 Reagent | Thermo Fisher Scientific | Cat# L3000008 | |
| Commercial assay or kit | Qiagen RNeasy Mini Kit | Qiagen | Cat# 74104 | |
| Commercial assay or kit | SuperScript VILO cDNA Synthesis Kit | Thermo Fisher Scientific | Cat# 11754050 | |
| Commercial assay or kit | PowerUp SYBR Green Master Mix | Thermo Fisher Scientific | Cat# A25776 | |
| Commercial assay or kit | LIVE/DEAD Fixable Dead Cell Stain Kits | Invitrogen | Cat# L34975 | |
| Commercial assay or kit | Q5 Site-Directed Mutagenesis Kit | New England BioLabs | Cat# E0554S | |
| Chemical compound, drug | DMXAA | InvivoGen | Cat# tlrl-dmx | (1 ug/ml) for macrophages, (2 ug/ml) for fibroblasts |
| Chemical compound, drug | 2’3’-cGAMP | InvivoGen | Cat# tlrl-nacga23 | (10 ug/ml) |
| Chemical compound, drug | ISD | InvivoGen | Cat# tlrl-isdn | (10 ug/ml) |
| Chemical compound, drug | poly(dA:dT) | InvivoGen | Cat# tlrl-patn-1 | (1 ug/ml) |
| Chemical compound, drug | poly(I:C) | Invivogen | Cat# tlrl-picw | (1 ug/ml) |
| Chemical compound, drug | D-Luciferin, Potassium Salt | GOLDBIO | Cat# LUCK | (150 mg/kg) |
| Chemical compound, drug | Menadione | Sigma | Cat# M9429 | (10 mg/kg) for mice, concentration for cells were indicated in each experiment |
| Chemical compound, drug | Hydrogen peroxide solution | Sigma | Cat# 216763 | Concentration for cells were indicated in each experiment |
| Chemical compound, drug | N-Acetyl-L-cysteine | Sigma | Cat# A7250 | 2 mM for macrophages, 5 mM in drinking water with 2% sucrose |
| Chemical compound, drug | Iodoacetamide | Sigma | Cat# I1149 | 100 mM |
| Chemical compound, drug | N-ethylmaleimide | Sigma | Cat# E3876 | 50 mM |
| Chemical compound, drug | Elastase | Worthington | Cat# LS006363 | 1 mg/ml |
| Chemical compound, drug | 5-(Iodoacetamido) fluorescein | Sigma | Cat# I9271 | 5 ug/ml |
| Chemical compound, drug | Diamide | Sigma | Cat# D3648 | 200 uM |
| Software, algorithm | GraphPad Prism 8 | GraphPad | www.graphpad.com; RRID:SCR_002798 | |
| Software, algorithm | FlowJo software | FlowJo | www.flowjo.com; RRID:SCR_008520 | |
| Software, algorithm | Live Image Software | Perkin Elmer | www.perkinelmer.com; RRID:SCR_014247 |
Additional files
-
Source data 1
All source data.
- https://cdn.elifesciences.org/articles/57837/elife-57837-data1-v1.pzfx.zip
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/57837/elife-57837-transrepform-v1.docx