Transient role of the middle ear as a lower jaw support across mammals
Figures
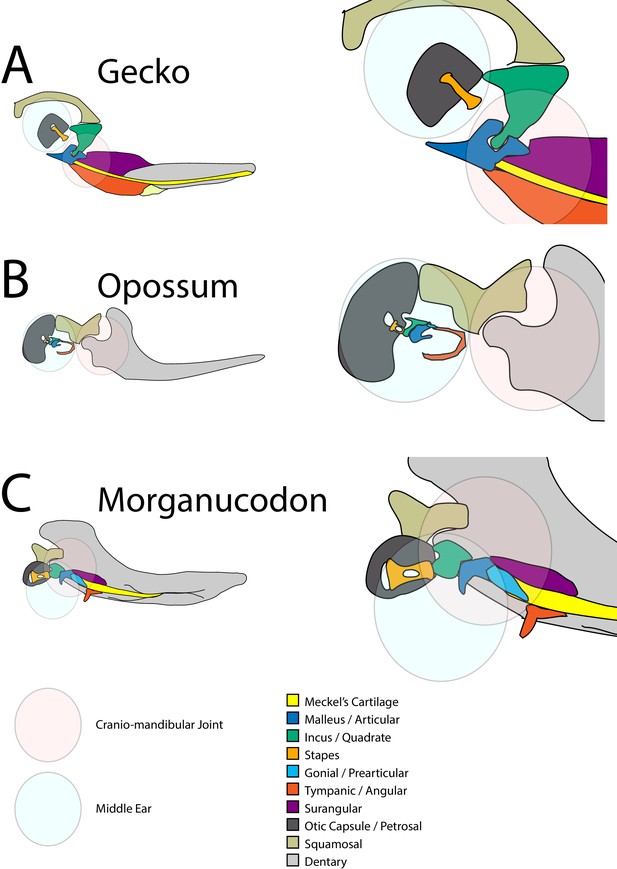
Schematic of cranial-mandibular jaw articulation showing the roles of the quadrate/incus and articular/malleus in the hearing and jaw joint modules in (A) reptile gecko, (B) mammal opossum, (C) mammal-like reptile Morganucodon.
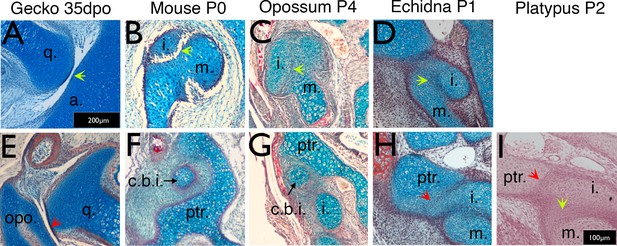
Timing of the development of the quadrate-articular/malleus incus, and cranio-incudo joints.
Histological sections stained with alcian blue and picrosirius red. (A) The primarily jaw articulation is formed by 35 days of post-oviposition (35dpo) during in ovo development in geckos. (B) The malleus-incus joint, the homologue of the quadrate-articular joint, is formed during in utero development in mice, and is fully formed at birth (Postnatal day (P) 0). (C-D) The malleus incus joint is still partially fused in 4 day postnatal (P4) opossum pups (C) and 1 day post-hatching echidna young (P1) (D). (E) During development the gecko quadrate forms a joint with the opisthotic (structurally equivalent to the mammalian petrosal). (F) At birth there is no articulation between the crus breve of the incus and the surrounding crista parotica of the petrosal in mice (P0). (G) The crus breve of the incus sits in close proximity to the petrosal in opossums at P4 (G). (H-I) The incus is fused with the petrosal in both P1 echidna (H) and the P2 platypus (I). Green arrows highlight Q-A/M-I interaction. Red arrows highlight Incus/Q-petrosal/opisthotic interaction. a. articular; c.b.i crus breve of the incus; (i) incus; m. malleus; opo, opisthotic; ptr. petrosal; q. quadrate. Scale in A = 200 microns, same scale in E. Scale bar in I = 100 microns, same scale in B-D, F-H.
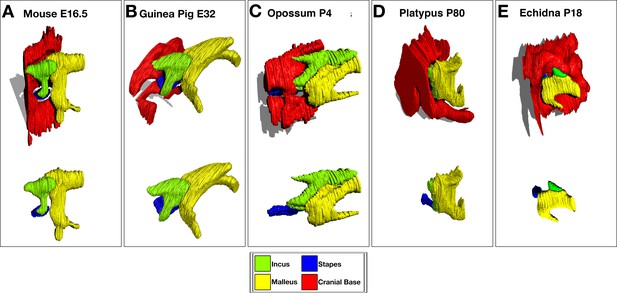
3D Reconstruction of cartilaginous middle ear ossicles and cranial base from histological sections shows differences in anatomy in different groups of mammals during development.
E = embryonic day, p=days postnatal/post-hatching development.
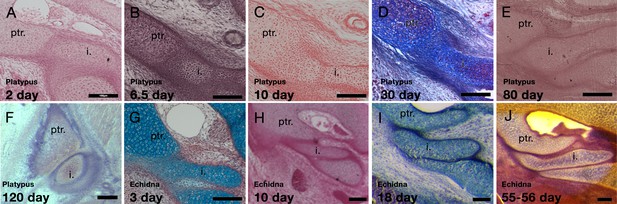
Development of the incus-petrosal joint in monotremes.
(A-B) The platypus incus is fused to the petrosal by immature chondrocytes at 2 days (A) and 6.5 days (B) post-hatching. (C) At 10 days post-hatching, the fusion persists, with mature chondrocytes forming the connection. (D) A similar morphology is seen at 30 post-hatching. (E) At 80 days post-hatching the incus and petrosal are no longer fused, but instead the two cartilages abut each other. (F) At 120 days post-hatching the incus and petrosal have begun to ossify, but the region of articulation in between the two elements remains cartilaginous. (G-H) In echidna the incus is fused to the petrosal by immature chondrocytes at 3 days (G) and 10 days (H) post-hatching. (I-J) By 18 days post-hatching the two elements are separated but remain abutted (I), This connection remains though to 55–65 days post-hatching (J). i: incus; ptr. petrosal. Scale bar = 100 microns.
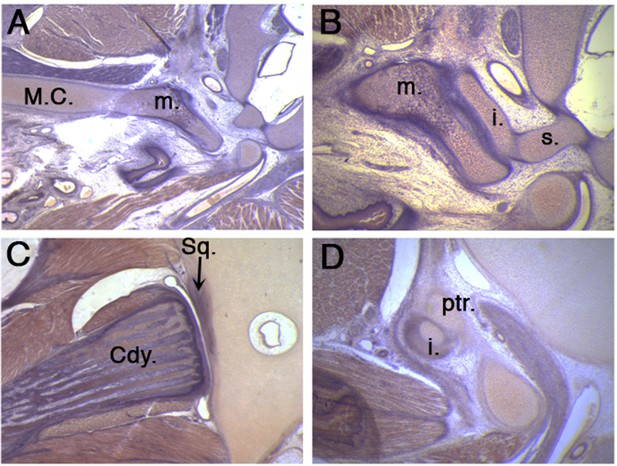
The middle ear and jaw joints of a 50 day old platypus.
(A) A robust connection is observed between the still persistent Meckel’s cartilage and the malleus in a un-cavitated middle ear. (B) The malleus connects to the incus which in turn connects to the stapes. (C) A fully formed synovial jaw joint forms between the condylar process of the dentary bone and the squamosal of the upper jaw. (D) At the same time, the incus additionally articulates with the petrosal in the cranial base, thereby linking the lower and upper jaw through the ossicle chain. Cdy. Condylar process of the dentary bone; i. incus; m. malleus; M.C. Meckel’s cartilage; ptr, petrosal; Sq. squamosal bone; s. stapes.
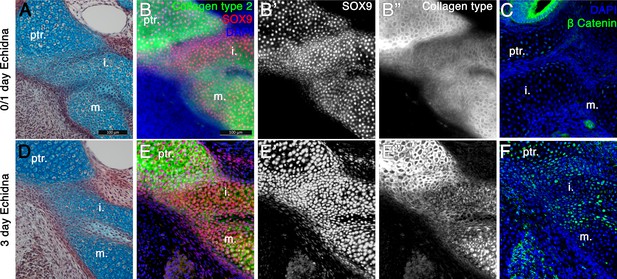
Fusion of the Incus with the petrosal in Echidna pouch young.
(A) Alcian blue/picrosirius red staining on the fusion between the incus and petrosal observed in the newly hatched echidna. (B) Immunofluorescence staining against the regulator of chondrogenesis Sox9 (red) (B,B’) and the marker of mature cartilage Collagen type 2 (green) (B,B”) demonstrates that the cartilaginous incus and petrosal bones are fully fused at post-hatching day 0/1 (P0/1). (C) Immunohfluorescence against β Catenin (green) shows no activity within the cartilages at this timepoint. Expression is observed in the neuroepithelium of the inner ear. (D) Alcian blue/picrosirius red staining on the fusion between the incus and petrosal observed in 3 day post-hatching echidna (P3) shows that the elements are now fused by fibrocartilage. (E) Immunofluorescence staining against the regulator of chondrogenesis Sox9 and the marker of mature cartilage collagen type 2 (E,E”). Sox9 is still continuously expressed between the elements (E,E’), but collagen type 2 is down regulated in the incus-petrosal and incus-malleus articulation region (E,E”). (F) Immunofluorescence against β Catenin shows nuclear localisation within the incus-petrosal and incus-malleus articulation regions, indicating active canonical Wnt signalling, an important step in suppression of chondrogenesis during joint formation. i. incus; m. malleus; ptr. petrosal.
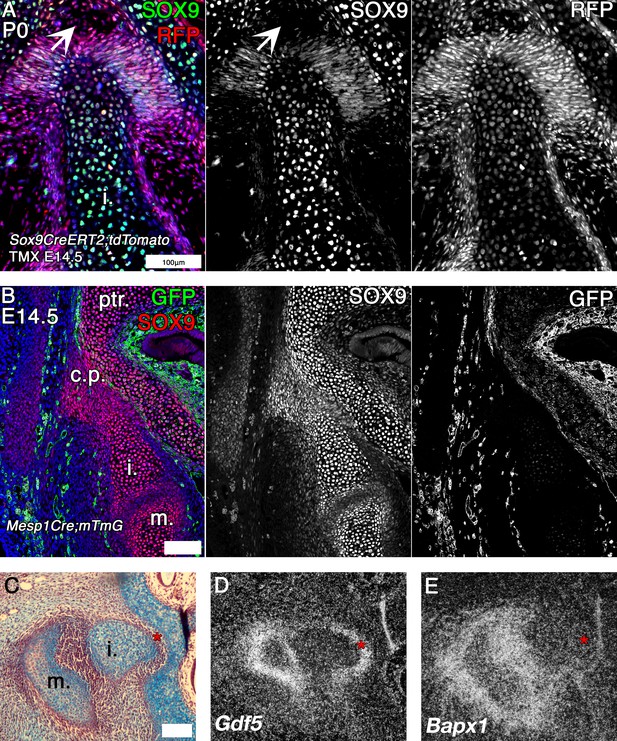
Mouse fate mapping studies demonstrate developmental fusion between incus and petrosal.
(A) Genetic tracing of chondrogenic Sox9 expression cells by inducible reporter mice at postnatal day 0 (P0). Sox9 lineage cells (red) (RFP) are observed in the mesenchyme and developing ligaments between the crus breve of the incus and the petrosal. Sox9 protein (green) is not expressed in the mesenchyme surrounding the incus at P0 (arrowhead). (B) Genetic tracing of mesoderm lineage cells (green) (GFP) and immunohistochemistry against Sox9 protein (red) at embryonic day 14.5 (E14.5). Sox9 expression at E14.5 confirms that the incus and petrosal are formed from a continuous chondrogenic mesenchyme, and that the incus joins with the petrosal at the crista parotica, which is not of mesodermal origin. (C-E) Expression by in situ hybridisation of joint markers in sagittal section of E14.5 mouse middle ears. Gdf5 mRNA is expressed with the malleus-incus joint, and between the incus and the petrosal (D), potentially acting to inhibit the Sox9 expressing mesenchyme between the ear and the cranial base from differentiating into cartilage. The middle ear joint marker Bapx1 is not expressed between the incus and the petrosal (E). * indicates space between of incus and petrosal in C-E. i. incus; m. malleus; ptr. petrosal. Scale bar in A,B = 100 microns.
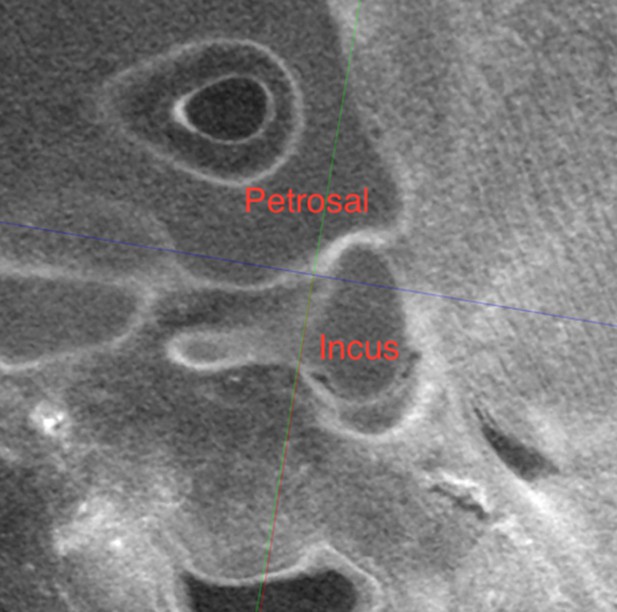
Contrast enhanced µCT of embryonic bat (Pterobnotus quadridens) middle ear at Carnegie Stage21, showing abutment of the crus breve of the incus against the petrosal.
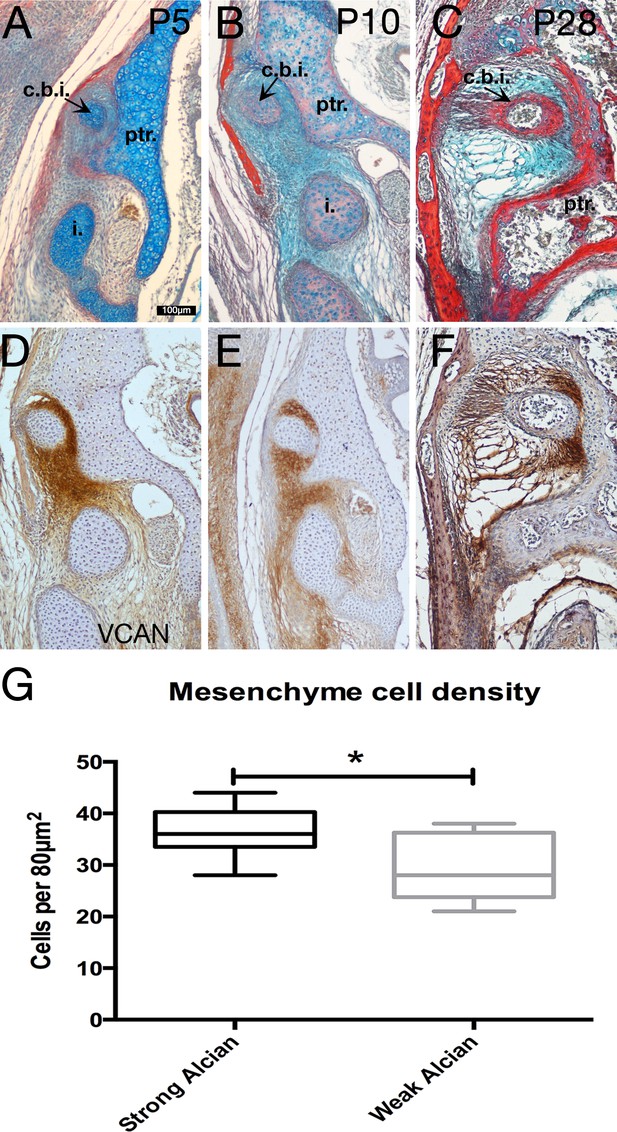
Specialist mesenchyme supports incus-petrosal connection in juvenile opossums.
(A-F) Mesenchyme surrounding the crus breve of the incus is rich in the proteoglycan Versican (Vcan) at postnatal day (P)5 (A,D) and P10 (B,E). During cavitation of the middle ear at P28 versican rich mesenchyme is concentrated between the crus breve of the incus and the petrosal (C,F). (G) At P5 the proteoglycan-rich regions surrounding the crus breve have a significantly greater cell density than the regions with less proteoglycan. *p=0.0152 unpaired two-tailed t-test. Error bars = 1 standard deviation. c.b.i crus breve of the incus; i. incus; ptr. petrosal. Scale bar in A = 100 microns, same scale in B-F.
-
Figure 6—source data 1
Statistical analysis of cell number in specialised mesenchyme surrounding the opossum incus.
- https://cdn.elifesciences.org/articles/57860/elife-57860-fig6-data1-v2.docx
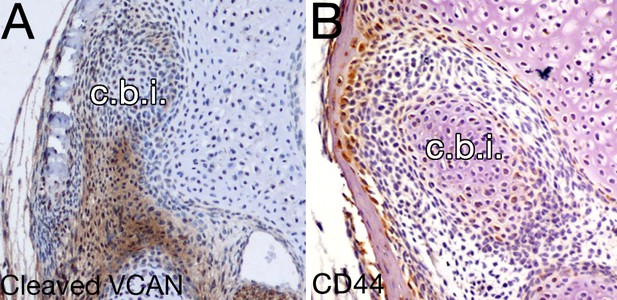
Versican signaling is not evident in the mesenchyme around the opossum incus.
(A-B) Expression by immunohistochemistry of cleaved veriscan DPEAAE (A) and CD44 (B) in 10 day opossums at the level of the crus breve of the incus (c.b.i.).
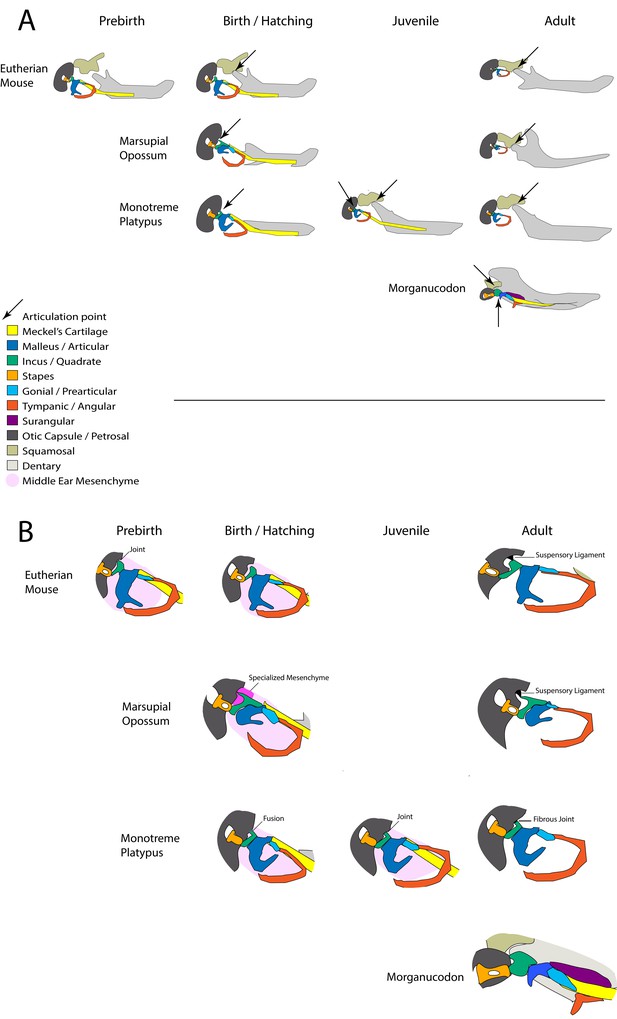
Summary of involvement of middle-ear ossicles in jaw articulation during development.
(A) The location of the jaw articulation in developing living mammals and in the extinct mammal-like reptile Morganucodon. Arrows indicate jaw articulation points. Eutherian mammals are born with a functional dentary-squamosal joint (TMJ), while young marsupials and monotremes use the middle ear bones due to a lack of this joint, which develops later. During postnatal development monotremes show evidence of a double jaw articulation, similar to fossil mammal-like reptiles such as Morganucodon. (B) The connections between the middle ear ossicles and the cranial base in developing mammals. The connections between the incus and cranial base differ in young marsupials and monotremes. The fusion followed by a joint seen in monotremes is also observed in pre-natal eutherians. Neonatal marsupials support the incus with a specialised middle-ear mesenchyme.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Monodelphis domestica) | short-tailed opossum | Anthwal et al., 2017. DOI:10.1038/s41559-017-0093; Urban et al., 2017. DOI:10.1098/rspb.2016.2416 | ||
| Strain, strain background (Cavia porcellus) | Guinea pig | Anthwal et al., 2015. DOI:10.1186/s13227-015-0030-6 | ||
| Strain, strain background (Paroedura picta) | ocelot gecko | Zahradnicek et al., 2012. DOI:10.1111/j.1469-7580.2012.01531.x | ||
| Strain, strain background (Mus musculus) | CD1 | King’s College London | ||
| Strain, strain background (Tachyglossus aculeatus) | short-beaked echidna | University of Melbourne | ||
| Genetic reagent (Mus musculus) | Sox9CreERT2:tdTomato | Other | RRID:MGI:4947114 | Prof Robin Lovell-Badge, Francis Crick Institute |
| Genetic reagent (Mus musculus) | Mesp1Cre; mTmG | RIKEN | Mesp1tm2(cre)Ysa:: Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo; RRID:MGI:3702469 | |
| Biological sample (Pterobnotus quadridens) | sooty mustached bat µCT scan | Other | Karen Sears, UCLA | |
| Biological sample (Ornithorhynchus anatinus) | platypus histological slides | Hill Collection, Museum für Naturkunde, Leibniz Institute for Research on Evolution and Biodiversity, Berlin; Cambridge University Museum of Zoology; Green, 1937; Presley and Steel, 1978; Watson, 1916 | Specimen W; M45; Specimen Delta; M038; HP; Specimen Beta; HX | Museum Samples |
| Biological sample (Tachyglossus aculeatus) | short-beaked echidna histological slides | Cambridge University Museum of Zoology; Green, 1937; Presley and Steel, 1978; Watson, 1916 | HX; Echidna H.SP EC5; Echidna H.SP EC4 | Museum Samples |
| Antibody | rabbit polyclonal anti Sox9 | Millipore | ab5535; RRID:AB_2239761 | IF 1/200 |
| Antibody | chicken polyclonal anti GFP | Abcam | ab13970; RRID:AB_300798 | IF 1/500 |
| Antibody | Rat monoclonal anti RFP | Chromotek | 5f8-100; RRID:AB_2336064 | IF 1/200 |
| Antibody | Rabbit polyclonal anti Beta-catenin | Santa Cruz | sc-7199; RRID:AB_634603 | IF 1/200 |
| Antibody | mouse monoclonal anti Tenascin C | DSHB | M1B4; RRID:AB_528488 | IF 1/40 |
| Antibody | mouse monoclonal anti type 2 collagen | DSHB | II-II6B3; RRID:AB_528165 | IF 1/50 |
| Antibody | mouse monoclonal anti CD44 | DSHB | HERMES-1; RRID:AB_528148 | IHC 1/50 |
| Antibody | mouse monoclonal anti Versican | DSHB | 12C5; RRID:AB_528503 | IHC 1/50 |
| Antibody | rabbit polyclonal anti Versican V1 (DPEAAE) | Abcam | ab19345; RRID:AB_444865 | IF 1/400 |
| Antibody | Donkey Polyclonal Alexa568 conjugated anti-Rabbit | Invitrogen | A10042; RRID:AB_2534017 | IF 1/300 |
| Antibody | Donkey Polyclonal Alexa 488 conjugated anti-Rabbit | Invitrogen | A-21206; RRID:AB_2535792 | IF 1/300 |
| Antibody | Donkey Polyclonal Alexa568 conjugated anti-Mouse | Invitrogen | A10037; RRID:AB_2534013 | IF 1/300 |
| Antibody | Goat Polyclonal Alexa488 conjugated anti-Chicken | Invitrogen | A-11039; RRID:AB_2534096 | IF 1/300 |
| Antibody | Goat Polyclonal Alexa568 conjugated anti-Rat | Invitrogen | A-11077; RRID:AB_2534121 | IF 1/300 |
| Antibody | Goat Polyclonal Biotin Anti-Mouse | Dako | E0433; RRID:AB_2687905 | IHC 1/400 |
| Recombinant DNA reagent | Mouse Gdf5 in situ hybridisation probe | Tucker et al., 2004. DOI:10.1242/dev.01017 | ||
| Recombinant DNA reagent | Mouse Bapx1 in situ hybridisation probe | Tucker et al., 2004. DOI:10.1242/dev.01017 | ||
| Commercial assay or kit | ABC-HRP streptavidin kit | Vector Labs | PK-6100; RRID:AB_2336819 | |
| Commercial assay or kit | ImmPACT DAB Peroxidase Substrate | Vector Labs | SK-4105; RRID:AB_2336520 | |
| Software, algorithm | FIJI | Schindelin et al., 2015; Schindelin et al., 2012 | RRID:SCR_002285 | |
| Software, algorithm | Prism 8 | Graphpad | RRID:SCR_002798 | |
| Other | Aqueous mounting medium with DAPI | Abcam | ab104139 | |
| Other | Picro‐sirius red, haematoxylin and alcian blue trichrome staining | CCRB Histology Core at King’s College London |
Museum held monotreme specimens used in the current study.
CRL – Crown rump length. *Estimate based (Ashwell, 2012). †Estimate based on Griffiths, 1978 and Rismiller and McKelvey, 2003.
| Species | Collection | ID | Estimated age | CRL/Max length |
|---|---|---|---|---|
| Ornithorhynchus anatinus | Cambridge | Specimen W | 2 days * | 16.5 mm |
| Ornithorhynchus anatinus | Berlin | M45 | 6.5 days * | 33 mm |
| Ornithorhynchus anatinus | Cambridge | Specimen Delta | 10 days * | 80 mm |
| Ornithorhynchus anatinus | Berlin | M038 | 30 days | Unknown |
| Ornithorhynchus anatinus | Cambridge | HP | 50 days * | 200 mm |
| Ornithorhynchus anatinus | Cambridge | Specimen Beta | 80 days* | 250 mm |
| Ornithorhynchus anatinus | Cambridge | HX | 120 days | 295 mm |
| Tachyglossus aculeatus | Cambridge | Echidna H.SP EC5 | 18 days† | 83 mm |
| Tachyglossus aculeatus | Cambridge | Echidna H.SP EC4 | 55–65 days † | 174 mm |





