Identification of specific Tie2 cleavage sites and therapeutic modulation in experimental sepsis
Figures
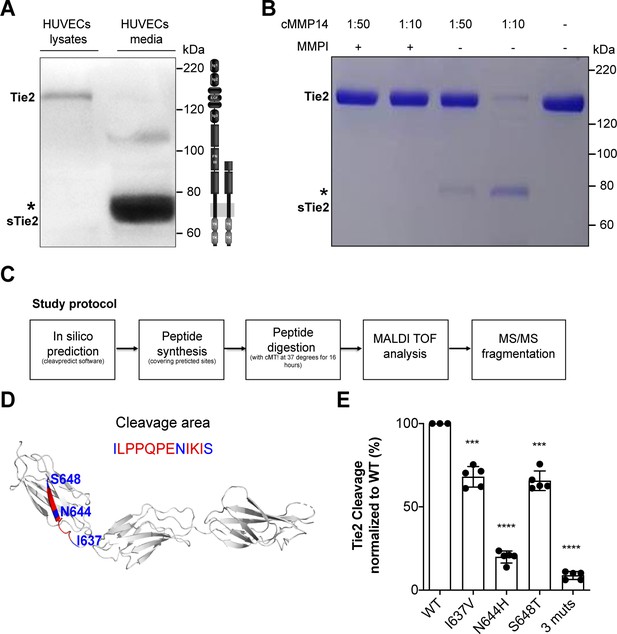
MMP14 cleaves Tie2 at the Fibronectin type-III domain on the cell surface.
(A) Representative Tie2 ectodomain (AB33) immunoblot of the total cell lysates and conditioned media from TNF-α (50 ng/ml) treated HUVECs. The asterisk indicates the truncated 75–80 kDa Tie2 fragments (sTie2). (B) Recombinant Tie2 (1 ug) was incubated with the recombinant catalytic domain of MMP14 (termed cMMP14) at two enzyme/substrate ratios (1:50, 1:10, and buffer only) in the presence or absence of GM6001 a general MMP inhibitor (MMPI). The protein mixture was subjected to Coomassie staining. The asterisk indicates the cleaved fragments of Tie2 (sTie2). (C) Schematic representation of the study protocol used for the detection of MMP14-mediated Tie2 cleavage sites. (D) Crystal structure of Fibronectin type III (FN3) and amino acid sequence of the cleavage area (red) and cleavage sites, I637, N644, S648 (blue) (E) Percentage soluble (s)Tie2 in wildtype (WT) or mutant transfected HEK293 cell culture supernatants treated with cMMP14. ***p<0.001, ****p<0.0001 compared with WT in t test (n = 3–5).

Recombinant MMP14 cleaves Tie2 polypeptides.
Mass spectrometry analyses after incubating two Tie2 polypeptides 630T–652H (peptide 1) and 736Q–745K (peptide 2) with the recombinant catalytic domain of MMP14 (cMMP14). (A) The intact peptide-one with MH+2588.3 (B) Peptide-one was completely digested into smaller fragments in the presence of recombinant MMP14. The fragments at MH+ 794.39, MH+ 925.53, MH+1703.80 and MH+2140.07 were further identified as LPPQPEN, IKISNITH, TAWTLSDILPPQPEN and TAWTLSDILPPQPENIKIS respectively, by tandem MS/MS. The signal at m/z 2491.26 is probably a product from incomplete synthesis, as it is already present in the synthetic peptide before digest. (C) Intact peptide-two with MH+ 913.4 (D) Peptide-two remained unchanged in the presence of recombinant MMP14.
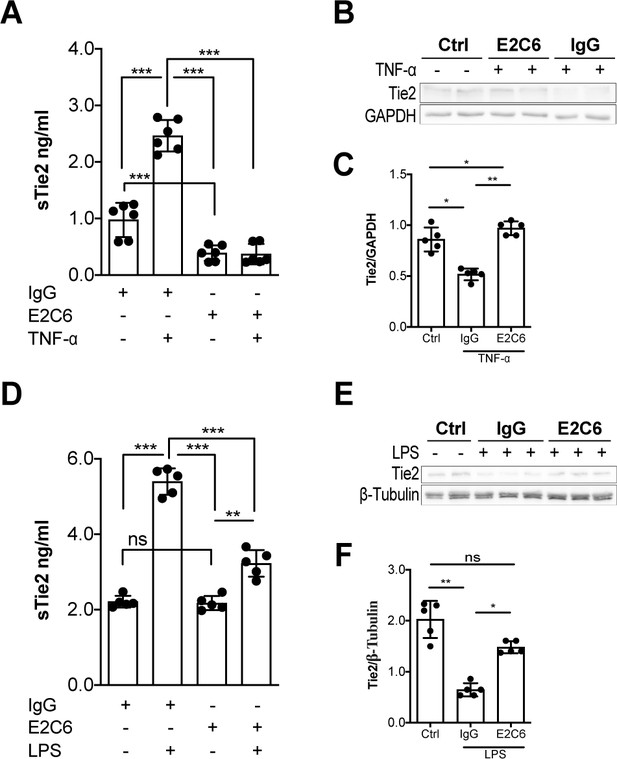
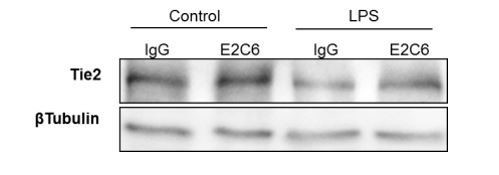
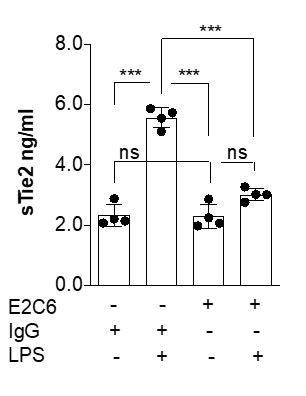
MMP14 blockade inhibits Tie2 cleavage both in vitro in HUVECs and in vivo in murine experimental sepsis.
Human umbilical vein endothelial cells (HUVECs) pretreated with or without E2C6 (100 nM) for 1 hr were analyzed 24 hr after stimulation with 50 ng/mL TNF-α. (A) sTie2 ELISA of supernatants (**p<0.01, ***p<0.001, n = 6) (B) Representative immunoblot of cell lysates and (C) densitometric quantification of blots (*p<0.05, **p<0.01, n = 5 per group). Mice pretreated with either IgG control or E2C6 for 1 hr were analyzed 16 hr after LPS induced endotoxemia. (D) sTie2 ELISA of murine serum (**p<0.01, ***<0.001, n = 5) (E) Representative immunoblot for Tie2 and beta-tubulin from the murine lung and (F) densitometry quantification of blots (*p<0.05, **p<0.01, n = 5 per group).
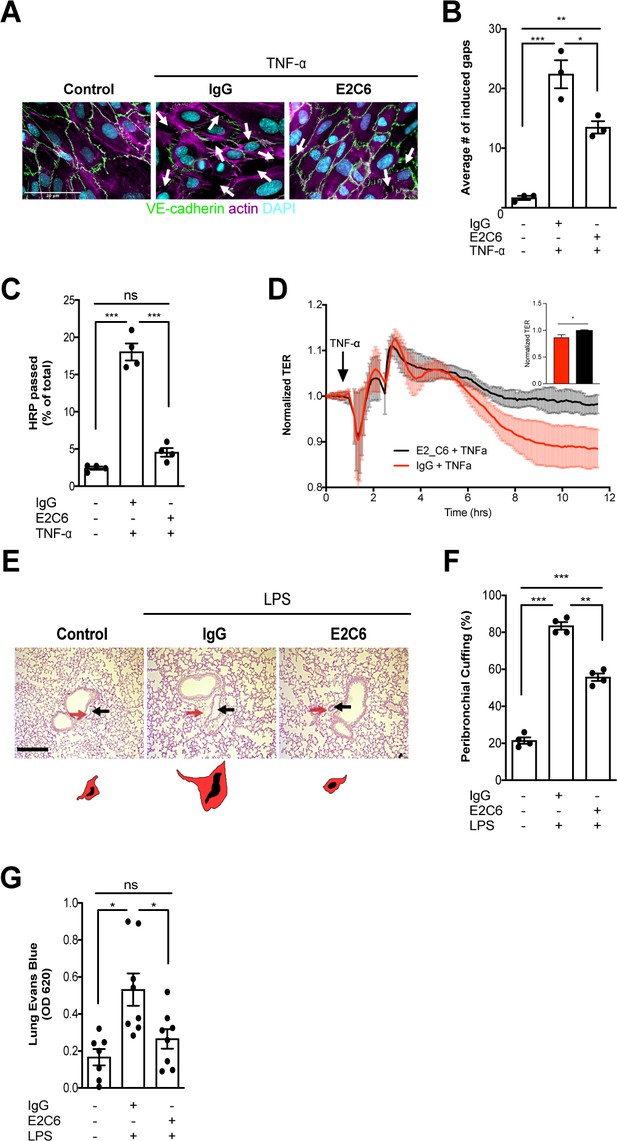
MMP14 blockade attenuates sepsis-induced hyperpermeability.
(A) Representative images of fluorescence immunocytochemistry staining for VE-cadherin (green), F-actin (magenta) and the nuclei (cyan) on confluent HUVECs, pretreated with or without E2C6 (100 nM) for 1 hr, were analyzed 6 hr after stimulation with TNF-α. Arrows (white) indicate inter-endothelial gaps. Scale bar 20 μm. (B) Average number (#) of intercellular gaps induced by TNF-α (*p<0.05, **p<0.01, ***p<0.001 in Turkey`s multiple comparisons test, n = 3 per group) (C) HRP leakage in confluent HUVECs, pretreated with or without E2C6 (100 nM) for 1 hr before TNF-α stimulation (50 ng/ml). (***p<0.001, n = 4) (D) Transendothelial Electrical Resistance (TER) was measured using an electrical cell-substrate impedance sensing system (ECIS) in HUVECs that were treated with E2C6 (100 nM) or control IgG and stimulated with 50 ng/ml TNF-α 60 min later. Inset, normalized TER measurement of TNF-α response in E2C6 treated vs control IgG treated cells at the 10 hr mark (*p<0.05, n = 4). (E) Periodic acid-Schiff (PAS) staining of paraffin-embedded lung tissue 16 hr after LPS-induced endotoxemia in E2C6 (10 mg/kg) or IgG control antibody (IgG) treatment groups. (n = 5 mice per group). All images show bronchus and their corresponding arteriola (as vasa vasorum of the bronchus surrounded by one common adventitia). The black arrows indicate the vessel area while the red arrows indicate the cuff area. Scale bar 20 μm. (F) Semi-quantification of peribronchial cuffing was performed by surveying whole lung sections. (***p<0.001, n = 4 per group). (G) In vivo pulmonary Evans blue permeability assay in mice pretreated with 10 mg/kg IgG control or E2C6 1 hr before LPS-induced endotoxemia (*p<0.05, n = 7–8 per group).
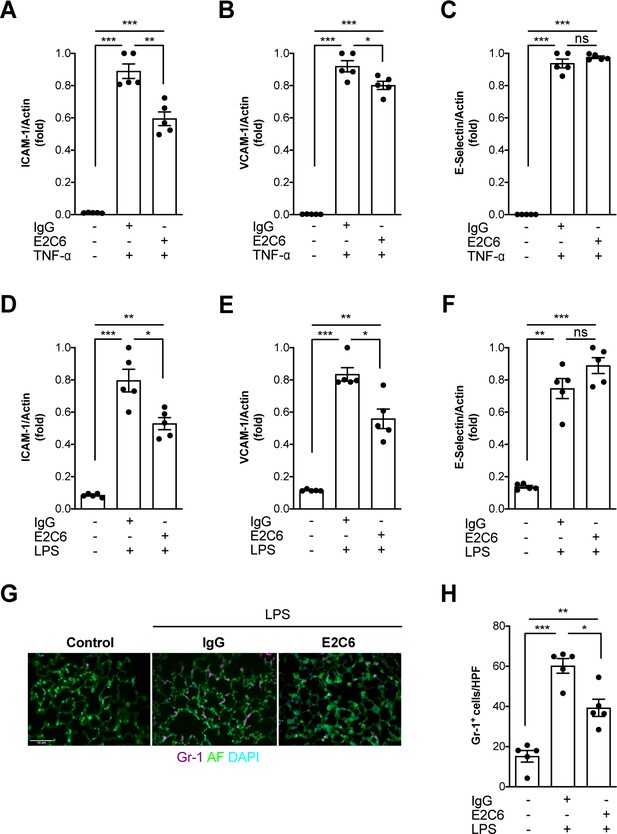
MMP14 blockade inhibits inflammation and neutrophil adhesion.
Messenger (m) RNA expression of (A) ICAM-1, (B) VCAM-1, and (C) E-selectin from HUVECs pretreated with either E2C6 or control IgG before TNF-α stimulation for 24 hr (*p<0.05, **p<0.01, ***p<0.001, n = 5 per group, ns = not significant). Lung mRNA 16 hr after LPS induced endotoxemia for markers of vascular inflammation (D) ICAM-1, (E) VCAM-1, (F) E-selectin. (*p<0.05, **p<0.01, ***p<0.001, n = 5 per group, ns = not significant,) (G) Representative lung immunostaining for granulocyte differentiation antigen (Gr)−1 (red) was performed 16 hr after cecal ligation puncture (CLP) or sham surgery in E2C6 (10 mg/kg) or IgG control antibody (IgG)-treated mice (nuclear staining with 4′,6-diamidino-2-phenylindole, (blue), autofluorescence is shown in green). (n = 5) Scale bar 50 μm. (H) Semiquantification of whole lung cross-sections by evaluating Gr-1+ cells per high power field (HPF) (HPF = 40 × magnification) (*p<0.05, **p<0.01, ***p<0.001, n = 5 per group).
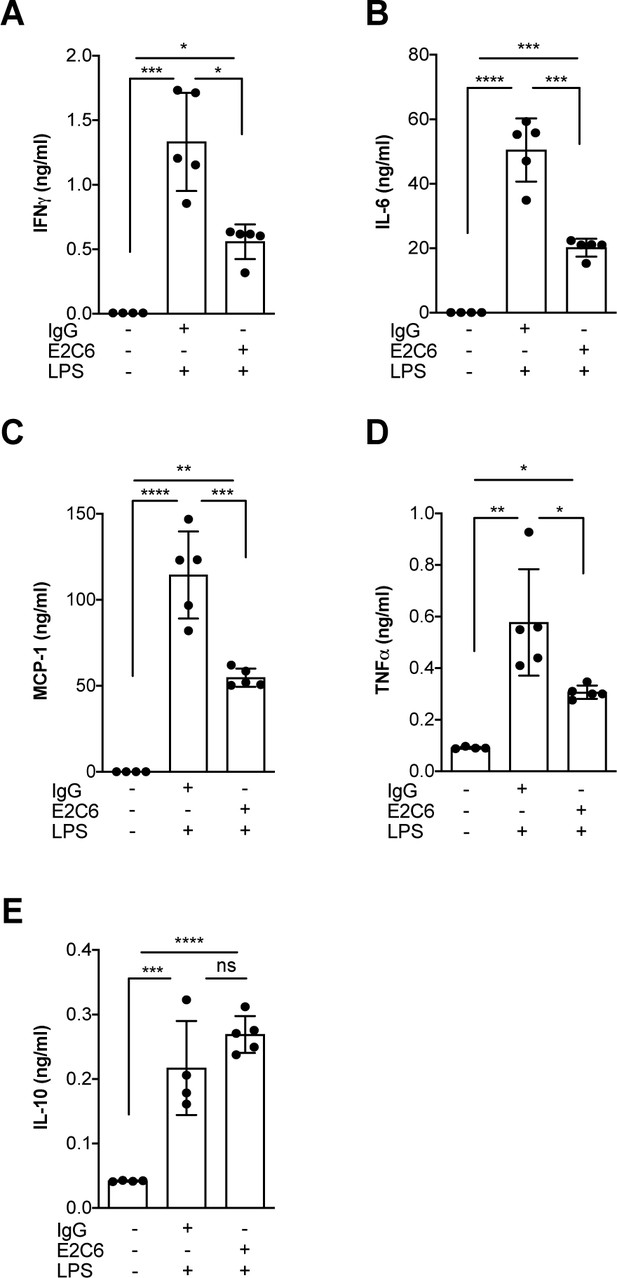
MMP14 blockade inhibits endotoxemia-induced pro-inflammatory cytokine release.
Quantification of circulating levels of (A) IFNy, (B) IL-6, (C) IL-1β, (D) TNF-α and (E) IL10 in the serum of mice pretreated with E2C6 (10 mg/kg) or control antibodies for 1 hr, followed by LPS for 16 hr (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n = 5 per group, ns = not significant). Data are the mean ± SD.
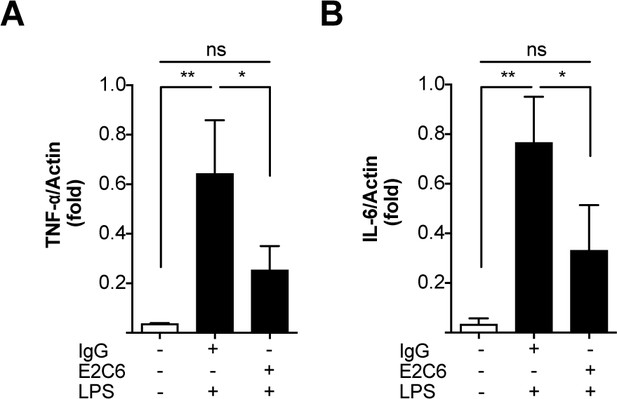
E2C6 attenuates LPS induced pulmonary proinflammatory cytokine transcription.
Adult male C57BL/6 J mice were pretreated with E2C6 (10 mg/kg) or control antibodies for 1 hr, followed by LPS from Escherichia coli i.p. for 16 hr. Messenger (m) RNA expression of septic and control mice lung homogenates for (A) Tumor necrosis factor α (TNF-α) and (B) interleukin-6 (IL-6). (Each group, n = 4–6, ns = not significant, *p<0.05, **p<0.01).
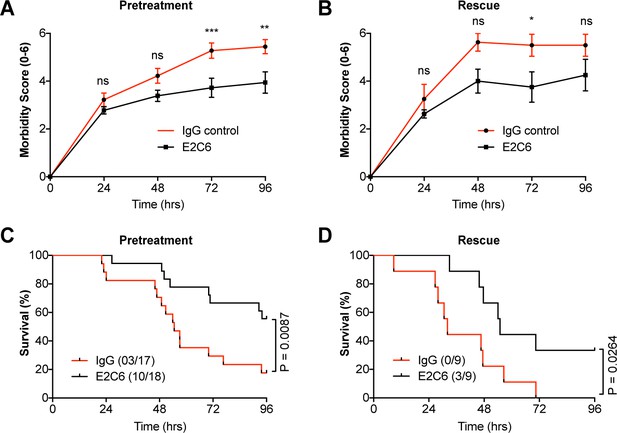
MMP14 blockade improves survival in experimental sepsis.
The morbidity of sepsis and severity of illness was semi-quantitatively assessed by an in-house scoring system (activity score, Table 1) (A) mice pretreated with either E2C6 (10 mg/kg) or control IgG intraperitoneally for 1 hr before CLP (n = 17–18 per group) (B) Mice subjected to CLP first and then treated with 10 mg/kg of E2C6 at 2, 24, and 48 hr after CLP. (n = 9 per group) (*p<0.05, **p<0.01, ***p<0.001, ns = non significant, in Bonferroni posttest of 2-way ANOVA). Kaplan-Meier survival analysis after CLP-induced sepsis in (C) mice pretreated with either E2C6 (10 mg/kg) or Control IgG intraperitoneally for 1 hr prior to CLP (D) Mice subjected to CLP first and then treated with 10 mg/kg of E2C6 at 2, 24, and 48 hr after CLP. Number in parentheses represents the number of surviving mice per each group. Statistical significance was analyzed by a log-rank test.
Tables
Activity score to evaluate severity of illness in septic mice.
| Score | Activity | General condition | Behaviour |
|---|---|---|---|
| 1 | Very active | Smooth fur, clear eyes, clean orifices (body openings) | Vigilant, curious, normal movements |
| 2 | Active | Smooth fur, clear eyes, clean orifices | Vigilant, normal movements |
| 3 | Less active | Matted fur, fur defects, eyes not completely open | Vigilant, quiet, reduced movements, normal posture, reduced corporal hygiene |
| 4 | Restricted | Dull fur, standing fur, eyes not completely open, unkempt orifices | Quiet, frequent persistence, restricted reaction on environmental stimuli, restricted body care |
| 5 | Apathetic | Dirty, dull fur, eyes closed, clogged or humid orifices, crooked posture | Self-isolation, no significant activity |
| 6 | Moribund (Death is expected) | Eyes closed, lateral position, shallow breathing, cramps, cold animal | No activity, no reaction on environmental stimuli |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Tie2 (C-20) (Rabbit polycloned IgG) | Santa Cruz | Cat#: SC-324 | WB: 1:1000 |
| Antibody | GAPDH (Rabbit polycloned IgG) | Santa Cruz | Cat#: SC-25778 | WB: 1:1000 |
| Antibody | β-Tubulin (Rabbit polycloned IgG) | Santa Cruz | Cat#: SC-9104 | WB: 1:1000 |
| Antibody | RAT anti Mouse Ly-6B.2 ALLOANTIGEN | Bio-Rad | Cat#: MCA771G | IF:1:200 |
| Antibody | CD144 (VE-cadherin) | BD Biosciences | Cat#: 555661 | IF:1:100 |
| Antibody | Human IgG2 isotype control | BioXcell | Cat# BE0301 | |
| Antibody | Anti-MMP14 (E2C6) | Botkjaer et al., 2016 | PMID:26934448 | A kind gift from Prof. Gillian Murphy and Dr. Yoshifumi Itoh |
| Commercial assay or kit | Human Tie-2 DuoSet | R and D | Cat#: DY5159 | |
| Commercial assay or kit | Mouse Tie-2 Quantikine ELISA Kit | R and D | Cat#: MTE200 | |
| Commercial assay or kit | CBA Mouse Inflammation Kit | BD | Cat#: 552364 | |
| Peptide, recombinant protein | Recombinant Human TNF-alpha | R and D | Cat#: 210-TA-020 | |
| Peptide, recombinant protein | Recombinant Human MMP14 (catalytic domain) | Enzo | Cat#: ALX-201–098 C010 | |
| Peptide, recombinant protein | Recombinant Human TIE2 protein | Sino Biological | Cat#: 10700-H03H | |
| Software, algorithm | ImageJ | NIH, Bethesda, MD (Version 1.52P) | https://imagej.nih.gov/ij/ | |
| Software, algorithm | Prism8 | Graphpad | https://www.graphpad.com/scientific-software/prism/ | |
| Software, algorithm | Flowjo | BD | https://www.flowjo.com/solutions/flowjo/downloads | |
| Other | LPS Escherichia coli serotype O111:B4 | Sigma-Aldrich | Cat#: L4130 |