Contribution of dorsal horn CGRP-expressing interneurons to mechanical sensitivity
Figures
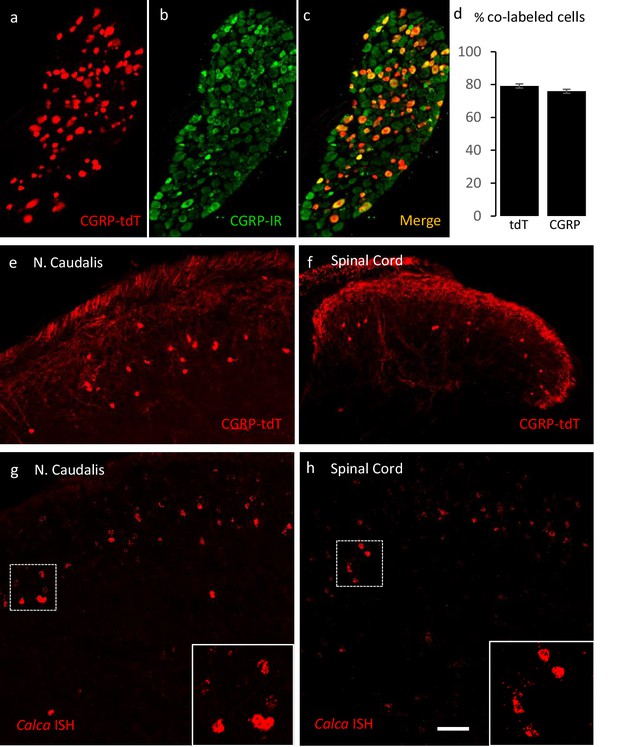
Validating the CalcaCreER transgenic mouse.
(a–c) Example of genetically labeled CGRP neurons from dorsal root ganglion of double transgenic CalcaCreER/tdTomato mice generated by crossing the CalcaCreER mouse line with a ROSA26fs-tdTomato (Ai14) mouse line (CGRP-tdTomato). Adult CalcaCreER/tdTomato mice received two injections of tamoxifen (150 mg/kg). Co-localization of tdTomato-(red) with CGRP- immunoreactivity (green) confirmed the specificity of CalcaCreER expression in trigeminal and dorsal root ganglia. (d) 80% of tdTomato-positive neurons were immunoreactive for CGRP (left bar) and 78% of CGRP-positive neurons were tdTomato-immunoreactive (right bar). Bars show mean and standard error (SEM) (three mice, four sections each). (e–f) CGRP-tdTomato expression was also detected in neurons of nucleus caudalis (e) and the spinal cord dorsal horn (f). The CGRP-tdTomato-immunoreactive neurons were concentrated in lamina III and occasionally observed in more superficial layers. The CGRP-tdTomato-labeled neurons were also abundant in regions of the central nervous system known to contain significant populations of CGRP-immunoreactive neurons or terminals (Figure 1—figure supplements 1–4). (g–h) In situ hybridization confirmed expression of Calca mRNA in the dorsal horn (g) and nucleus caudalis (h). Insets show higher magnification of the Calca mRNA expressing neurons. Scale bars: 100 µm.
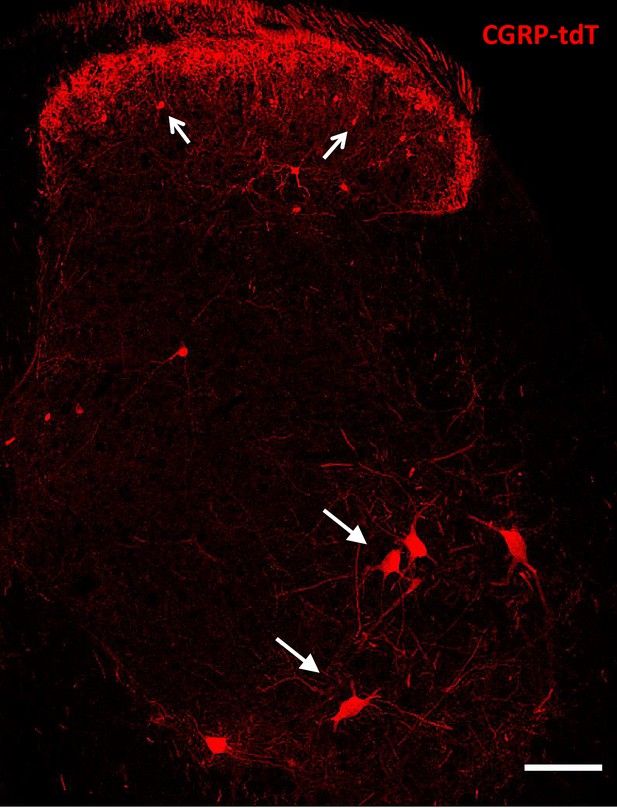
CGRP-tdTomato expression in the lumbar spinal cord.
Lumbar section from a CGRP-tdTomato mouse. Arrows point to intensely fluorescent CGRP-tdTomato neurons in lamina III of the dorsal horn and ventral horn motoneurons. Laminae I and II (substantia gelatinosa) contain a dense array of fluorescent processes originating from CGRP-expressing primary sensory neurons. Scale bars: 100 μm.
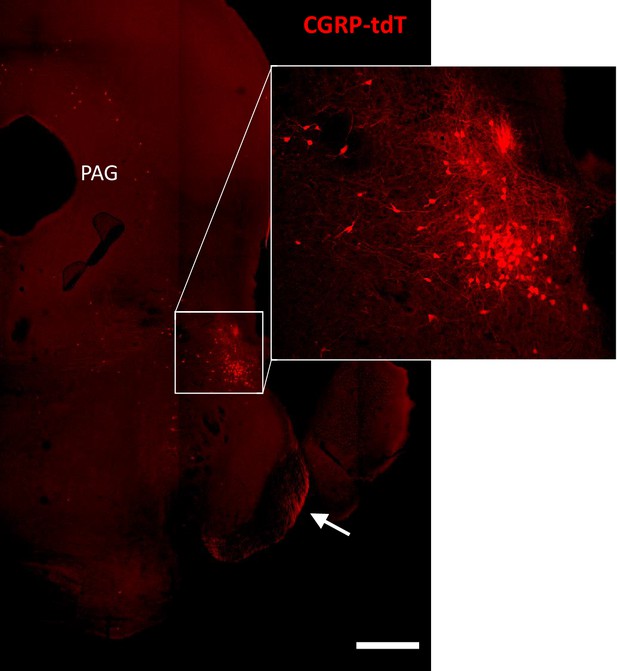
CGRP-tdTomato expression in the parabrachial nucleus.
CGRP-tdTomato fluorescence at a caudal midbrain/rostral pontine level. The boxed area in the main figure shows intensely labeled neurons in the external lateral parabrachial nucleus, which are shown at higher magnification in the inset. The periaqueductal gray (PAG) also contains scattered CGRP-tdTomato-expressing neurons. The arrow points to primary afferent axons originating from the trigeminal ganglion. Scale bar: 500 μm.
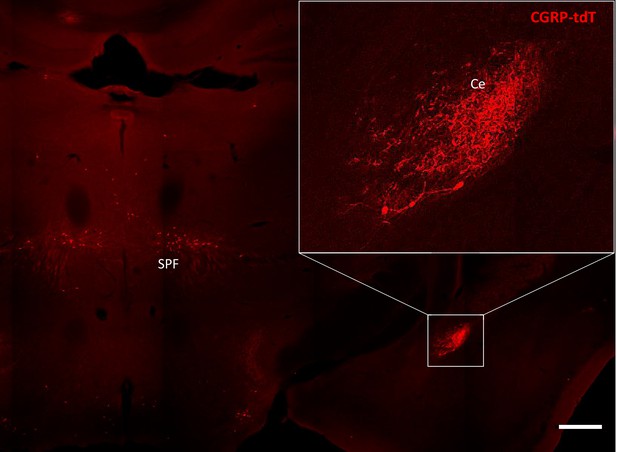
CGRP-tdTomato expression in the amygdala.
CGRP-tdTomato fluorescence at a thalamic level. The boxed area in the main figure shows the dense plexus of CGRP-tdTomato fluorescent processes in the central nucleus of the amygdala (Ce). The section also illustrates labeled neurons in the subparafascicular nucleus of the thalamus (SPF). Scale bar: 500 μm.
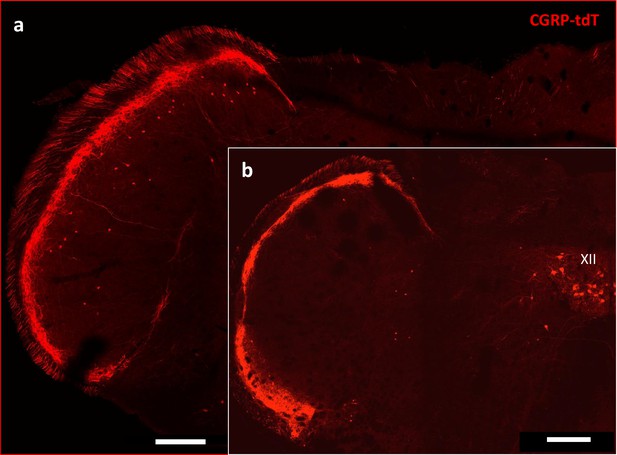
CGRP-tdTomato expression in the trigeminal nucleus caudalis.
Caudal levels of the medulla (a) Contain greater numbers of CGRP-tdTomato neurons in lamina III of the nucleus caudalis compared to more rostral levels (b). The latter level includes CGRP-tdTomato-expressing motoneurons in the hypoglossal nucleus (XII), Scale bars: 500 μm.
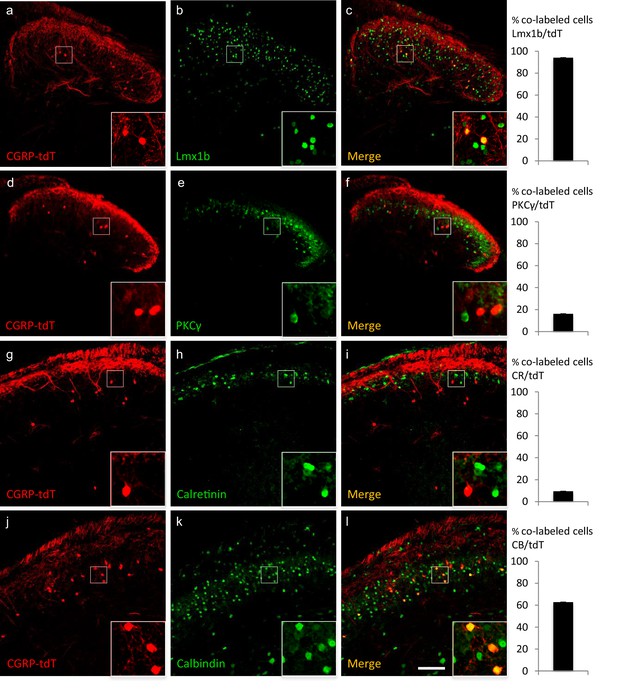
CGRP-expressing neurons in the dorsal horn (a–f) and nucleus caudalis (g–l) are a distinct class of excitatory (Lmx1b+) interneurons.
(a–l) Immunohistochemistry showed that CGRP-tdTomato fluorescent neurons (red) co-express many markers (green) of excitatory, but not inhibitory (e.g. Pax2, Figure 2—figure supplement 1) interneurons in the dorsal horn (a–f) and nucleus caudalis (g–l). Ninety-eight percent of CGRP-tdTomato neurons co-expressed Lmx1b (a–c), 16% co-expressed PKCγ (d–f), 9% co-expressed calretinin (g–i), and 63% co-expressed calbindin (j–l). Insets show higher magnification views of boxed areas in respective images. Graphs illustrate mean percentages ± SEM of CGRP-tdTomato neurons that were double-labeled with the indicated antibody (~100 cells per antibody). Scale bar: 100 µm.
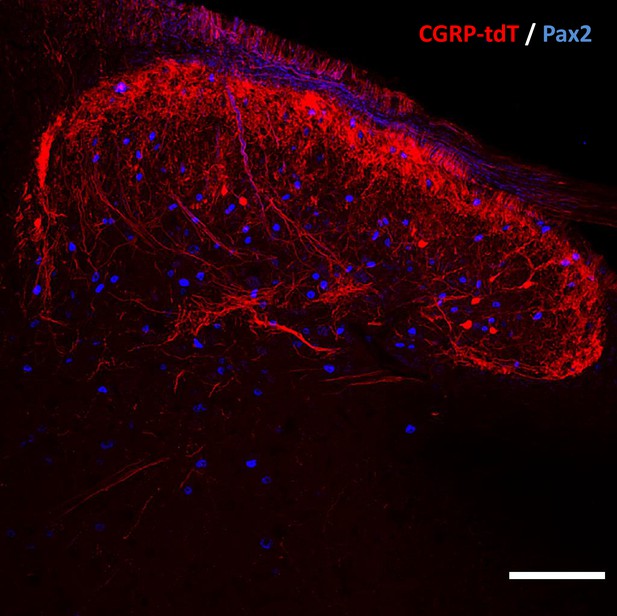
CGRP tdTomato interneurons are Pax2-negative.
In lumbar dorsal horn, the absence of double labeling for CGRP-tdTomato (red) and Pax2 (blue)-immunoreactivity, a marker of inhibitory interneurons, indicates that the dorsal horn CGRP-tdTomato neurons are excitatory. Scale bar: 100 μm.
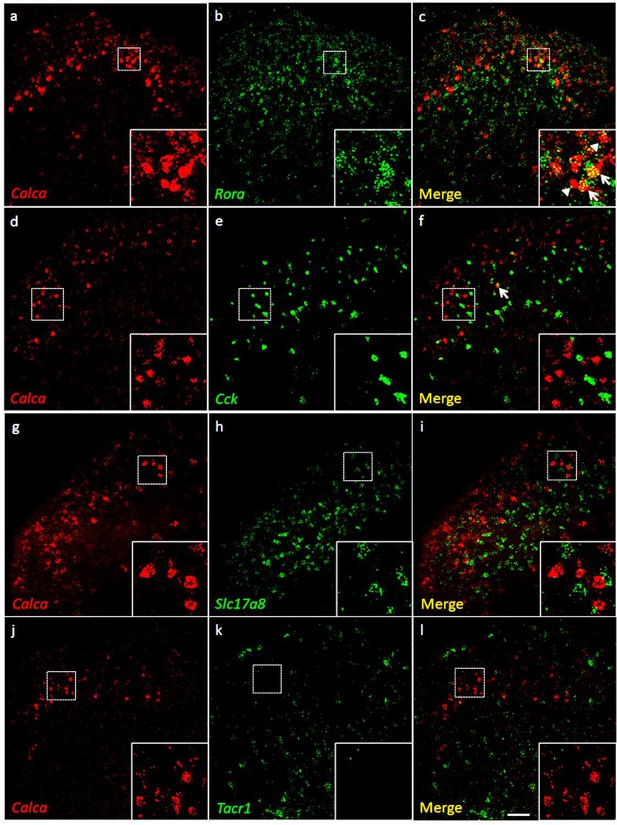
Coexpression of Calca mRNA with Rora mRNA, but with neither Cck nor Tacr1 mRNA.
(a-l) Co-expression of Calca mRNA (Calca; red), with other markers (green) in subsets of dorsal horn (a-c; j–l) and nucleus caudalis (d–i) neurons. Of Calca-expressing cells, 56% express Rora mRNA (a–c), but only 4.4% express Cck mRNA (d–f). Similarly, there was minimal overlap of Calca and Slc17a8, the gene coding for VGLUT3 (g–i), or Calca and Tacr1, the gene coding for the NK1 receptor (j–l). Insets show higher magnification images of boxed areas. Scale bar: 100 µm.
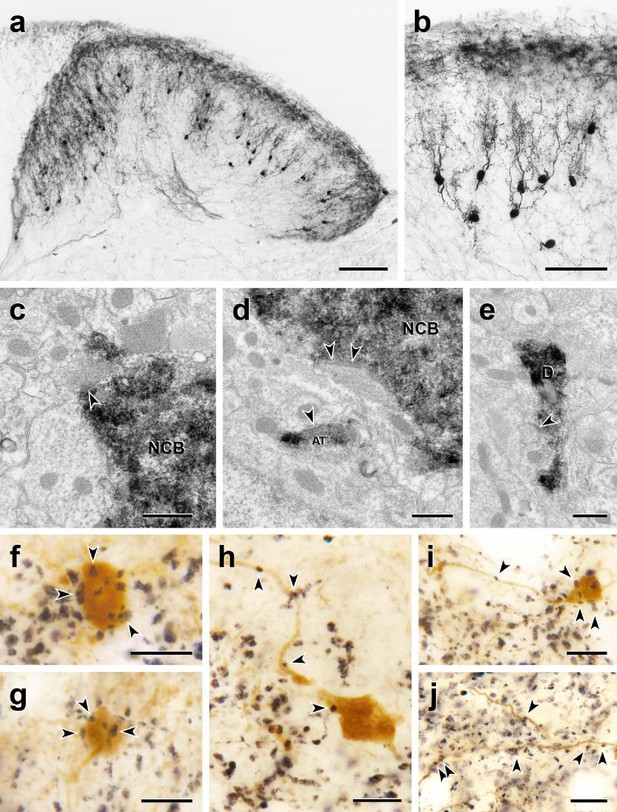
Morphology and VGLUT1 innervation of dorsal horn CGRP interneurons.
(a, b) Most tdTomato-immunoreactive CGRP interneurons (black) are located in lamina III and have a relatively uniform morphology with many spiny, dorsally projecting dendrites. Scale bars: 100 µm in a; 50 µm in b. (c-e) Electron microscopic analysis revealed unlabeled host synapses (arrowheads) presynaptic to the cell bodies (NCB in c and d) and dendrites (D in e) of tdTomato-immunoreactive (black) CGRP interneurons. d also shows an asymmetric presynaptic input (AT) from a presumptive CGRP interneuron to an unlabeled host dendrite. (f – j) Black VGLUT1-immunoreactive varicosities form close appositions (arrowheads) with the cell bodies (f and g) and dendrites (h – j) of brown tdTomato-immunoreactive CGRP interneurons. Scale bars: 500 nm in c – e, 10 µm in f – j.
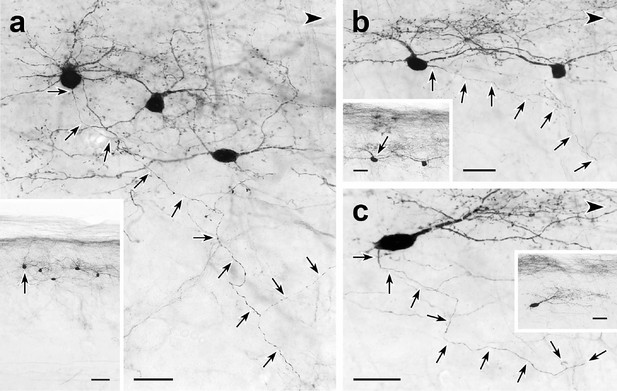
Trajectories of axons of CGRP-tdTomato interneurons.
tdT-immunoreactive CGRP interneurons (black) in 50 µm parasagittal sections from the lumbar dorsal horn of CGRP-tdTomato mice in which an intrathecal injection of capsaicin reduced primary afferent-derived CGRP. The CGRP-tdTomato neurons have spiny, dorsally directed dendrites and their axons (arrows) course ventrally and often caudally (large arrowhead) (Figure 5—figure supplements 1 and 2). Arrows in insets indicate location of the neurons whose axons are shown in a, b and c. Scale bars: 20 µm in a–c, 50 µm in inset a, 20 µm in insets b and c.
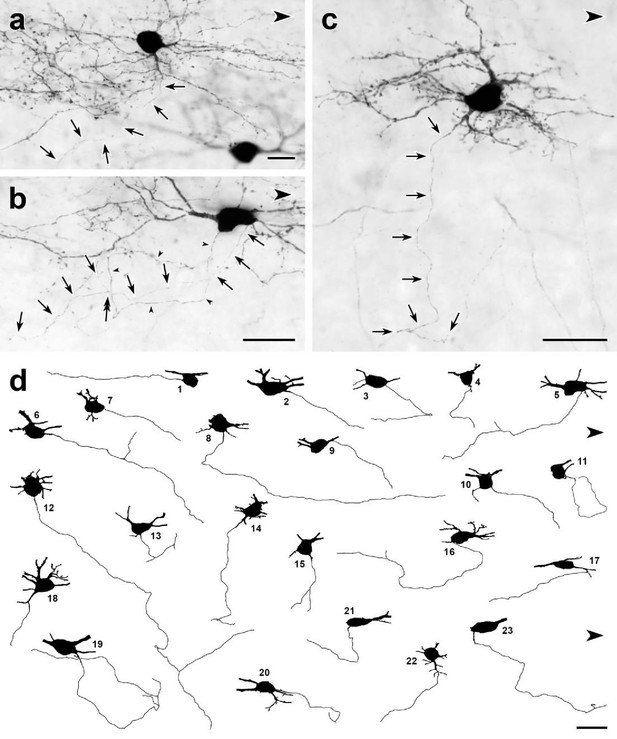
Dorsal horn CGRP interneurons have ventrally directed axons.
(a–c) Parasagittal 50-μm-thick sections of lumbar enlargement from CalcaCreER/tdTomato mice that received intrathecal capsaicin treatment to reduce tdTomato-immunoreactivity from primary afferents. In most cases, the axons (arrows) of the dorsal horn CGRP-tdTomato interneurons travel ventrally. (a) An axon arises from a ventrally-projecting primary dendrite, rather than the cell body. (b) The axon (arrows) of this tdTomato-positive neuron arises from the caudal ventral surface of the cell body and travels ventrally and rostrally. The cell body also emits a very fine dendritic process, defined by the presence of spines (double-headed arrow). (c) This heavily spine-laden, multipolar CGRP-tdTomato interneuron emits a ventrally directed axon from one of its dendrites. (d) Drawings of 23 dorsal horn CGRP-tdTomato interneurons whose axons could be identified and traced. Each drawing shows the neuronal cell body and its axon as well as initial portions of its major primary dendrites. Nineteen of the axons originate from the ventral region of the cell body; 3 (d14, d18, d22), from a ventrally projecting primary dendrite and one (d17) from a secondary dendrite close to its branch point off a primary dendrite. Most of the axons travel ventrally and caudally; some travel rostrally (e.g. d1, d5) and an occasional axon courses directly ventral (e.g., d15). After initially travelling ventro-caudally, 2 of the axons (d11, d19) looped dorsally and then began to travel rostrally. Eight of the axons bifurcated (d5, d6, d12, d13, d14, d15, d16, d19), all within 120 μm of their origin from a cell body. Scale bars: 20 μm.
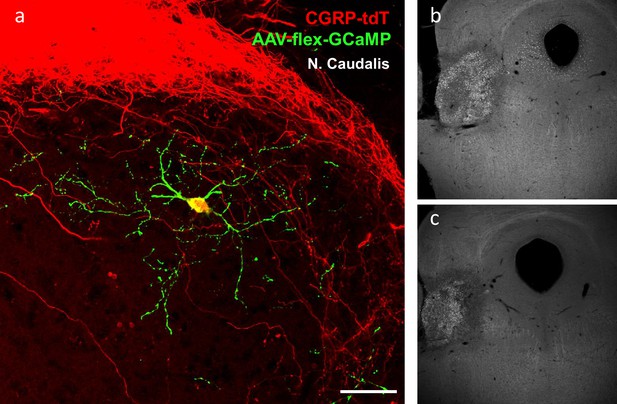
Radial morphology of the CGRP-tdTomato interneurons revealed after AAV injection.
(a) A transverse section of a CGRP-tdTomato neuron after injection of Cre-dependent AAV1-GCaMP6 (green) into the nucleus caudalis. This approach revealed a comparable morphology of the CGRP-tdTomato interneuron to that illustrated in Figure 4 and Figure 5—figure supplement 1a–c, but did not reveal distant axonal projections. Scale bar: 50 μm. (b–c) Two levels illustrating a Fluorogold injection site in the parabrachial nucleus.
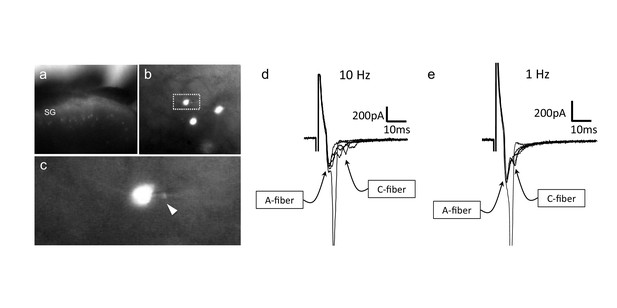
CGRP-tdTomato interneurons receive low threshold sensory inputs.
Low (a) and high (b) magnification micrographs of endogenous fluorescent CGRP-tdTomato neurons in a spinal cord slice. The boxed neuron in (b) is shown at high magnification in c; arrowhead points to the recording pipette in a whole cell configuration. (d,e) Responses of CGRP-tdTomato interneuron to dorsal root stimulation at 10 Hz (d) or 1 Hz (e). An early, persistent component likely corresponds to a monosynaptic A-fiber input. The late component, with variable latency and failures, likely reflects polysynaptic C-fiber input (electrophysiological properties: Figure 6—source data 1 table).
-
Figure 6—source data 1
Electrophysiological properties of CGRP-tdTomato interneurons in the dorsal horn and nucleus caudalis.
Most CGRP-tdTomato neurons showed delayed firing patterns (delayed 19, tonic 1, reluctant 2, single 2, no response 3). Based on electrical stimulation of dorsal roots, we conclude that CGRP interneurons in the lumbar cord predominantly receive monosynaptic input from Aβ primary afferent fibers.
- https://cdn.elifesciences.org/articles/59751/elife-59751-fig6-data1-v2.docx
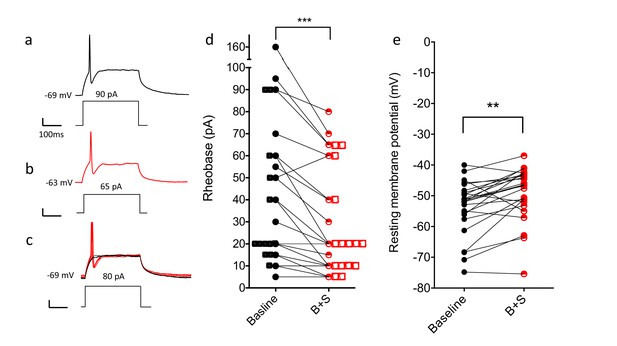
Blocking GABA and glycine receptors increases the excitability of the CGRP interneurons.
Representative traces show current evoked action potential before (a) and after (b) bicuculline and strychnine. (c) Administration of antagonists when current application was 10 pA below rheobase threshold (black) also induced an action potential (red). (d) Rheobase values before and after antagonist treatment (n = 25; two-tailed, paired T-test, p=0.0005). (e) Resting membrane potentials before and after bicuculline (B) and strychinine (S) treatment (n = 25).
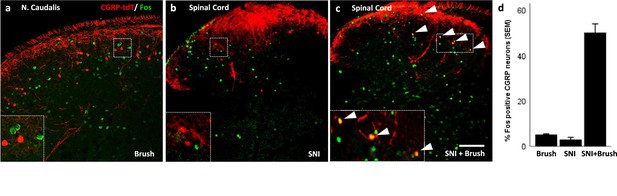
Peripheral innocuous stimuli activate CGRP interneurons but only after spared nerve injury (SNI).
(a) Fos-immunoreactive neurons in nucleus caudalis after brushing the cheek of a naive uninjured mouse. (b) Fos expression in the lumbar dorsal horn 6 days after SNI without additional peripheral stimulation. (c) Fos expression in the lumbar dorsal horn 6 days after SNI with additional brush stimulation of the hindpaw. Insets: high-magnification images of the boxed areas in the respective micrographs. Arrowheads indicate double-labeled cells. Scale bar: 100 µm. (d) Mean percentages ± SEM of CGRP-tdTomato neurons that are Fos-immunoreactive in the different conditions (Figure 8—figure supplements 1 and 2).
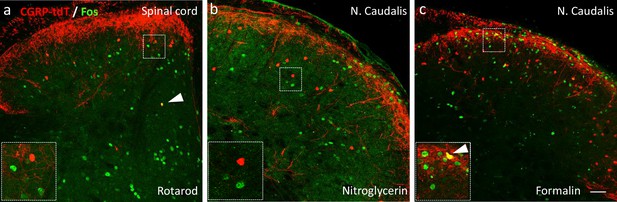
Neither noxious nor innocuous stimuli induce Fos expression in CGRP-tdTomato interneurons in control mice.
(a) Fos expression-immunoreactivity (green) in neurons of the lumbar dorsal horn after walking on a rotarod (90 min). (b) Fos-immunoreactivity in the neurons of the nucleus caudalis after systemic nitroglycerin injection (10 mg/kg, i.p.) or after a 2% formalin (10 μl) injection into the cheek (c) in unanesthetized mice. Insets illustrate higher magnification images of separate populations of Fos-immunoreactive and CGRP-tdTomato interneurons. Arrows in a and c point to rare double-labeled cells outside lamina III. Scale bar: 100 μm.
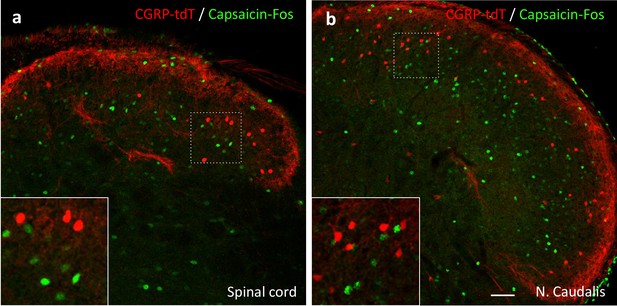
Capsaicin does not activate CGRP-tdTomato interneurons in the lumbar spinal cord or trigeminal nucleus caudalis.
In anesthetized mice (2% isoflurane), a unilateral injection of 20 µl of capsaicin (1.0 µg/µl) into the hindpaw (a) or the cheek (b) did not induce Fos expression (green) in CGRP-tdTomato interneurons in the dorsal horn of the lumbar spinal cord (a) or in the nucleus caudalis (b). Insets show higher magnification views of boxed areas. Scale bar: 50 μm.
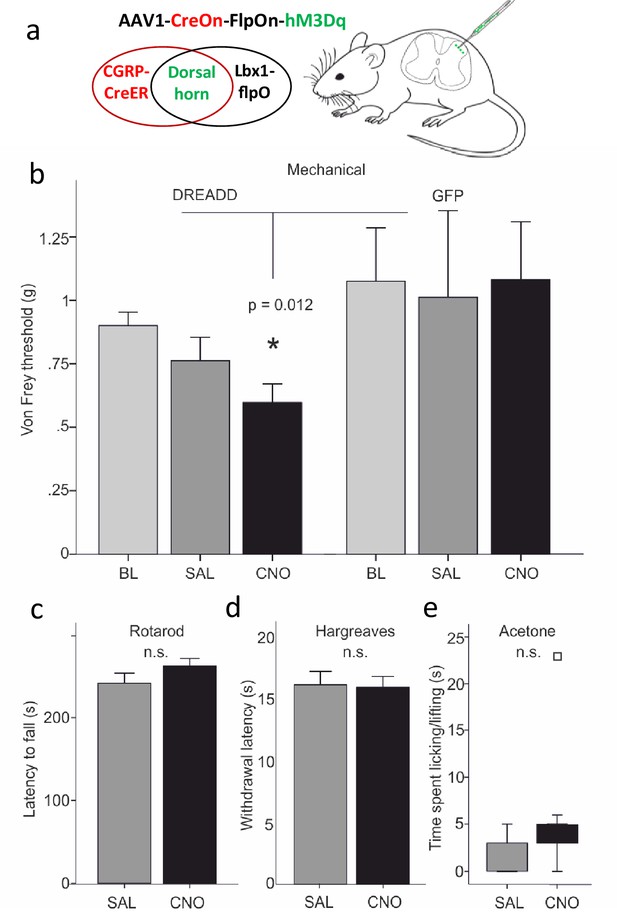
Dorsal horn CGRP interneurons contribute to mechanical sensitivity in vivo.
(a) CalcaCreER mice were crossed to an Lbx1-driven FLPo mouse line, which restricts Cre expression to Lbx1-expressing neurons in the dorsal spinal cord and hindbrain. We then injected a Cre and FLP-dependent DREADD (hM3Dq) virus (AAV1-CreOn-FlpOn-hM3Dq) or a GFP-expressing AAV into the lumbar dorsal horn. (b) Baseline (BL) von Frey mechanical thresholds of the DREADD-expressing mice (n = 16; light grey bars) did not differ from baseline thresholds of mice injected with the AAV-GFP (GFP) control virus (n = 6). In contrast, CNO injection significantly reduced von Frey thresholds (CNO, black bars) of the ipsilateral hindpaw in the DREADD-injected mice, compared either to their baseline, to the GFP controls or to saline (SAL; light grey bars)-injected mice (Repeated measures Two-way ANOVA, p=0.012). Neither latency to fall from a rotarod (c), withdrawal to noxious heat in the Hargreaves test (d), nor time spent paw lifting after exposure of the paw to a cold stimulus (acetone) (e) differed when comparing CNO and the control saline injection (p>0.05, Students T-test and Wilcoxon Signed Ranks Test, respectively). Square in (e) indicates an outlier.
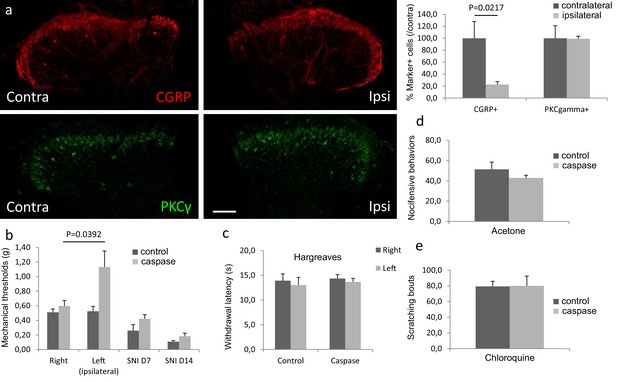
Ablation of dorsal horn CGRP interneurons decreases mechanical sensitivity in vivo.
(a) The number of CGRP (red)-expressing interneurons was significantly decreased 4 weeks after injection of a Cre-dependent caspase-expressing viral vector into the superficial dorsal horn of CalcaCreER/tdTomato mice. In contrast, the number of PKCγ (green)-expressing interneurons did not change. Scale bar: 100 μm. (b) The von Frey mechanical thresholds were significantly higher ipsilateral to the injection side (left) after the CGRP interneuron ablation (n = 8; light grey bars), compared to the contralateral (right) side. In contrast, the von Frey mechanical thresholds did not differ in control mice (n = 6; dark gray bars). Nevertheless, CGRP-ablated and control mice exhibited similar levels of mechanical hypersensitivity 7 and 14 days after SNI. (c–e) Ablation of spinal cord CGRP-expressing interneurons did not change the withdrawal latencies in the Hargreaves test (c), the number of paw lifts in response to a cold stimulus (acetone) (d) or the number of scratching bouts evoked by a subcutaneous calf injection of chloroquine (unpaired Students T-test).
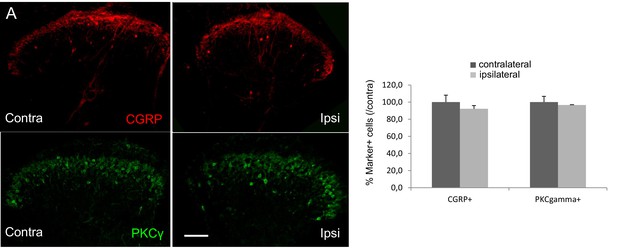
The number of CGRP (red)- or PKCγ (green)-expressing interneurons did not change 4 weeks after injection of saline into the superficial dorsal horn of CalcaCreER/tdTomato mice (unpaired Students T-test).
Scale bar: 100 μm.
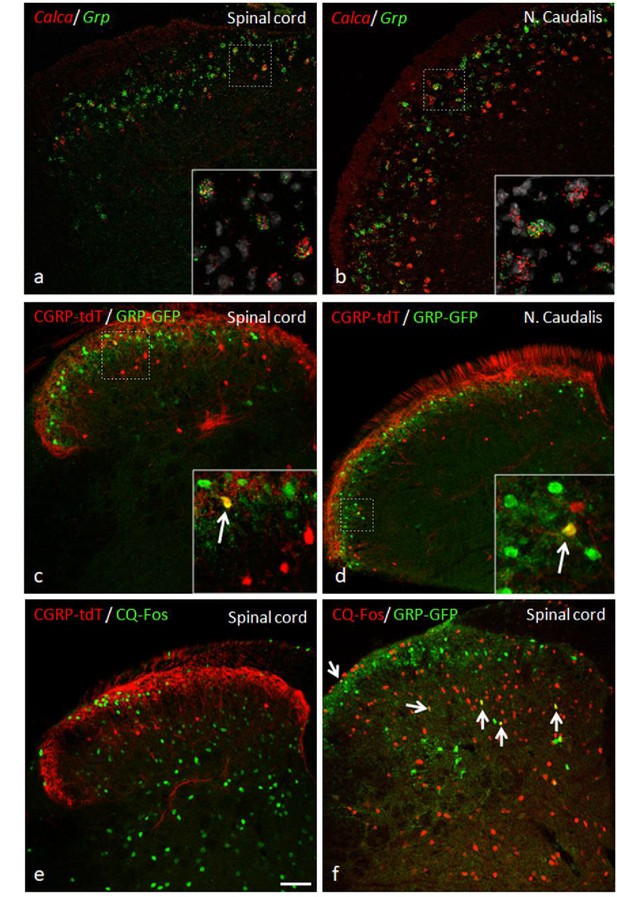
GRP, CGRP, and pruritogen-evoked Fos expression.
Double in situ hybridization for tdTomato (red) and Grp (green) illustrates considerable mRNA co-expression in neurons of the dorsal horn (a) and nucleus caudalis (b) of CGRP-tdTomato mice. In contrast, immunocytochemical localization of GRP and tdTomato in a tamoxifen-treated CalcaCreER/tdTomato mouse that was crossed with a GRP-GFP reporter mouse revealed only occasional double labeling (arrow in inset) in the dorsal horn (c) or nucleus caudalis (d). Consistent with this minimal overlap, Fos expression in tdTomato-labeled CGRP interneurons was rare in response to a hindpaw injection of chloroquine (CQ; e). In contrast, many GRP-GFP interneurons were immunostained for Fos in response to CQ (arrows in f). As the mice were anesthetized the CQ-induced Fos was scratching-independent. Scale bar: 100 μm.





