Mechanistic insights into volatile anesthetic modulation of K2P channels
Figures
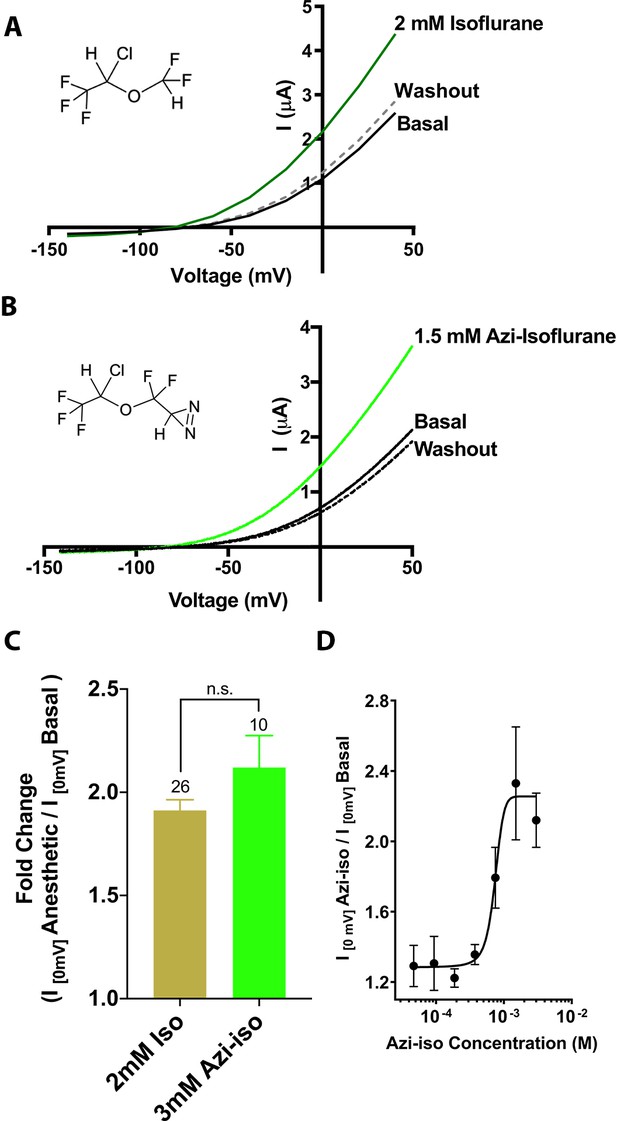
Functional validation of azi-isoflurane activity on TREK1 channels.
Representative two electrode voltage clamp recordings demonstrate the potentiating effects of saturating doses of isolflurane (A) and azi-isoflurane (B). Chemical structures of isoflurane and azi-isoflurane are shown. (C) Fold effect of administration of saturating doses of either isoflurane or azi-isoflurane on TREK1 outward current, as determined by the ratio of the recorded current at a voltage of 0 mV, immediately prior to and following administration of volatile anesthetic (VA) agent. No significant difference was found between the responses of TREK1 to isoflurane versus azi-isoflurane, unpaired two tailed t-test p value of 0.11 (D) Dose response curve for azi-isoflurane activation of TREK1. Data derived from n > 6, N > 2 experimental observations. Error bars in panels C and D are mean ± SEM.
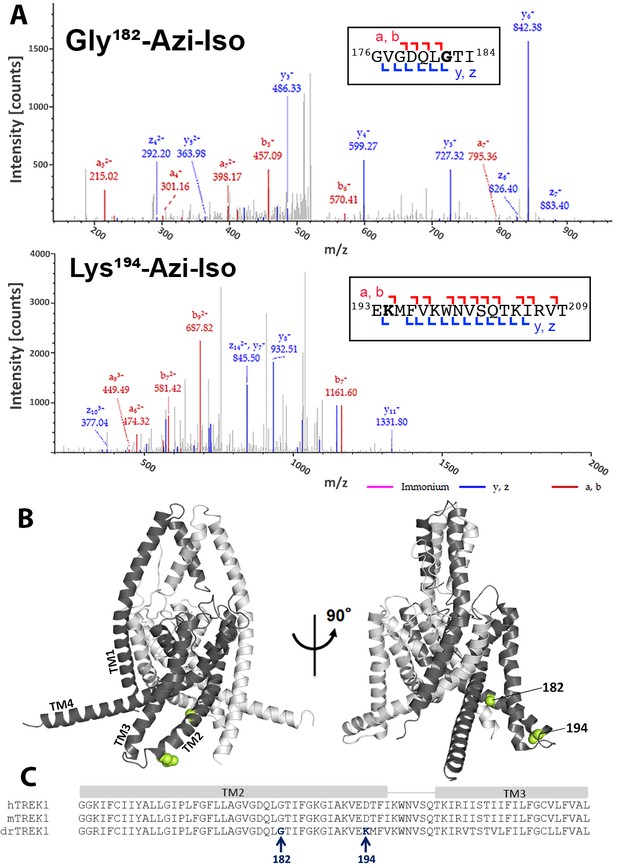
Azi-isoflurane photolabeling of TREK1.
(A) Mass spectra of drTREK1 photoaffinity labeled peptides labeled at Glycine182 (top) and Lysine 194 (bottom). Colored intensities denote the identified peptide a, b, z, and y ion fragments for the sequence assignment, as shown in the inset boxes. See Figure 2—figure supplement 3 for corresponding peptide tables. (B) A structural model of mouse TREK1 (PDBID 6CQ6), showing the positions of residues G182 and K194 (labeled lime spheres) along the TM2 helix. (C) Alignment of the TREK1 TM2 and TM3 helixes from human (hTREK1), mouse (mTREK1), and zebrafish (drTREK1).
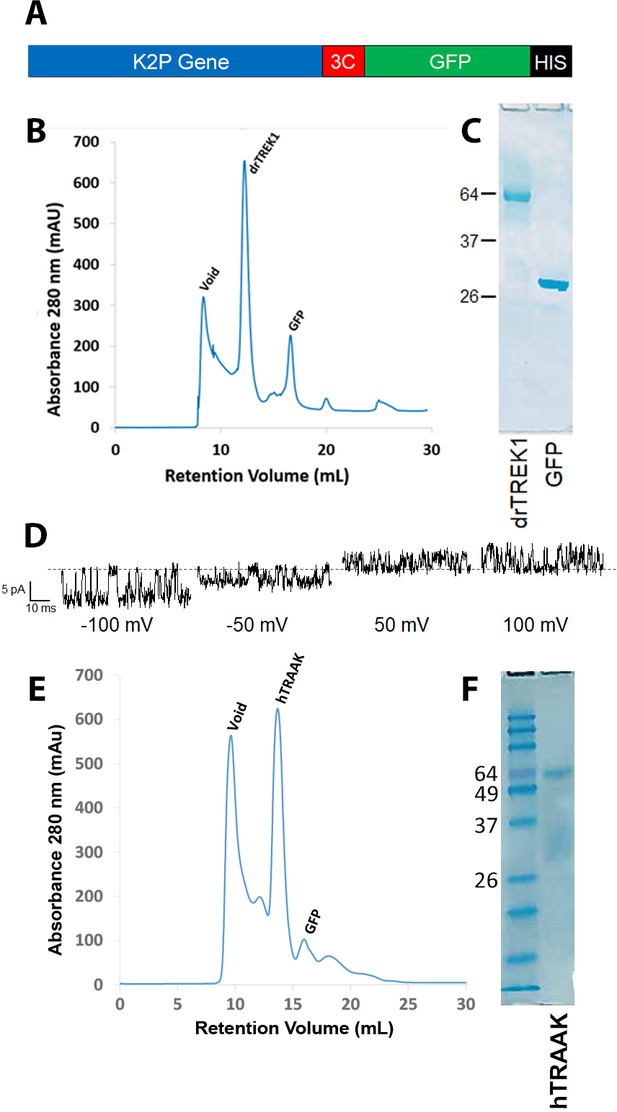
Purification of TREK1 and TRAAK proteins.
(A) Zebrafish TREK1 or human TRAAK DNA sequences were purified by metal chromatography via a C-terminal HIS tag, and expressed as fusion proteins with GFP, cleaved off via a 3C protease cleavage site prior to (B,E) final size exclusion chromatography. (C, F) After SDS PAGE electrophoresis, purified protein ran at a molecular weight of approximately 65 kDa, consistent with a K2P dimer. Prior to photolabeling experiments, purified TREK1 protein was assessed for functional integrity by reconstitution into planar lipid bilayers to measure single-channel activity at the indicated holding potentials. Recordings were performed in symmetrical 150 mM KCl solution (D). hTRAAK protein was similarly active when reconstituted into bilayers (not shown).
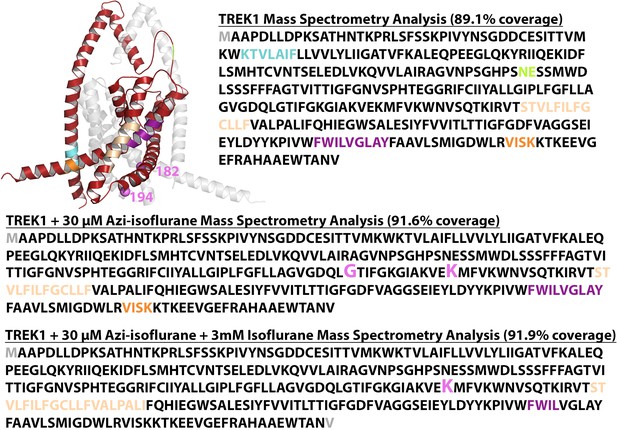
Mass spectrometry (MS) analysis of purified drTREK1.
Results of MS analysis of TREK1 wildtype (WT) in the (top) absence of reaction with azi-isoflurane, (second) following reaction with 30 μM azi-isoflurane, (third) following reaction with 30 μM azi-isoflurane in the presence of 3 mM Isoflurane, or (bottom) TREK1 G182W following reaction with 30 μM azi-isoflurane. Regions positively identified by MS analysis are shown in red in the TREK1 structural model (PDB ID 6CQ6) and in black font in the sequence data. Regions absent from MS data occurred in five distinct regions, all of which are displayed in matching color in both the structural model and the sequence data. The G182 and K194 residues found to be modified by azi-isoflurane in TREK1 WT are shown as pink spheres in the structural model, and positive photolabeling is denoted in the sequence data by enlarged font and pink color. The A67 and T303 residues modified by azi-isoflurane in TREK1 G182W are similarly denoted in blue. The initial and final residues in the TREK1 protein were not identified in the majority of the MS results and are shown in gray to denote absence from positive MS identification. These residues are not present in the TREK1 structural model.
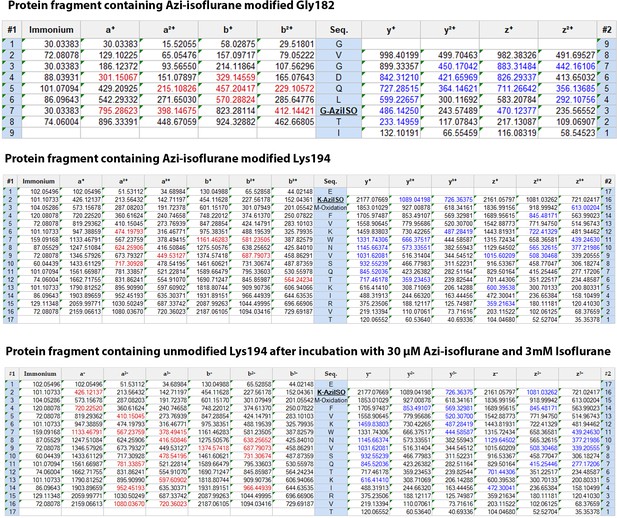
Mass spectrometry protein fragment tables.
Shown are the fragmentation tables of the (top) 176-GVGDQLGTI-184 photolabeled peptide in the presence of 30 μM azi-isoflurane (middle) the 193-EKMFVKWNVSQTKIRVT-209 photolabeled peptide in the presence of 30 μM azi-isoflurane, and (bottom) the 193-EKMFVKWNVSQTKIRVT-209 photolabeled peptide in the presence of 30 μM azi-isoflurane (AziISO) and 3 mM isoflurane. Detected identified a, b (red) and z, y (blue) ions are colored red and blue, respectively. Residues detected with a modification are noted, and those modified by azi-isoflurane are additionally noted in bold and underlined.
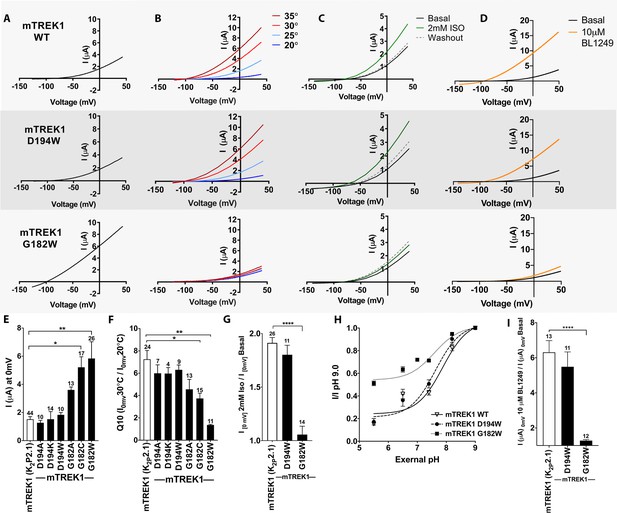
Functional assessment of mTREK1 residues identified by azi-isoflurane photolabeling.
Representative two electrode voltage clamp recordings of mTREK1 wildtype (WT) and channel mutants G182W and D194W. (A) Basal current measured 24 hr after microinjection of 2.4 ng cRNA. (B) Temperature dependence of TREK1 currents, measured at temperatures of 20–35°C, in 5°C increments. (C) Response to administration of 2 mM isoflurane, followed by washout. (D) Response to administration of 10 μM BL1249. For temperature, isoflurane, and BL1249, experiments performed on TREK1 channels bearing mutations that alter basal current density, the concentration of microinjected cRNA was titrated to achieve 1 μA of current at 20°C, to approximate WT channel current density. (E) Quantification of TREK1 channel activity on basal current level, (F) temperature dependence as measured by Q10 (30°C/20°C), (G) response to isoflurane administration, (H) changes in external pH, or (I) BL1249 administration, as measured by TREK1 current at 0 mV. Number of replicate experiments indicated. Statistical significance was determined by one-way ANOVA combined with a Dunnetts multiple comparison test against mTREK1 WT data, results indicated, *p<0.5, **p<0.05, ****p<0.0005. Error bars are mean ± SEM.
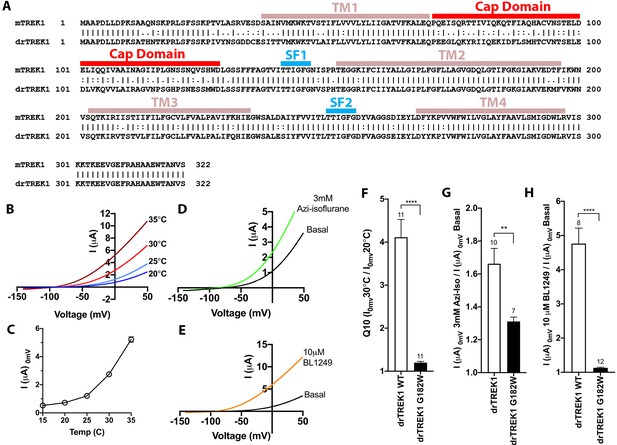
Function assessment of the drTREK1 isoform.
(A) Pairwise alignment of the mouse and zebrafish TREK1 protein sequences, with the transmembrane domains (TM), extracellular cap (CAP), and selectivity filter (SF) sequences denoted. (B) Representative traces showing temperature dependence of drTREK1 current, measured at temperatures of 20–35°C, in 5°C increments and (C) quantification of temperature responsiveness as measured by drTREK1 current at 0 mV. (D) Representative traces of drTREK1 response to administration of 2 mM Isoflurane or (E) 10 μM BL1249. Experiments performed on mTREK1 channels bearing mutations that alter basal current density, the concentration of microinjected cRNA was titrated to achieve 1 µA of current at 20°C, to approximate wild-type channel current density. (E) Quantification of mTREK1 channel activity on basal current level, (F) temperature dependence as measured by Q10 (30°C/20°C), (G) response to isoflurane administration, (H) changes in external pH, or (I) BL1249 administration, as measured by mTREK1 current at 0 mV. Number of replicate experiments indicated. Error bars are mean ± SEM. Statistically significance was determined in panels F–H by unpaired two tailed t-tests. Results indicated, **p<0.05, ****p<0.0005.
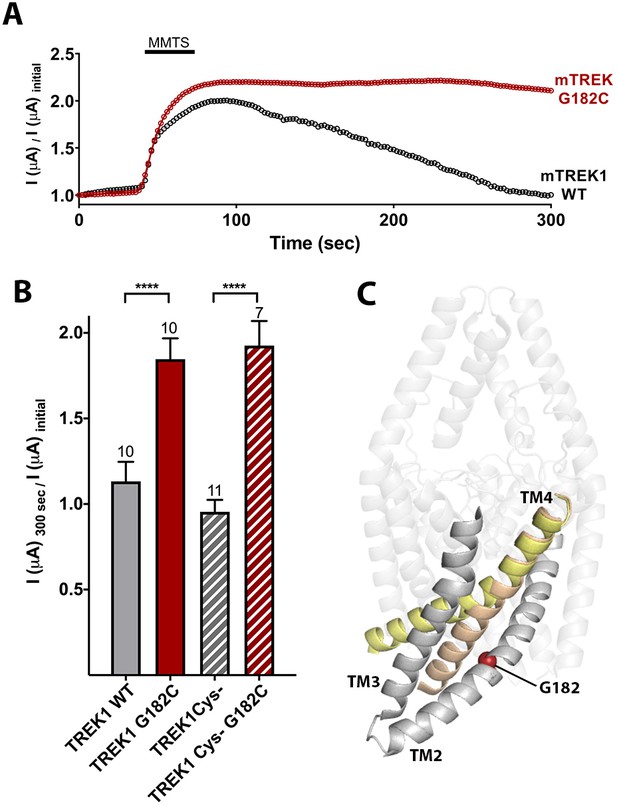
Cysteine modification at the G182 position leads to TREK1 activation.
(A) Representative time courses of the response of TREK1 wildtype (WT) or TREK1 G182C channels to treatment with 2 mM MMTS. Shown is the recorded current at 0 mV holding potential, measured every 5 s for 5 min, with current values normalized to the initial value recorded at the beginning of the time course (B) Quantification of effect size at the end of the time course (300 s), for TREK1 WT and TREK1 Cys-, a mutant TREK1 channel lacking all five of the endogenous TREK1 cysteine residues, and for G182 mutants in both of the above backgrounds. Number of replicate experiments indicated. Error bars are mean ± SEM. Statistically significance determined by one-way ANOVA combined with a Sidak multiple comparison test of TREK1 WT versus TREK1 G182C and TREK1 Cys- versus TREK1 cys- G182C. Results indicated, ****p<0.0005. (C) Crystallographically defined structural models of TREK2 in the TM4 up (PDB ID 4xdl, yellow) and TM4 down (PDB ID 4bw5, tan) conformations, highlighting the TM2, TM3, and TM4 helices from only a single subunit. The position of G182 (red sphere) is noted.
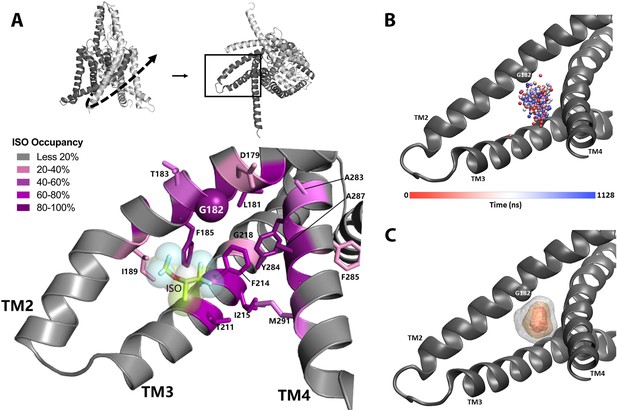
Molecular dynamics (MD) simulation studies define an isoflurane-binding pocket in TREK1 channels.
(A) By rotating the TREK1 structure shown in Figure 2 (top left, dashed arrow), we demonstrate the region of TM2, TM3, and TM4 that forms the isoflurane-binding site (top right, boxed). Below, a representative MD simulation snapshot of the isoflurane binding pocket, highlighting the G182 residue (sphere) and additional positions found to exhibit high isoflurane occupancy (sticks). Relative isoflurane occupancy for each residue (see Table 1) is shown as quartiles, as described on left (B) Position of the oxygen atom at the center of the isoflurane molecule during equilibrium MD simulation trajectory 2 of mTREK1 WT in the presence of isoflurane (C) Density map of the position of the bound isoflurane. Isosurfaces represent 10% (gray), 30% (yellow), and 50% (orange) isoflurane occupancy.
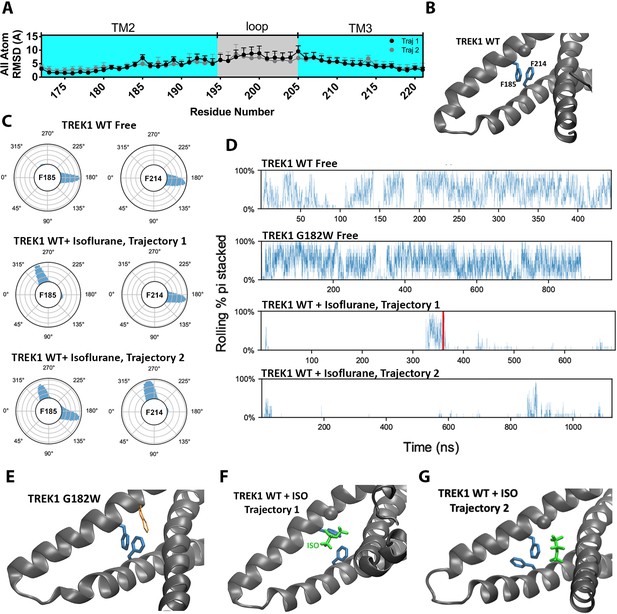
The presence of the isoflurane ligand disrupts a key TM2/TM3 interaction.
(A) All atom per residue RMSD within the TREK1 TM2/TM3 loop from TREK1 wildtype (WT) + Isoflurane Trajectory 1 (black symbols) or Trajectory 2 (gray symbols), as compared with the final frame of the equilibrated TREK1 WT-Free simulation. (B) A representative equilibrium MD simulation snapshot of TREK1 WT. The G182 residue is represented as a sphere and the pi-stacking interaction between F185 and F214 is shown. (C) Side chain χ 1 dihedral angle residence plots for residue F185 and F214 during the TREK1 WT-free (top) and TREK1 WT + isoflurane simulation trajectories (middle, bottom) (D) Pi-stacking plots for the F185/F214 residue interaction, examined by sampling the average number of pi-stacked snapshots over a rolling window of 10 snapshots spanning every 200 fs across the entire simulation timescale. The red bar in the TREK1 WT + Isoflurane trajectory one panel represents the time when isoflurane escaped the binding pocket during this simulation. (E) Representative equilibrium MD simulations snapshots of G182W, showing retained pi-stacking of the F185 and F214 residues or (G,H) simulation snapshots of TREK1 in the presence of isoflurane with disrupted F185/F214 pi-stacking.
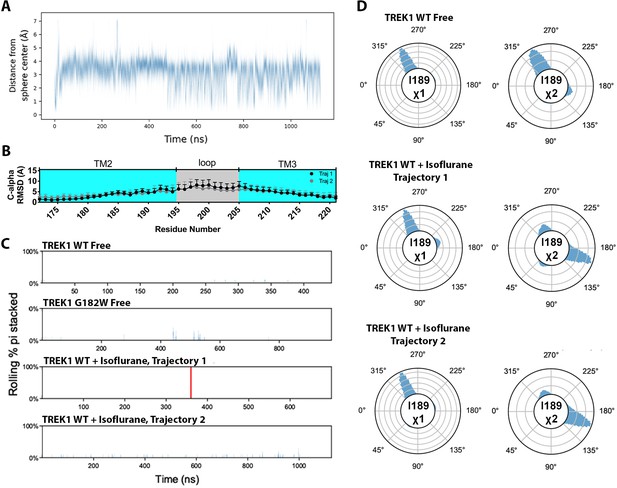
Additional molecular dynamics simulation data.
(A) Distance of the center of geometry of the isoflurane ligand from the center of the spherical restraint, of radius 7A, as a function of simulation time during TREK1 wildtype (WT) + isoflurane trajectory 2 (B) C-alpha limited per residue RMSD within the TREK1 TM2/TM3 loop from TREK1 WT + isoflurane trajectory 1 (black symbols) or trajectory 2 (gray symbols), as compared with the final frame of the equilibrated TREK1 WT-free simulation. (C) Pi-stacking plots, as described in the Figure 6 legend, for the F185/F214 residue interaction in the unliganded neighboring TREK1 subunit. (D) Side chain χ 1 and χ 2 dihedral angle residence plots for mTREK1 I189 during the TREK1 WT-free (top) and TREK1 WT + isoflurane simulation trajectories (middle, bottom).
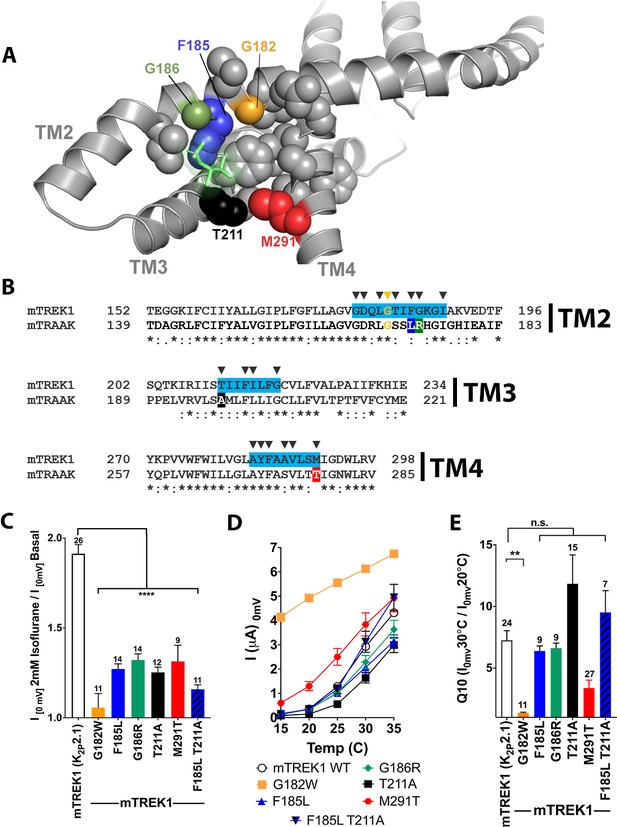
Mutations in the isoflurane-binding site alter anesthetic sensitivity without perturbing global channel function.
(A) Representative MD) snapshot showing the isoflurane-binding site, including residues predicted to have >20% occupancy by isoflurane shown in sphere representation and isoflurane shown in stick representation (lime). (B) Alignments of mouse TREK1 and TRAAK sequences, with the isoflurane-binding domain regions of TREK1 TM2, TM3, and TM4 (as identified by MD simulation) highlighted in blue. Arrows denote positions of high isoflurane occupancy. Poorly conserved residues are color coded throughout the figure [F185 (blue), G186 (green), T211 (red), and M291 (black)]. (C) Quantification of TREK1 wildtype and mutant responses to 2 mM isoflurane administration, (D) temperature, as measured by TREK1 current at 0 mV, or (E) temperature dependence as measured by Q10 (30°C/20°C). Number of replicate experiments indicated. Error bars are mean ± SEM. Statistically significance was determined by one-way ANOVA combined with a Dunnetts multiple comparison test against mTREK1 WT data, results indicated, **p<0.05,****p<0.0005.
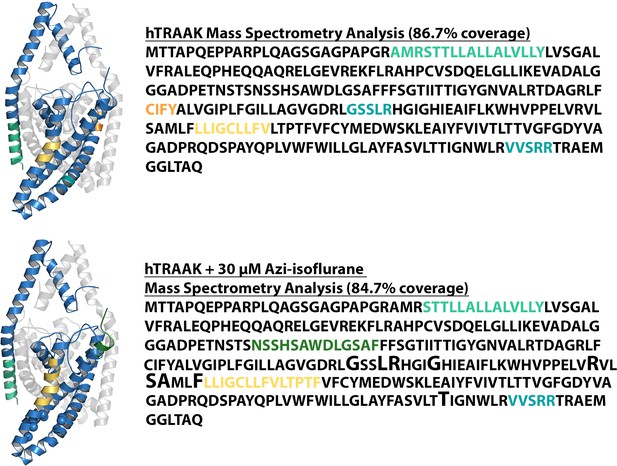
Mass spectrometry (MS) analysis of purified human TRAAK.
Results of MS analysis of TRAAK in the absence of reaction with azi-isoflurane (top) or following reaction with 30 μM azi-isoflurane (bottom). Regions positively identified by MS analysis are shown in blue in the TRAAK structural model (PDB ID 4WFE) and in black font in the sequence data. Regions absent from MS data are displayed in matching color in both the structural model and the sequence data. TRAAK residues homologous to TREK1 positions that exhibit high isoflurane occupancy in MD simulation are displayed as spheres in the structural model of TRAAK and are denoted in the sequence data by an enlarged font. All these residues are identified by MS, but none show evidence of azi-isoflurane labeling. A group of residues in the C-terminal region of TRAAK (marked in gray in the sequence data) were absent from our MS analysis but are not present in the TRAAK structural model.
Tables
Occupancy of residues in G182 isoflurane-binding pocket, defined as the percentage of snapshots where isoflurane within 7 Å of the given residue during two independent molecular dynamics (MD) simulation trajectories.
Residues with occupancy of less than 20% in both trajectories are omitted. TREK1 residues homologous to positions previously found to mediate volatile anesthetic (VA) sensitivity in TASK K2P channels are annotated (*).
| Mouse TREK1 residue number | Percent occupancy during MD trajectory 1 | Percent occupancy during MD trajectory 2 | Transmembrane domain |
|---|---|---|---|
| GLY178 | 47.65% | 79.20% | TM2 |
| ASP179 | 35.19% | 35.36% | TM2 |
| LEU181 | 68.40% | 91.72% | TM2 |
| GLY182 | 94.01% | 98.65% | TM2 |
| THR183 | 69.07% | 41.84% | TM2 |
| PHE185* | 92.17% | 97.89% | TM2 |
| GLY186 | 89.16% | 57.30% | TM2 |
| ILE189 | 52.96% | 10.07% | TM2 |
| THR211* | 56.20% | 88.69% | TM3 |
| PHE214 | 44.39% | 82.77% | TM3 |
| ILE215 | 25.65% | 95.46% | TM3 |
| GLY218 | 9.25% | 61.26% | TM3 |
| ALA283 | 28.07% | 61.74% | TM4 |
| TYR284 | 67.95% | 96.27% | TM4 |
| PHE285 | 27.41% | 41.74% | TM4 |
| ALA287 | 88.05% | 99.48% | TM4 |
| VAL288 | 78.20% | 94.79% | TM4 |
| MET291* | 56.11% | 37.02% | TM4 |





