Artemisinin exposure at the ring or trophozoite stage impacts Plasmodium falciparum sexual conversion differently
Figures
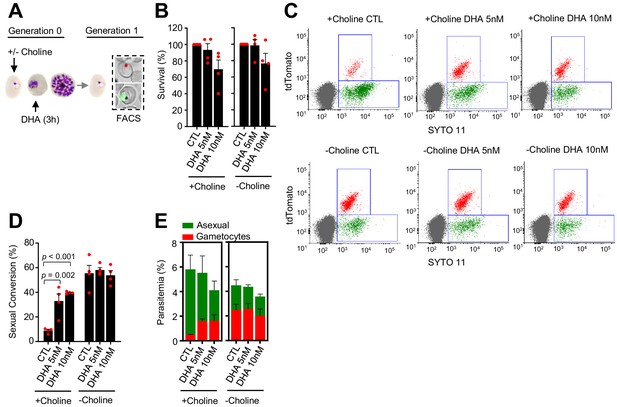
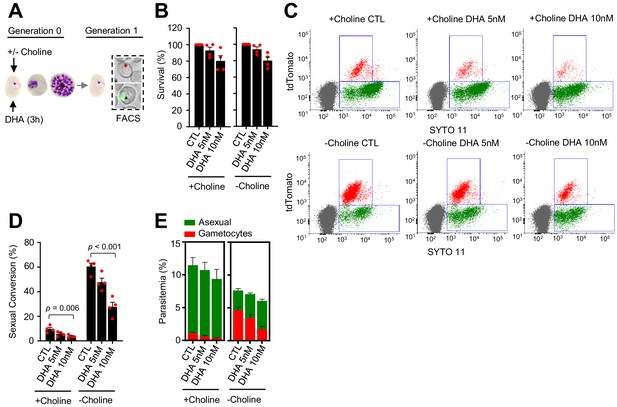
Effect of a dihydroartemisinin (DHA) pulse at the trophozoite stage on sexual conversion.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Representative SYTO 11 (stains parasite DNA) versus TdTomato (marks gametocytes) flow cytometry plots. (D) Sexual conversion rate determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (E) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel D). In all panels, data are presented as the average and s.e.m. of four independent biological replicates.
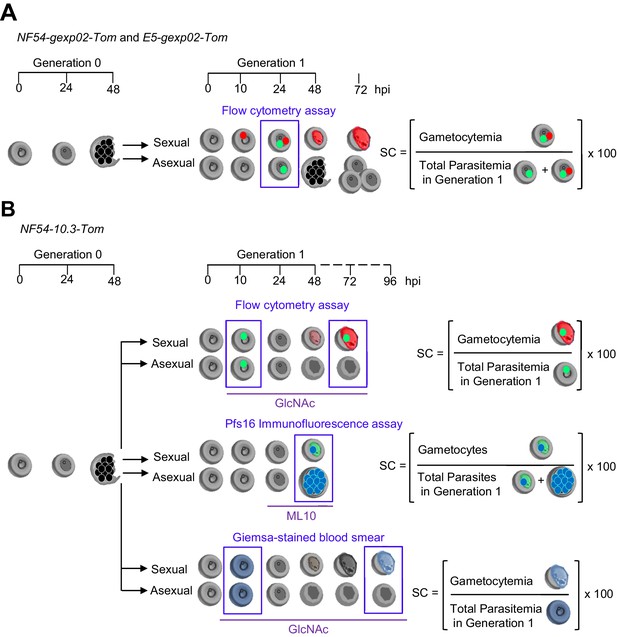
Schematic representation of the assays to determine sexual conversion rates.
We used parasite lines expressing the fluorescent reporter tdTomato under the control of the gexp02 (A) or etramp10.3 (B) promoters. Generation 0 is the growth cycle at which the culture is exposed to different conditions (e.g., choline removal, or drugs), with parasite age indicated in h post-invasion (hpi). In all cases, the sexual conversion rate (SC) is calculated as the percentage of Generation 1 parasites that develop into non-replicative sexual forms (gametocytes). The total parasitemia (sexual + asexual parasites) at Generation 1 is measured by flow cytometry using fluorescent markers that stain DNA in all parasites (green dots) or active mitochondria in viable parasites (not represented here), by immunofluorescence assay using a fluorescent DNA stain (blue dots) or by light microscopy using Giemsa-stained smears (pale blue marks). Gametocytemia is measured by flow cytometry based on tdTomato signal (red marks), by immunofluorescence based on Pfs16 signal (pale green marks), or by light microscopy using Giemsa-stained smears (gametocyte-shaped pale blue marks). In immunofluorescence assays, the number of parasites and gametocytes in a given number of fields is determined instead of parasitemia and gametocytemia, but since both total parasites and gametocytes are determined simultaneously from the same samples (i.e., the total erythrocytes denominator is the same for both parameters), this does not affect the sexual conversion rate calculation. Detection of gametocytes based on tdTomato expression in the NF54-gexp02-Tom and E5-gexp02-Tom lines is performed as early as 24 hpi in Generation 1. Detection based on Pfs16 expression is performed at 48 hpi, and requires addition of ML10 to prevent schizont bursting and reinvasion, which would increase the number of asexual parasites observed. Detection of gametocytes based on tdTomato expression or light microscopy analysis of Giemsa-stained smears in the NF54-10.3-Tom line is performed at 72 or 96 hpi, respectively, and requires the addition of N-acetyl-D-glucosamine (GlcNAc) to prevent multiplication of asexual parasites, which would collapse the culture.
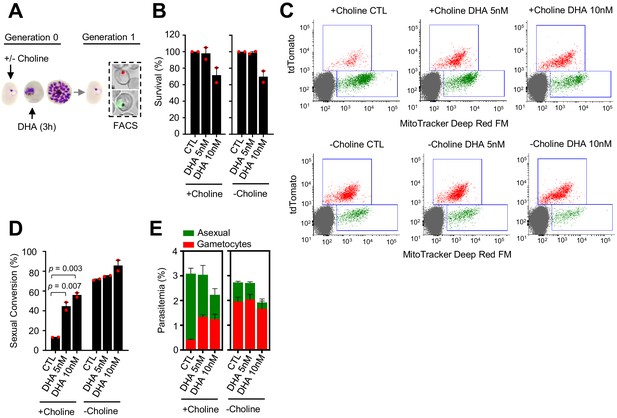
Effect of a dihydroartemisinin (DHA) pulse at the trophozoite stage on sexual conversion, determined using MitoTracker to identify viable parasites.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia of live parasites (asexual + sexual parasites) determined with a mitochondrial membrane potential stain (MitoTracker Deep Red FM). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Representative MitoTracker Deep Red FM versus TdTomato (marks gametocytes) flow cytometry dot plots. (D) Sexual conversion rates determined by flow cytometry, calculated using MitoTracker-positive cells only. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (E) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel D). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.

Effect of a dihydroartemisinin (DHA) pulse at the trophozoite stage on sexual conversion in the E5-gexp02-Tom line.
(A) Schematic representation of the assay. Tightly synchronized cultures of the E5-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites) based on identification of all parasites with SYTO 11 or viable parasites only with MitoTracker Deep Red FM. For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
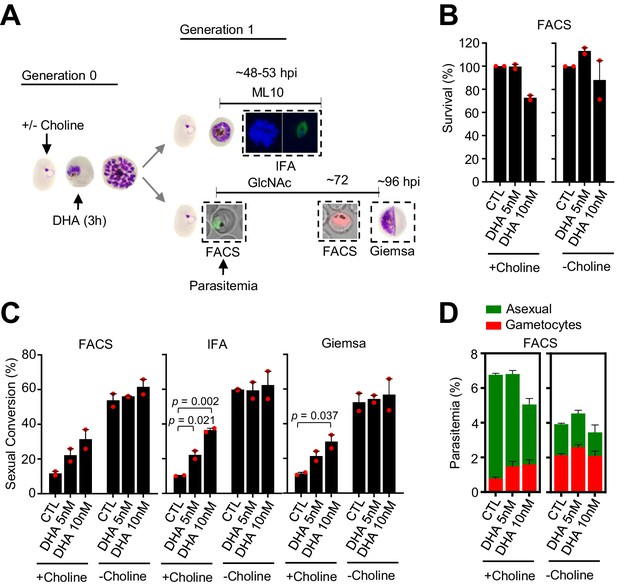
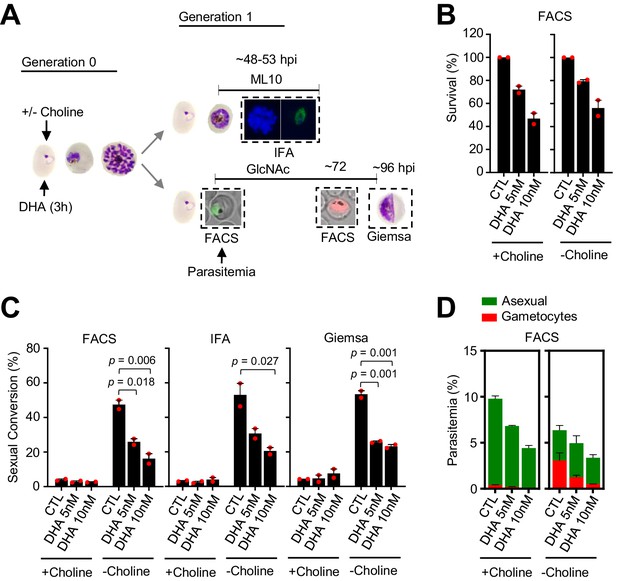
Effect of a dihydroartemisinin (DHA) pulse at the trophozoite stage in the NF54-10.3-Tom line on sexual conversion, determined by three different methods.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-10.3-Tom line (expression of the fluorescence reporter starts later during gametocyte development than in the NF54-gexp02-Tom line) under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by (i) flow cytometry analysis (FACS) on D1 (D0 is the first day of Generation 1) to determine the initial parasitemia (using the SYTO 11 stain), and on D3 to determine the gametocytemia (SYTO 11 and TdTomato signal) in cultures treated with N-acetylglucosamine (GlcNAc); (ii) immunofluorescence assay (IFA) analysis of cultures treated with ML10 using the Pfs16 marker; (iii) flow cytometry analysis on D1 to determine the initial parasitemia, and on D4 light microscopy analysis of Giemsa-stained blood smears (Giemsa) to determine the gametocytemia in cultures treated with GlcNAc. (B) Survival rate of cultures exposed to the different drug doses, using total D1 parasitemia values (asexual + sexual parasites) determined by flow cytometry (FACS). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by FACS, IFA, and Giemsa-stained blood smears. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites, determined by flow cytometry (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
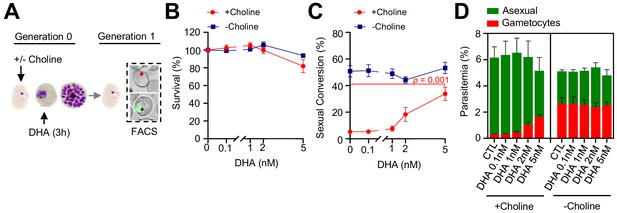
Effect of a low concentration dihydroartemisinin (DHA) pulse at the trophozoite stage on sexual conversion.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at various low doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites) based on identification of all parasites using SYTO 11. For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.
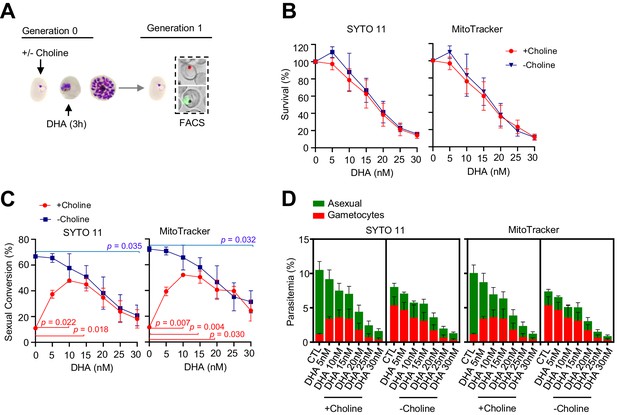
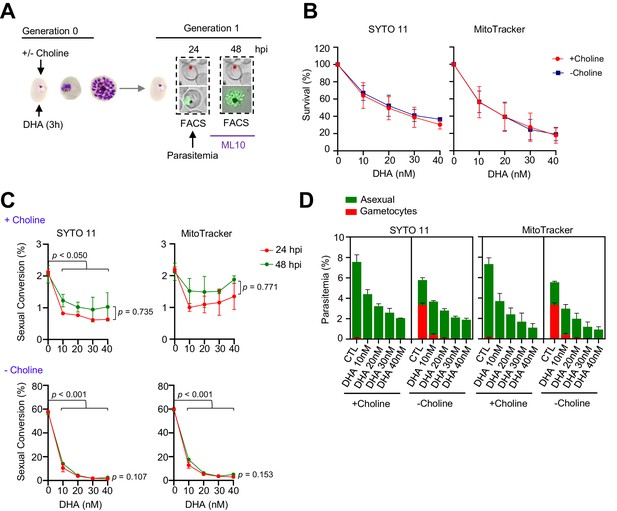
Effect on sexual conversion of a dihydroartemisinin (DHA) pulse at 5–30 nM concentrations during the trophozoite stage.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at various doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites) based on identification of all parasites or viable parasites only, with SYTO 11 or MitoTracker Deep Red FM, respectively. For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.
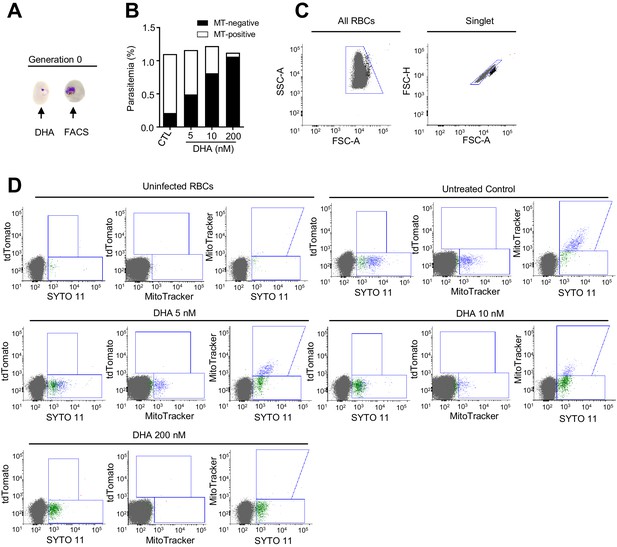
Flow cytometry set-up for the identification of viable parasites in the 3D7-A parasite line using MitoTracker.
(A) Schematic representation of the assay. Tightly synchronized cultures of the 3D7-A wild-type parasite line (does not have the TdTomato gene, does not produce gametocytes) were exposed to a 3 hr dihydroartemisinin (DHA) pulse at subcurative doses at the ring stage (0–20 hpi) or maintained under a lethal dose (200 nM, ‘kill’ control) for ~24 hr. Flow cytometry measurements were performed the next day, within the same asexual cycle. (B) Total parasitemia as determined using SYTO-11, and the distribution of MitoTracker (MT) Deep Red FM-positive (viable) and –negative (non-viable and non-stained) parasites after DHA exposure. (C) Dot plots of the initial gating strategy. The red blood cell (RBC) population was gated first for cell granularity and size (SSC-A versus FSC-A plot) and then for a defined singlet population (FSC-H versus FSC-A plot). (D) Flow cytometry dot plots for MitoTracker Deep Red FM, SYTO 11, and TdTomato (marks gametocytes in the gametocyte-reporter lines). Some presumably healthy parasites were not stained with MitoTracker, as revealed by the presence of MitoTracker-negative/SYTO 11-positive parasites in the no-drug control.
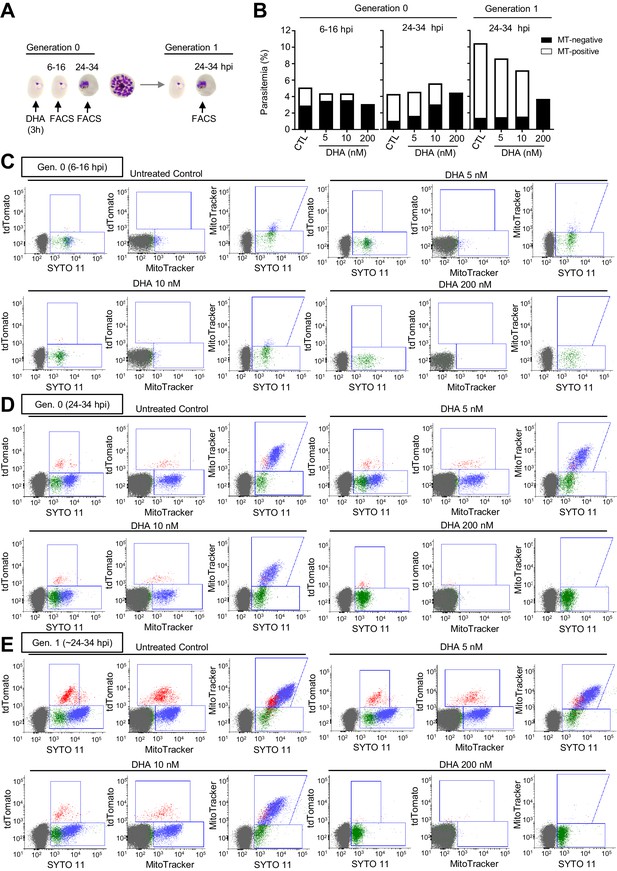
Flow cytometry set-up for the identification of viable parasites in the NF54-gexp02-Tom line using MitoTracker.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line were exposed to a 3 hr dihydroartemisinin (DHA) pulse at subcurative doses at the ring stage (0–10 hpi) or maintained under a lethal dose (200 nM, ‘kill’ control) for up to 48 hr. Flow cytometry measurements were performed at different times within the same cycle, and after reinvasion, as indicated. (B) Total parasitemia as determined using SYTO-11, and distribution of MitoTracker (MT) Deep Red FM-positive (viable) and –negative (non-viable and non-stained) parasites after DHA exposure. (C–E) Flow cytometry dot plots for MitoTracker Deep Red FM, SYTO 11, and TdTomato (marks gametocytes) at 6–16 hpi (C) and 24–34 hpi (D) of the same cycle of DHA treatment, and ~24–34 hpi of the next cycle (E). Some presumably healthy parasites were not stained with MitoTracker, as revealed by the presence of MitoTracker-negative/SYTO 11-positive parasites in the no-drug control (especially in ring-stage cultures).
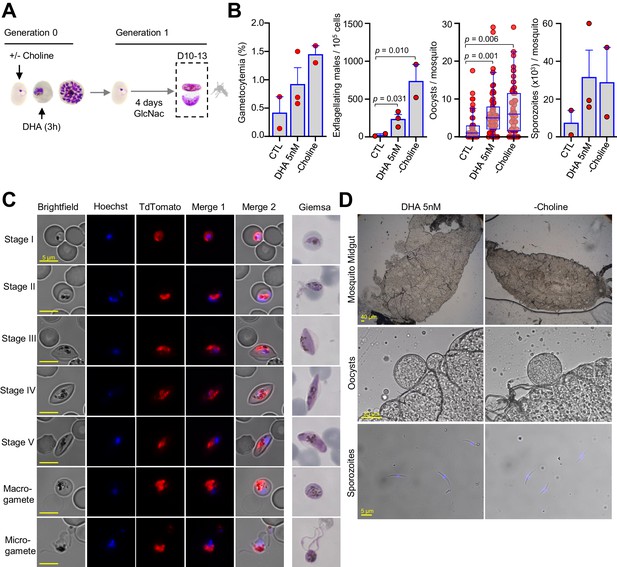
Mosquito infection by gametocytes from cultures exposed to dihydroartemisinin (DHA).
(A) Schematic representation of the assay. Sorbitol-synchronized cultures of the NF54-gexp02-Tom line were maintained under control non-inducing conditions (+ choline, CTL), exposed to a 3 hr 5 nM DHA pulse (in the presence of choline) at the trophozoite stage, or maintained in the absence of choline (– choline, used as a positive control for a gametocyte-inducing condition). On the first day of Generation 1, N-acetylglucosamine (GlcNAc) was added and maintained for 4 d to eliminate asexual parasites and obtain pure gametocyte cultures. The different cultures were used to infect Anopheles mosquitoes by standard membrane feeding. (B) Gametocytemia at the time of mosquito infection (10–13 d after DHA treatment), exflagellation levels (after 10 min of activation with fetal calf serum), number of oocysts/mosquito (n = 53 for CTL, n = 103 for DHA 5 nM, and n = 79 for – choline, data for all individually dissected mosquitoes from all replicates is shown) and average number of sporozoites/mosquito in each independent biological replicate (obtained from pooled dissections; in total, n = 65 for CTL; n = 111 for DHA; n = 123 for – choline). Results are from three independent biological replicates, but in one experiment the CTL culture was lost and in another one the – choline control was not included. Data are presented as the average and s.e.m. of the independent biological replicates, except for oocysts/mosquito results that are presented as standard box and whisker plots. The p-value is indicated only for significant differences (p<0.05) between conditions. (C) Representative images of gametocytes at different stages and activated gametes from DHA-treated cultures, showing no apparent abnormality. Images from live cell fluorescence analysis (Hoechst stains nuclei; TdTomato is expressed under the control of the gexp02 promoter) and Giemsa-stained smears are shown. (D) Representative images of mosquito midguts (transparent, circular structures are oocysts), oocysts, and sporozoites from DHA-treated and – choline cultures, showing no apparent abnormality.
Effect of a dihydroartemisinin (DHA) pulse at the ring stage on sexual conversion.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the early ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–40 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Representative SYTO 11 (stains parasite DNA) versus TdTomato (marks gametocytes) flow cytometry plots. (D) Sexual conversion rate determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (E) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel D). In all panels, data are presented as the average and s.e.m. of four independent biological replicates.

Effect of a dihydroartemisinin (DHA) pulse at the ring stage on sexual conversion, determined using MitoTracker to identify viable parasites.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the early ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–40 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia of live parasites (asexual + sexual parasites) determined with a mitochondrial membrane potential stain (MitoTracker Deep Red FM). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Representative MitoTracker Deep Red FM versus TdTomato (marks gametocytes) flow cytometry dot plots. (D) Sexual conversion rates determined by flow cytometry, calculated using MitoTracker-positive cells only. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (E) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel D). In all panels, data are presented as the average and s.e.m. of four independent biological replicates.
Effect on sexual conversion of a dihydroartemisinin (DHA) pulse at the ring stage in the NF54-10.3-Tom line, determined by three different methods.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-10.3-Tom line (expression of the fluorescence reporter starts later during gametocyte development than in the NF54-gexp02-Tom line) under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the ring stage (1–6 hpi). Sexual conversion was measured by (i) flow cytometry analysis (FACS) on D1 (D0 is the first day of Generation 1) to determine the initial parasitemia (using the SYTO 11 stain), and on D3 to determine the gametocytemia (SYTO 11 and TdTomato signal) in cultures treated with N-acetylglucosamine (GlcNAc); (ii) immunofluorescence assay (IFA) analysis of cultures treated with ML10 using the Pfs16 marker; (iii) flow cytometry analysis on D1 to determine the initial parasitemia, and on D4 light microscopy analysis of Giemsa-stained blood smears (Giemsa) to determine the gametocytemia in cultures treated with GlcNAc. (B) Survival rate of cultures exposed to different drug doses, using total D1 parasitemia values (asexual + sexual parasites) determined by flow cytometry (FACS). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates as determined by FACS, IFA, and Giemsa-stained blood smears. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites, determined by flow cytometry (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
Effect on sexual conversion of a dihydroartemisinin (DHA) pulse at 10–40 nM concentrations at the ring stage.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at the early ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion, at 24 or 48 hpi of the next multiplication cycle. To measure sexual conversion at 48 hpi, cultures were treated with ML10 to prevent an additional round of reinvasion that would increase the asexual parasitemia. (B) Survival rate of cultures exposed to the different drug doses, using 24 hpi total parasitemia values (asexual + sexual parasites) based on identification of all parasites or viable parasites only, with SYTO 11 or MitoTracker Deep Red FM, respectively. For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by flow cytometry. The p-value is indicated only for comparison between 24 and 48 hpi readouts of sexual conversion and for treatment versus control (no drug) significant differences (p<0.05); a single value is shown when all drug concentrations tested yielded a similar p-value. A two-way ANOVA test was used (time of readout was included as a variable). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
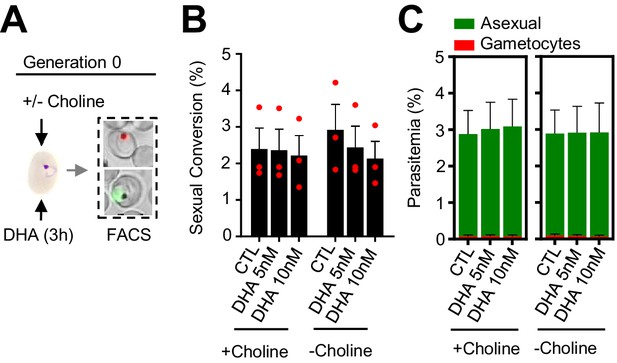
Effect of a dihydroartemisinin (DHA) pulse at the ring stage on sexual conversion by the same cycle conversion (SCC) route.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the early ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) within the same multiplication cycle (~30–40 hpi) to determine the effect of the drug pulse only on production of new gametocytes by the SSC route. (B) Sexual conversion rate determined by flow cytometry. No significant difference (p<0.05) with the control (no drug) was observed for any treatment condition. (C) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel B). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.
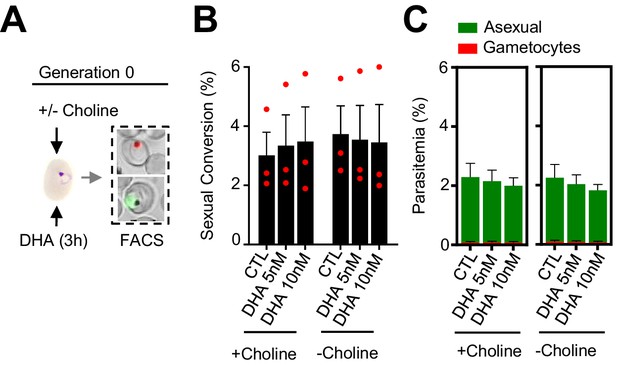
Effect of a dihydroartemisinin (DHA) pulse at the ring stage on sexual conversion by the same cycle conversion (SCC) route, determined using MitoTracker to identify viable parasites.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the early ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) within the same cycle (~30–40 hpi) to determine the effect of the drug pulse only on production of new gametocytes by the SSC route. (B) Sexual conversion rates determined by flow cytometry, calculated using MitoTracker-positive cells only. No significant difference (p<0.05) with the control (no drug) was observed for any treatment condition. (C) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel B). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.
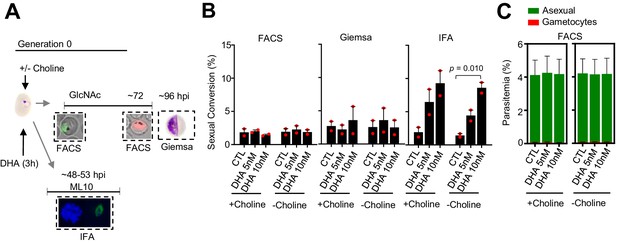
Effect of a dihydroartemisinin (DHA) pulse at the ring stage on sexual conversion by the same cycle conversion (SCC) route in the NF54-10.3-Tom line, determined by three different methods.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-10.3-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the early ring stage (1–6 hpi). Sexual conversion was measured by: (i) flow cytometry analysis (FACS) on D1 (D0 is the first day of Generation 0, that is, the day of drug treatment) to determine the initial parasitemia (using the SYTO 11 stain), and on D3 to determine the gametocytemia (SYTO 11 and TdTomato signal) in cultures treated with N-acetylglucosamine (GlcNAc); (ii) immunofluorescence assay (IFA) analysis of cultures treated with ML10 using the Pfs16 marker; (iii) flow cytometry analysis on D1 to determine the initial parasitemia, and on D4 light microscopy analysis of Giemsa-stained blood smears (Giemsa) to determine the gametocytemia in cultures treated with GlcNAc. (B) Sexual conversion rates as determined by FACS, IFA, and Giemsa-stained blood smears. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (C) Distribution of absolute parasitemia of asexual and sexual parasites, determined by flow cytometry (from the same flow cytometry measurements as in panel B). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
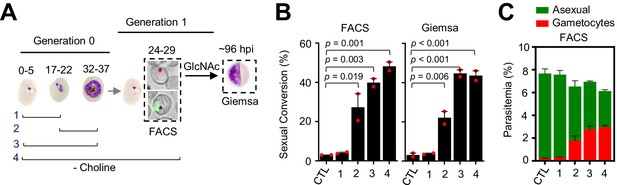
Changes in sexual conversion rates after choline depletion at different parasite stages.
(A) Schematic representation of the assay. Choline was removed from tightly synchronized cultures of the NF54-gexp02-Tom line for the periods indicated, and sexual conversion rates measured after reinvasion by flow cytometry (FACS;~24–29 hpi of the following multiplication cycle) or by light microscopy analysis of Giemsa-stained smears (Giemsa;~96 hpi) in cultures treated with GlcNac. Control (CTL) cultures were maintained with choline all the time. (B) Sexual conversion rate for cultures under different conditions. The p-value is indicated only for choline depletion versus control significant differences (p<0.05). (C) Distribution of absolute parasitemia of asexual and sexual parasites, determined by flow cytometry (from the same flow cytometry measurements as in panel B). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
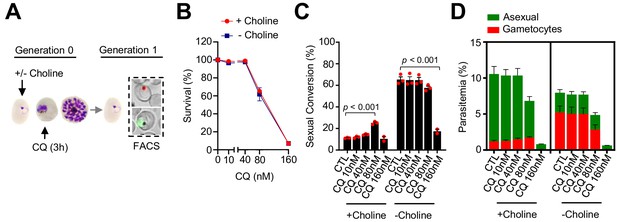
Effect of a chloroquine (CQ) pulse at the trophozoite stage on sexual conversion.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr CQ pulse at subcurative doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rate determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.

Effect of a chloroquine (CQ) pulse at the trophozoite stage on sexual conversion, determined using MitoTracker to identify viable parasites.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr CQ pulse at subcurative doses at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia of live parasites (asexual + sexual parasites) determined with a mitochondrial membrane potential stain (MitoTracker Deep Red FM). For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by flow cytometry, calculated using MitoTracker-positive cells only. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.
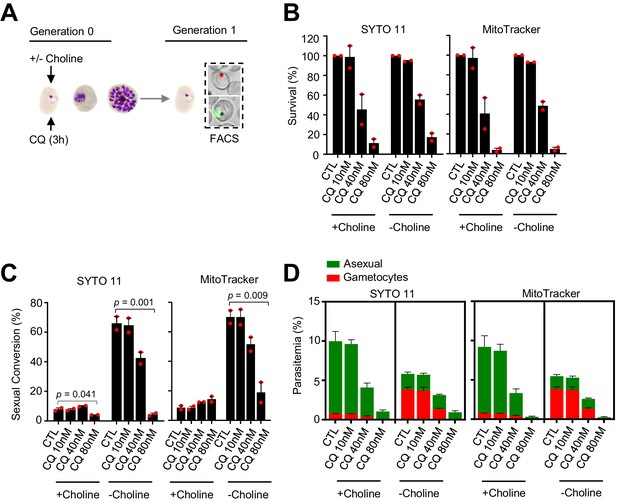
Effect of a chloroquine (CQ) pulse at the ring stage on sexual conversion.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr CQ pulse at subcurative doses at the ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–40 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to the different drug doses, using total parasitemia values (asexual + sexual parasites) based on identification of all parasites with SYTO 11 or viable parasites only with MitoTracker Deep Red FM. For each choline condition, values are presented relative to the parasitemia in the control cultures (no drug). (C) Sexual conversion rates determined by flow cytometry. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.

Effect of a chloroquine (CQ) pulse at the ring stage on sexual conversion by the same cycle conversion (SCC) route.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr CQ pulse at subcurative doses at the early ring stage (0–10 hpi). Sexual conversion was measured by flow cytometry (FACS) within the same cycle (~30–40 hpi) to determine the effect of the drug pulse only on the production of new gametocytes by the SSC route. (B) Sexual conversion rates as determined by flow cytometry using SYTO 11 or MitoTracker Deep Red FM to identify all parasites or viable parasites only, respectively, in addition to TdTomato to identify gametocytes. No significant difference (p<0.05) with the control (no drug) was observed for any treatment condition. (C) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel B). In all panels, data are presented as the average and s.e.m. of two independent biological replicates.
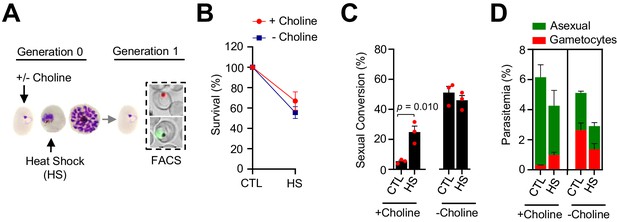
Effect of heat shock at the trophozoite stage on sexual conversion.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr heat shock (41.5°C) at the trophozoite stage (25–30 hpi). Sexual conversion was measured by flow cytometry (FACS) after reinvasion (~30–35 hpi of the next multiplication cycle). (B) Survival rate of cultures exposed to heat shock (HS) or maintained at 37°C (CTL), using total parasitemia values (asexual + sexual parasites). For each choline condition, values are presented relative to the parasitemia in the control cultures. (C) Sexual conversion rate determined by flow cytometry. The p-value is indicated only for heat shock versus control significant differences (p<0.05). (D) Distribution of absolute parasitemia of asexual and sexual parasites (from the same flow cytometry measurements as in panel C). In all panels, data are presented as the average and s.e.m. of three independent biological replicates.
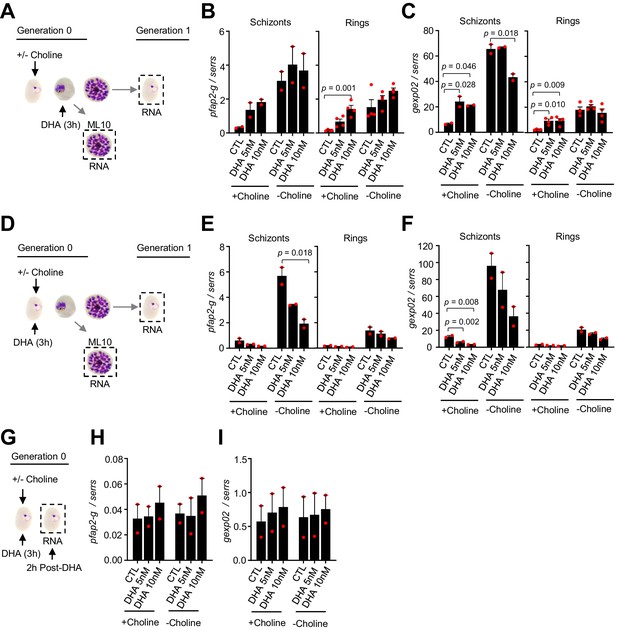
Changes in the expression of pfap2-g and gexp02 after a dihydroartemisinin (DHA) pulse.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the trophozoite stage (25–30 hpi). RNA for transcriptional analysis was collected from ML10-treated cultures at the mature schizont stage (48–53 hpi) and, after reinvasion, from cultures at the early ring stage (cultures not treated with ML10,~5 hpi). (B–C) Transcript levels of pfap2-g (B) or gexp02 (C) normalised against the serine-tRNA ligase (serrs) gene. (D–F) Same as panels A-C, but cultures were exposed to DHA at the ring stage (0–10 hpi). (G–I) Same as panels D-F, but RNA for transcriptional analysis was collected only 2 hr after completing the drug pulse. Data are presented as the average and s.e.m. of four (panels B-C, rings) or two (other panels) independent biological replicates. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05).

Changes in the transcript levels of pfap2-g and gexp02 after a dihydroartemisinin (DHA) pulse, normalized against the uce gene.
(A) Schematic representation of the assay. Tightly synchronized cultures of the NF54-gexp02-Tom line under non-inducing (+ choline) or inducing (– choline) conditions were exposed to a 3 hr DHA pulse at subcurative doses at the trophozoite stage (25–30 hpi). RNA for transcriptional analysis was collected from ML10-treated cultures at the mature schizont stage (48–53 hpi) and, after reinvasion, from cultures at the early ring stage (cultures not treated with ML10,~5 hpi). (B–C) Transcript levels of pfap2-g (B) or gexp02 (C) normalized against the ubiquitin-conjugating enzyme (uce) gene. (D–F) Same as panels A-C, but cultures were exposed to DHA at the ring stage (0–10 hpi). (G–I) Same as panels D-F, but RNA for transcriptional analysis was collected only 2 hr after completing the drug pulse. Data are presented as the average and s.e.m. of four (panels B-C, rings) or two (other panels) independent biological replicates. The p-value is indicated only for treatment versus control (no drug) significant differences (p<0.05).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Plasmodium falciparum) | pfap2-g | PlasmoDB | PF3D7_1222600 | |
| Gene (Plasmodium falciparum) | gexp02 | PlasmoDB | PF3D7_1102500 | |
| Cell line (Plasmodium falciparum) | NF54-gexp02-Tom | PMID:31601834 | Maintained in culture with 2 mM choline | |
| Cell line (Plasmodium falciparum) | E5-gexp02-Tom | PMID:31601834 | Maintained in culture with 2 mM choline | |
| Cell line (Plasmodium falciparum) | NF54-10.3-Tom | PMID:31601834 | Maintained in culture with 2 mM choline | |
| Commercial assay or kit | RNeasy Mini Kit | Qiagen | Cat. No. 74104 | |
| Chemical compound, drug | ML10 | PMID:28874661; S. Osborne (LifeArc) and D. Baker (LSHTM) | cGMP-dependent protein kinase inhibitor | |
| Chemical compound, drug | Dihydroartemisinin (DHA) | Sigma-Aldrich | Cat. No. D7439 | |
| Chemical compound, drug | Chloroquine | Sigma-Aldrich | Cat. No. C6628 | |
| Chemical compound, drug | Choline chloride | Sigma-Aldrich | Cat. No. C7527 | |
| Chemical compound, drug | N-acetyl-d-glucosamine | Sigma-Aldrich | Cat. No. A8625 | |
| Software, algorithm | BD FACSDiva Software | BD Biosciences | RRID:SCR_001456 | Flow cytometryacquisition andanalysis using BD LSRFortessa machine |
| Software, algorithm | Flowing Software version 2.5.1 | Perttu Terho | RRID:SCR_015781 | Flow cytometry data analysis |
| Software, algorithm | Prism 8 | GraphPad | RRID:SCR_002798 | |
| Antibody | Pfs16 (mouse, monoclonal) | R.Sauerwein, Radboud University | 32F717:B02 | IFA (1:400) |
| Antibody | Goat-anti-mouse IgG–Alexa Fluor 488 | Thermo Fisher | Cat. No. A11029 | IFA (1:1000) |
| Other | SYTO 11 | Life Technologies | Cat. No. S7573 | Flow cytometry (0.016 μM) |
| Other | MitoTracker Deep Red FM | Invitrogen | Cat. No. M22426 | Flow cytometry (0.6 µM) |
| Other | DAPI | Applichem lifescience | Cat. No. A4099.0005 | IFA (5 μg/mL) |
| Other | Hoechst 33258 | Thermo Fisher | Cat. No. H3569 | Live cellfluorescence microscopy (2 μM) |





