Distinct effects of complement and of NLRP3- and non-NLRP3 inflammasomes for choroidal neovascularization
Figures
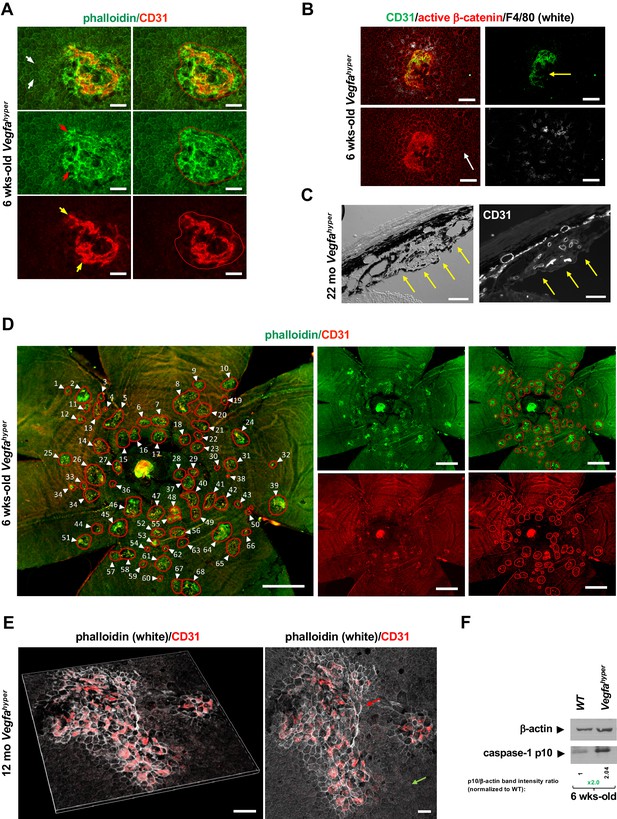
Quantifications of CNV lesions in Vegfahyper mice.
(A) Disrupted honeycomb pattern morphology of RPE cells and sub-RPE protrusions of CD31+ neovessels demarcate a choroidal neovascular lesion in choroidal flat mounts of Vegfahyper mice. A choroidal flat mount of a Vegfahyper mouse is shown. Phalloidin staining highlights RPE cell membranes and shows the regular honeycomb pattern of RPE cells at sites devoid of CNV lesions (white arrows). At the site of a CNV lesion, the typical RPE honeycomb pattern is disrupted (phalloidin, green; red arrows). CD31 (red) staining identifies neovessels of a CNV lesion that protrude into the sub-RPE space (yellow arrows). Disruption of regular RPE cell morphology and CD31 staining of neovessels determine the size of the lesion. A polygon is used to quantify the lesion area. Scale bars, 50 µm. 6-weeks-old mouse. (B) CNV lesion in a 6-weeks-old Vegfahyper mouse shows that RPE disruption occurs only at sites of choroidal neovascularization due to CD31+ neovessels (green) protruding into the sub-RPE space, whereas RPE cells show a normal honeycomb pattern cellular morphology at sites without CNV lesions (white arrow). Labeling for active β-catenin outlines RPE cell membranes (red), similar to phalloidin staining. F4/80+ cells (white) infiltrate sites of CNV lesions. Pigmented cells cover part of the CNV lesion (yellow arrow). Scale bars, 100 µm. (C) Section through a CNV lesion (yellow arrows) in an aged Vegfahyper mouse eye (22-months-old) shows CD31+ neovessels in CNV lesions. Scale bars, 50 µm. (D) Representative image of how CNV lesions were quantified in choroidal flat mounts in mice with the Vegfahyper allele. Measurements of multiple lesions in a choroidal flat mount. A choroidal flat mount of a Vegfahyper mouse is shown in which each CNV lesion is counted (numbered, arrows) and each lesion area shown by a polygon. Phalloidin staining (green) outlines CNV lesions and CD31 immunolabeling (red) detects CD31+ neovessels. Increased phalloidin signal at sites of disruption of the regular RPE morphology occurs only at sites of CD31+ neovessels. Scale bars, 500 µm. 6-weeks-old mouse. (E) Enlarged confluent CNV lesions can be observed in aged Vegfahyper mice. Even at an advanced age, disruption of the honeycomb pattern RPE morphology is strictly localized to the site of CD31+ neovessel protrusions into the sub-RPE space in CNV lesions (red arrow), whereas RPE areas with no CNV maintain their honeycomb pattern (green arrow). The left image shows Z-stack 3D-rendering of CNV lesions in a 12-months-old Vegfahyper mouse eye and the right image shows the projection of this z-stack. CD31+ neovessels of CNV lesions in red; phalloidin in white. Scale bars, 50 µm. (F) RPE/choroid lysates from 6-weeks-old Vegfahyper mice and their WT littermates show that the inflammasome activation product caspase-1 p10 can already be detected at this young age at which CNV lesions were quantified. A > 2 fold increase in p10 levels relative to WT (normalized to β-actin levels) are observed at 6 weeks of age in RPE/choroid lysates of Vegfahyper mice. RPE/choroids of both eyes from four Vegfahyper mice versus four of their WT littermates were pooled. An anti-caspase-1 p10 antibody from Thermo Fisher Scientific was used (PA5-105049) to detect p10.
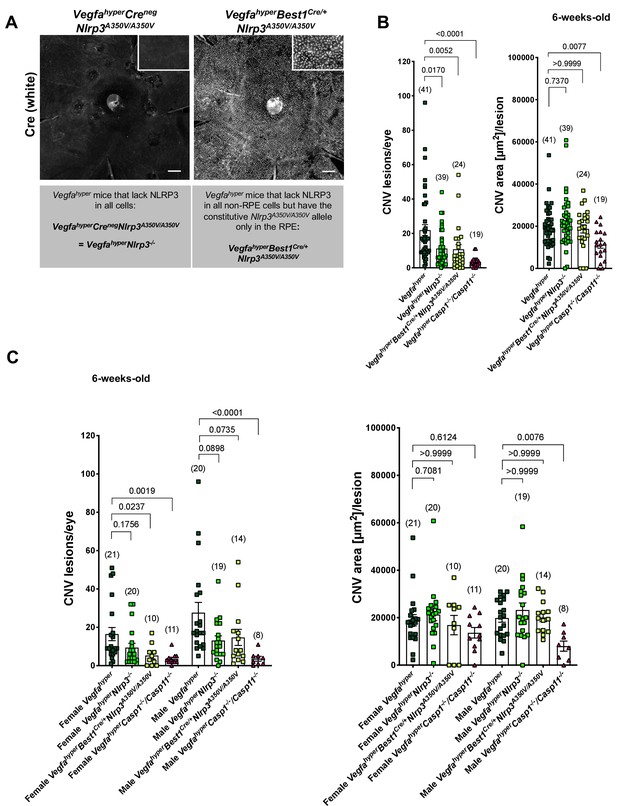
NLRP3 inflammasome activation in non-RPE cells but not in RPE cells promotes CNV.
(A) Nuclear Cre immunostaining (white) in choroidal flat mounts from 6-weeks-old VegfahyperBest1Cre/+Nlrp3A350V/A350V mice confirms uniform Cre expression in the RPE. Inset shows higher magnification image. No Cre staining is seen in VegfahyperCrenegNlrp3A350V/A350V mice ( = VegfahyperNlrp3-/- mice). Scale bars, 200 μm. (B-C). CNV lesion numbers and average CNV lesion area (in μm2/lesion) were measured in 6-weeks-old mice. VegfahyperNlrp3-/- mice (group 2) and VegfahyperBest1Cre/+Nlrp3A350V/A350V mice (group 3) show significantly decreased CNV lesion numbers compared to Vegfahyper mice (group 1), but no significant difference in CNV lesion sizes. CNV lesion sizes and numbers between VegfahyperNlrp3-/- mice and VegfahyperBest1Cre/+Nlrp3A350V/A350V mice are similar. No marked differences in lesion sizes or lesion numbers were observed among male or female groups of VegfahyperNlrp3-/- mice and VegfahyperBest1Cre/+Nlrp3A350V/A350V mice. VegfahyperCasp1-/-Casp11-/- mice (group 4) have fewer CNV lesions than VegfahyperNlrp3-/- mice or VegfahyperBest1Cre/+Nlrp3A350V/A350V mice. Values represent total CNV lesion numbers/eye for each mouse and average lesion area/eye for each mouse. Absolute numbers of mice per group are indicated in parentheses. Graphs show mean ± SEM. P-values are shown and were determined by a Kruskal-Wallis test followed by a Dunn’s test.
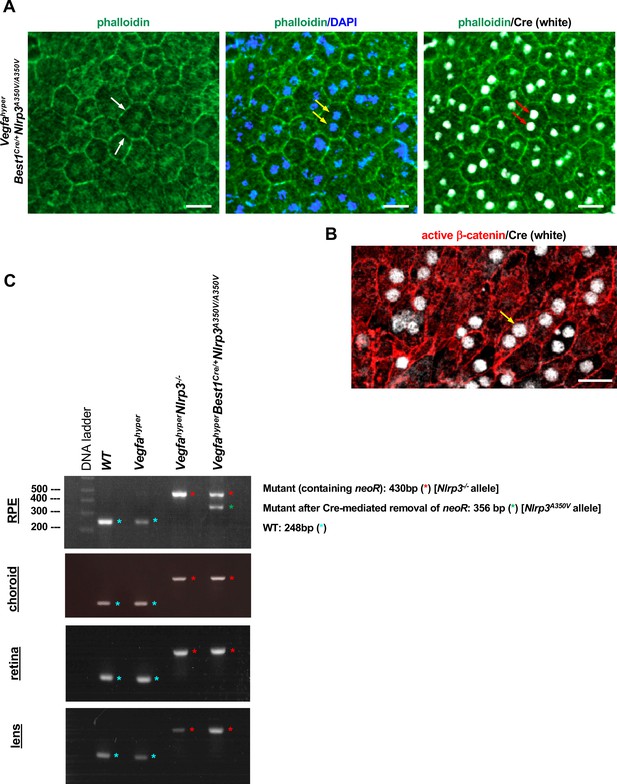
RPE-specific Cre expression in Best1Cre/+ mice mediates the removal of floxed alleles in the RPE.
(A) A choroidal flat mount of a Best1Cre/+ mouse is shown (VegfahyperBest1Cre/+Nlrp3A350V/A350V mouse) that was labeled with phalloidin-Alexa488 (green) and with an anti-Cre antibody (white). Nuclear staining was performed with DAPI. Phalloidin staining (green) highlights cell membranes and shows the honeycomb pattern of RPE cells (white arrows). Best1 expression occurs in the eye only in RPE cells, which can be unambiguously identified by their hexagonal cell structure and the doublet cell nuclei (yellow arrows). Nuclear Best1-specific Cre signal (white; red arrows) is only seen in RPE cells. Scale bars, 20 µm. 6-weeks-old mouse. (B) Nuclear Cre+ immunolabeling (white, yellow arrow) is seen in RPE cells of Best1Cre/+ mice, outlined by immunolabeling for active β-catenin (red). Scale bar, 20 µm. 6-weeks-old mouse. (C) VegfahyperBest1Cre/+Nlrp3A350V/A350V mice show Cre-mediated removal of the floxed neoR cassette in their RPE cells. RPE cells were isolated from the eyes of an adult mouse of each strain: WT, Vegfahyper, VegfahyperNlrp3-/-, and VegfahyperBest1Cre/+Nlrp3A350V/A350V. PCR result of isolated genomic DNA from RPE cells using primers that flank the floxed neoR cassette in intron 2 of Nlrp3 shows the presence of a PCR product without the neoR cassette (Nlrp3A350V allele from the subset of RPE cells in which Cre was active) in addition to a PCR product containing the neoR cassette (Nlrp3-/- allele from the subset of RPE cells in which Cre was not active) in VegfahyperBest1Cre/+Nlrp3A350V/A350V mice. In contrast, RPE cells from VegfahyperNlrp3-/- mice without the Cre show only the PCR product containing the floxed neoR cassette in intron 2 of the Nlrp3 gene (Nlrp3-/- allele). RPE cells from WT and Vegfahyper mice show neither PCR product (only WT allele is seen). This demonstrates that Cre recombinase is not only expressed in the RPE of VegfahyperBest1Cre/+Nlrp3A350V/A350V mice but also active and mediates Cre-mediated excision of floxed alleles that lead to the Nlrp3A350V/A350V mutant alleles. Here, result for a VegfahyperBest1Cre/+Nlrp3A350V/A350V mouse is shown where Cre activity occurs only in a subset of RPE cells to demonstrate the size difference between the mutant alleles with and without the neoR cassette, but for CNV lesion quantifications we only used eyes of VegfahyperBest1Cre/+Nlrp3A350V/A350V mice that showed uniform Cre-expression in the RPE (confirmed by immunolabeling for Cre). No Cre-mediated excision of the floxed neoR cassette was observed in the retina, lens, or choroid in eyes of the identical mice and only the Nlrp3-/- allele was detected in these other eye tissues of VegfahyperBest1Cre/+Nlrp3A350V/A350V mice and VegfahyperNlrp3-/- mice, consistent with an RPE-specific Cre activity in eyes of VegfahyperBest1Cre/+Nlrp3A350V/A350V mice. 2% agarose gel is shown.
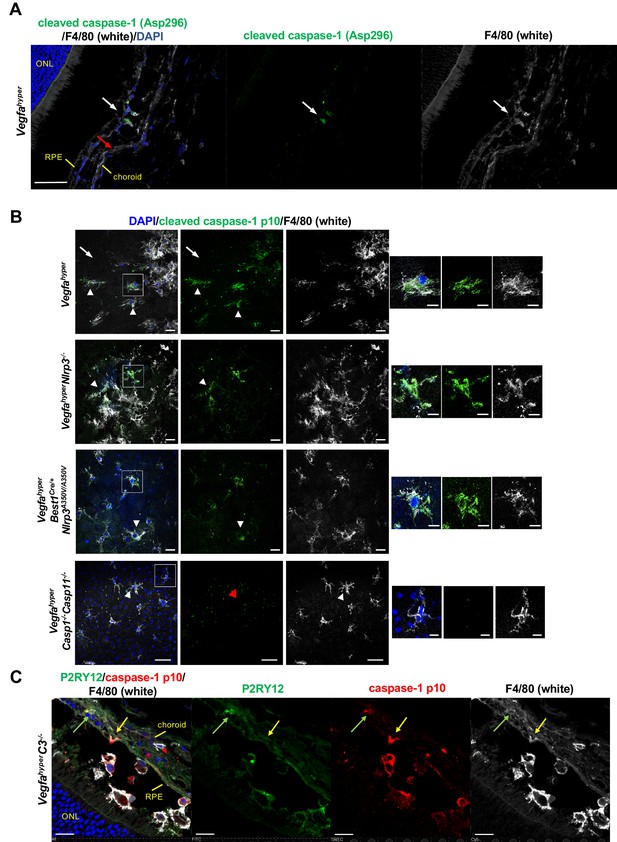
F4/80+ cells in CNV lesions have high levels of caspase-1 protein.
(A) Immunolabeling of eye sections of Vegfahyper mice with an antibody that recognizes endogenous levels of caspase-1 protein only when cleaved at Asp296 that occurs with inflammasome activation but does not detect full-length caspase-1 (Cell Signaling Technology Cat# 89332). F4/80+ cells at the site of a CNV lesion (white) show the presence of cleaved caspase-1 (green, white arrow), whereas this staining is not observed in RPE cells (red arrow) in eyes of Vegfahyper mice. DAPI nuclear staining in blue. Scale bar, 50 μm. Location of the outer nuclear layer of the retina (ONL), choroid, and RPE are shown. (B) Immunolabeling of choroidal flat mounts for caspase-1 p10 (green) shows predominant staining in F4/80+ cells (white, arrowheads) but not in RPE cells (arrows) in CNV lesions. The antibody (sc-22166, Santa Cruz Biotechnology) detects cleaved caspase-1 p10 (inflammasome activation marker) and was raised against a short amino acid sequence containing the neoepitope at Gly315 of caspase-1 of mouse origin. Proteolytic cleavage of the precursor caspase-1 at Gly315 generates the functional caspase-1 subunits, known as p20 and p10 subunits. Choroidal flat mounts from 3-months-old Vegfahyper, VegfahyperNlrp3-/-, and VegfahyperBest1Cre/+Nlrp3A350V/A350V mice are shown. No caspase-1 staining is observed even in aged VegfahyperCasp1-/-Casp11-/- mice (red arrowhead), confirming the specificity of the caspase-1 immunolabeling (a representative flat mount of an 18-months-old mouse is shown). DAPI nuclear staining in blue. Right: Higher magnification images of F4/80+ cells (highlighted in boxes of left images). Scale bars, 20 μm (left) and 10 μm (right). (C) At sites of CNV lesions in mice with the Vegfahyper allele, a subset of infiltrating F4/80+ cells shows immunolabeling for caspase-1 p10 and is also positive for the microglial marker P2RY12 (green arrows). In contrast, other F4/80+p10+ cells show no staining for P2RY12 (yellow arrows). Representative image of a 12-months-old mouse eye with the Vegfahyper allele is shown (here an eye of a VegfahyperC3-/- mouse is shown). Scale bars, 50 μm. DAPI nuclear staining in blue. Location of ONL, choroid, and RPE are shown.
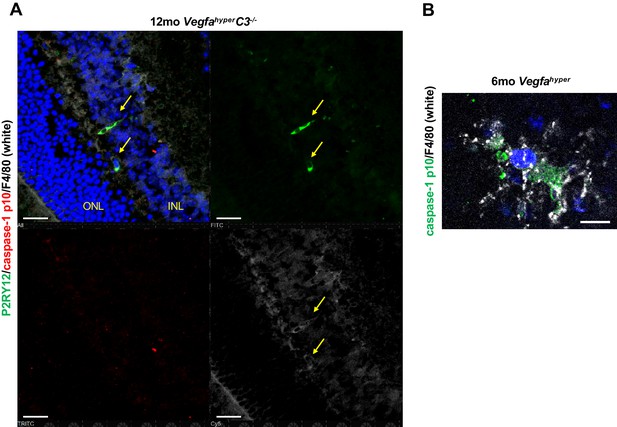
Non-lesional retinal microglia show no caspase-1 p10 immunolabeling.
(A) Immunolabeling for the microglial marker P2RY12, caspase-1 p10, and F4/80 show that at sites devoid of CNV lesions retinal microglia (F4/80+P2RY12+) cells do not show positive labeling for p10. Non-lesional area of the same retina as shown in Figure 3C from a 12-months-old VegfahyperC3-/- mouse is shown. Scale bars, 50 µm. Location of ONL and the inner nuclear layer of the retina (INL) are shown. (B) F4/80+ cells infiltrating CNV lesions in Vegfahyper mice show strong staining for caspase-1 p10 but not adjacent RPE cells. Choroidal flat mount of a 6-months-old Vegfahyper mouse is shown. Scale bar, 10 µm.
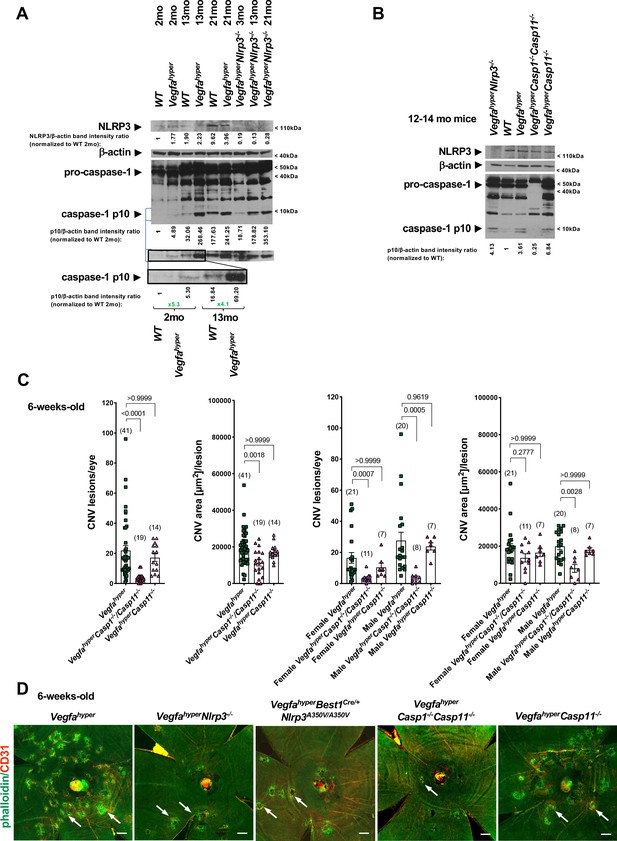
Progressive inflammasome activation in eyes of Vegfahyper mice occurs through both NLRP3-dependent as well as through NLRP3-independent mechanisms.
(A) RPE/choroid lysates of Vegfahyper mice show increased levels of the inflammasome activation marker cleaved caspase-1 p10 compared to age-matched WT mice both at 2 months of age as well as at 13 months of age. Caspase-1 p10 levels increase with progressive age in eyes of both WT and Vegfahyper mice and maintain their relative difference with age. Caspase-1 p10 levels are already markedly increased in RPE/choroid lysates from 2-months-old Vegfahyper mice when compared to their WT littermates (~5 fold). They are further increased in 13-months-old mice with a similar relative increase in Vegfahyper mice when compared to their WT littermates (~4 fold). NLRP3 immunoblotting confirms the presence of NLRP3 in RPE/choroid lysates of Vegfahyper mice already at 2 months of age and shows the lack of NLRP3 protein in VegfahyperNlrp3-/- mice, validating the specificity of the NLRP3 antibody. Lack of NLRP3 does not prevent inflammasome activation in RPE/choroid lysates and VegfahyperNlrp3-/- mice show caspase-1 p10 levels in their eyes that increase with progressive age, similarly as observed in Vegfahyper mice. Four RPE/choroid tissues from four independent mice were pooled for each of the groups indicated. Age of groups are indicated. Ratios of p10/β-actin or NLRP3/β-actin band intensities normalized to 2-months-old WT values are shown. Blue lines indicate p10 bands from the same western blot after re-probing with secondary antibody and longer exposure of the film. Boxed area is magnified and p10 bands are quantified, demonstrating that p10 levels are already increased in 2-months-old Vegfahyper mouse groups. (B) RPE/choroid lysates of Vegfahyper mice, VegfahyperNlrp3-/- mice and VegfahyperCasp11-/- mice show increased levels of caspase-1 p10 compared to those of WT mice. No pro-caspase-1 or p10 were observed in lysates of VegfahyperCasp1-/-Casp11-/- mice, confirming the specificity of the antibody used (Santa Cruz Biotechnology Cat# sc-514). WT mice, Vegfahyper mice and VegfahyperCasp1-/-Casp11-/- mice express NLRP3. Lysates of RPE/choroid tissues from 12 to 14 months-old mice (four RPE/choroids pooled for each group). Ratios of p10/β-actin band intensities normalized to WT values are shown. (C) VegfahyperCasp1-/-Casp11-/- mice show significantly reduced CNV lesion numbers compared to Vegfahyper mice. Significantly smaller CNV lesion sizes were observed in male but not in female VegfahyperCasp1-/-Casp11-/- mice compared to male and female Vegfahyper mice respectively. Comparing VegfahyperCasp11-/- with Vegfahyper mice shows no significant difference in either CNV lesion numbers or their sizes. 6-weeks-old mice. Values represent total CNV lesion numbers/eye for each mouse and average lesion area/eye for each mouse. Absolute numbers of mice per group are indicated in parentheses. Graphs show mean ± SEM. P-values are shown and were determined with a Kruskal-Wallis test followed by a Dunn’s test. (D) Representative choroidal flat mount images show multiple CD31+ CNV lesions (white arrows) in 6-weeks-old Vegfahyper and VegfahyperCasp11-/- mice and much fewer lesions in VegfahyperNlrp3-/-, VegfahyperBest1Cre/+Nlrp3A350V/A350V, and VegfahyperCasp1-/-Casp11-/- mice. Staining for phalloidin in green (outlining CNV lesions prominently) and for CD31 in red. Scale bars, 200 μm. Phalloidin-marked loss of honeycomb morphology of RPE occurs only at sites of CD31+ neovessel protrusion into the sub-RPE space. This can also be seen when assessing red and green channels of these images separately (shown in Figure 4—figure supplement 1).
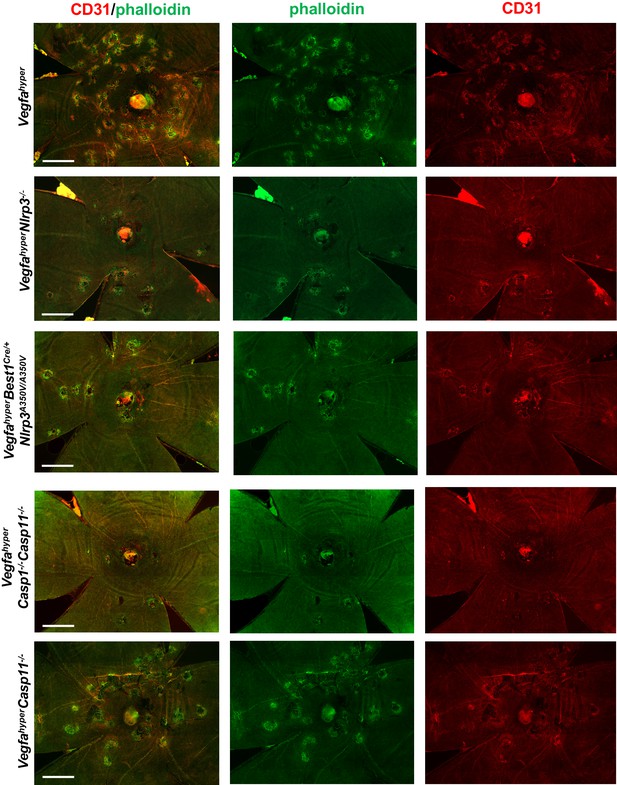
Disruption of honeycomb pattern RPE morphology occurs only at sites of CD31 neovessel protrusions into the sub-RPE space.
Merged and single-channel images (CD31 in red; phalloidin in green) are shown from choroidal flat mounts shown in Figure 4D to demonstrate strict co-localization of RPE abnormalities with CD31 neovessels that protrude into the sub-RPE space. Scale bars, 500 µm.
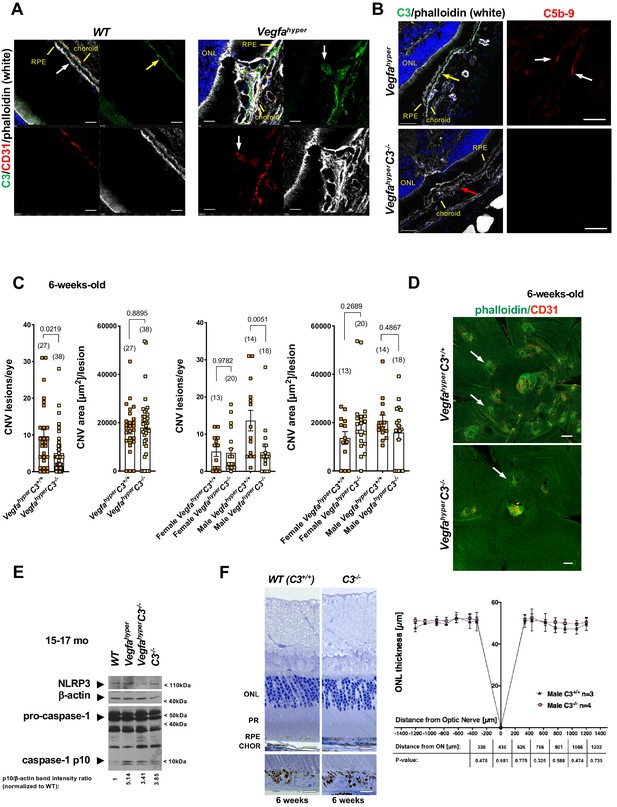
Targeting complement-mediated inflammation inhibits CNV without preventing inflammasome activation.
(A) Left: Complement C3 (yellow arrow, green) is localized particularly along Bruch’s membrane under RPE cells (white arrow) in WT mice. Right: Strong C3 immunolabeling (green) is also observed in CNV lesions of Vegfahyper mice, where it co-localizes with CD31+ neovessel protrusions (red; white arrows). 12-months-old mice. Scale bars, 50 μm. Location of ONL, choroid, and RPE are shown. (B) Left: C3 (green, yellow arrow) is detected in CNV lesions in eyes of Vegfahyper mice but not in CNV lesions in VegfahyperC3-/- mice (CNV lesion shown by a red arrow), confirming loss of C3 protein in C3-/- mice. Right: CNV lesions in Vegfahyper mice show immunolabeling for C5b-9 (red, white arrows), whereas no C5b-9 immunolabeling is detected in CNV lesions in VegfahyperC3-/- mice. 12-months-old mice. Scale bars, 50 μm (left), 20 μm (right). C5b-9 immunolabeling (red) from co-immunolabeling image shown in Figure 5—figure supplement 1. Locations of ONL, choroid, and RPE are shown. (C) VegfahyperC3-/- mice show significantly fewer CNV lesions compared to littermate Vegfahyper mice (VegfahyperC3+/+ mice), but this difference was only observed in male mice. Male Vegfahyper mice have more CNV lesions compared to females, whereas there was no marked difference in lesion numbers for male and female VegfahyperC3-/- mice. Inactivation of C3 did not significantly affect CNV lesion sizes. 6-weeks-old mice. Values represent total CNV lesion numbers/eye for each mouse and average lesion area/eye for each mouse. Absolute numbers of mice per group are indicated in parentheses. Graphs show mean ± SEM. P-values are shown (two-tailed) and were determined with a Mann-Whitney test. (D) Representative choroidal flat mount images of 6-weeks-old male mice show multifocal CD31+ CNV lesions (white arrows) in Vegfahyper mice and significantly fewer CNV lesions in VegfahyperC3-/- mice. Staining with phalloidin in green and immunolabeling for CD31 in red. Scale bars, 200 μm. (E) Lack of complement C3 does not prevent inflammasome activation in eyes of VegfahyperC3-/- mice. Two RPE/choroid tissue lysates were pooled from 15 to 17 months-old mice. Ratios of p10/β-actin band intensities normalized to WT values are shown. (F) C3 deficiency does not cause significant retinal morphological defects at 6 weeks of age. Representative sections through posterior eyes of 6-weeks-old male WT mice and C3-/- mice are shown. Scale bars, top 20 μm, bottom 5 μm. Outer nuclear layer (ONL) thickness was measured at seven distances along the meridian in eyes of 6-weeks-old mice. Values represent average ONL thickness in μm per group. SEMs are shown for each mean. Student’s t-tests were performed for values measured at equal distances from the optic nerve (ON). No significant difference in ONL thickness between WT mice and C3-/- mice was observed. PR: photoreceptors; CHOR: choroid.
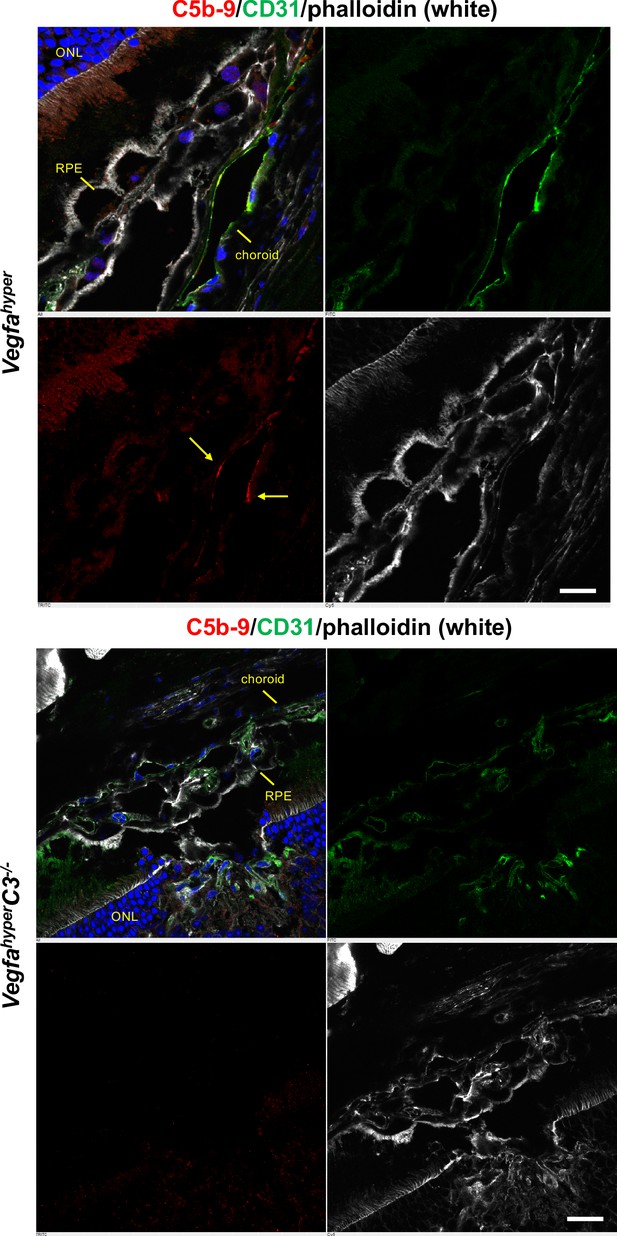
Complement C5b-9 is observed in CNV lesions of Vegfahyper mice, whereas no C5b-9 is observed in CNV lesions of VegfahyperC3-/- mice.
Immunolabeling of eye sections from Vegfahyper mice and VegfahyperC3-/- mice with antibodies against C5b-9 (red) and CD31 (green). Arrows show C5b-9 immunolabeling in CNV lesions of Vegfahyper mice but not in those of VegfahyperC3-/- mice. 12-months-old mice. Phalloidin in white and DAPI in blue (nuclei). Scale bars, 20 µm. Locations of ONL, choroid, and RPE are shown.
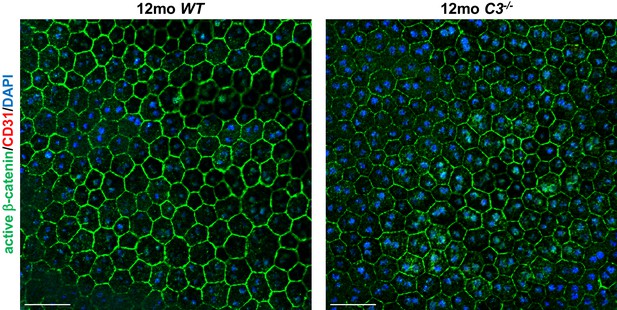
C3-/- mice maintain the typical honeycomb pattern RPE morphology even at 12 months of age.
Choroidal flat mounts of 12 months old C3-/- and littermate WT mice are shown that were immunolabeled for active β-catenin (outlining RPE cell membranes) and for CD31. No CNV lesions occur in 12 months old C3-/- mice. DAPI stains nuclei (blue). Scale bars, 50 µm.
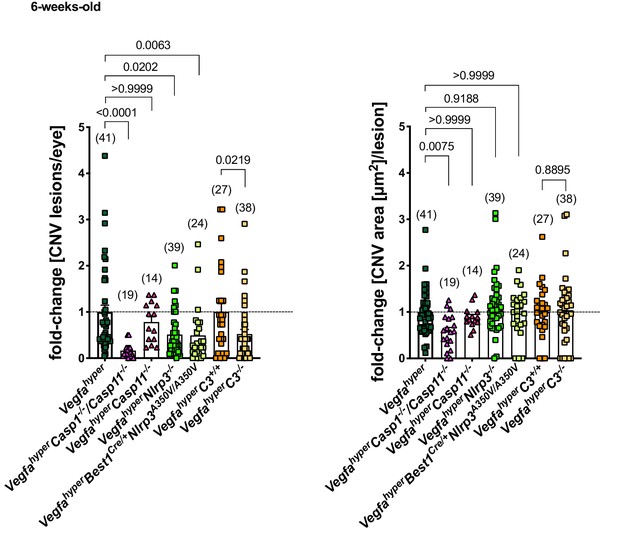
Inactivation of all inflammasomes inhibits CNV lesion formation significantly more than when targeting only NLRP3 or C3.
Comparison of fold-changes in CNV lesion numbers and sizes in all strains relative to Vegfahyper mice. 6-weeks-old mice. VegfahyperC3+/+ mice are littermates of VegfahyperC3-/- mice that are WT for C3. Graphs show mean ± SEM. Absolute numbers of mice per group are indicated in parentheses. P-values are shown. Kruskal-Wallis test followed by Dunn’s test.





