Synaptic memory requires CaMKII
Figures
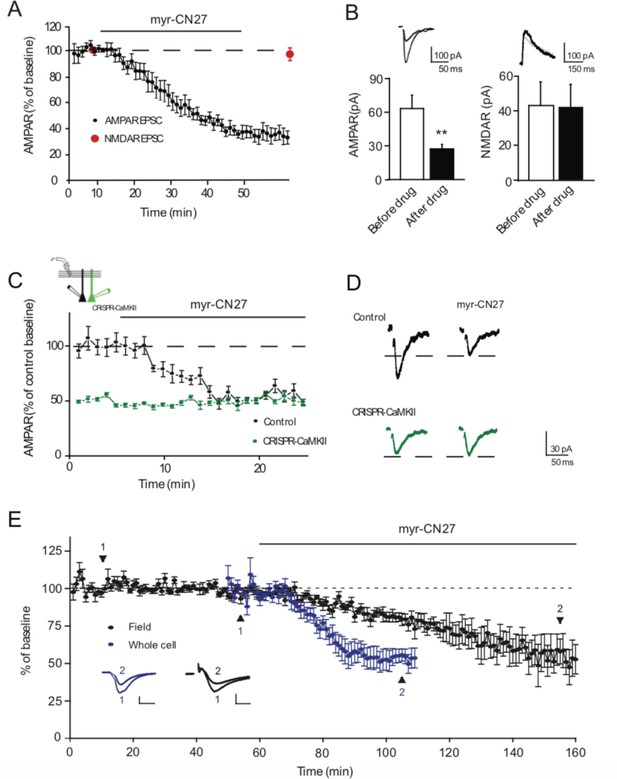
Ca2+-calmodulin-dependent kinase II (CaMKII) inhibitor myr-CN27 inhibits AMPAR synaptic transmission through CaMKIIα.
(A) Time course of myr-CN27 (1 µM) inhibition on AMPAR EPSCs and NMDAR EPSCs in acute slices. (B) Summary graphs showing myr-CN27 inhibited AMPAR EPSCs (before drug: 63.9 ± 12 pA; after drug: 27.6 ± 3.9 pA; n = 10, p < 0.01, two-tailed Wilcoxon Signed Rank Test), but not NMDAR EPSCs (before drug: 43.2 ± 13.7 pA; after drug: 42 ± 13.4 pA; n = 5, p > 0.05, two-tailed Wilcoxon Signed Rank Test). (C) Time course of the effect of myr-CN27 on AMPAR EPSCs in wt cells and simultaneously recorded CRISPR-CaMKIIα transfected cells, normalized to wt baseline (from culture slices). While myr-CN27 inhibited AMPAR EPSCs in wt cells, it had no effect on CRISPR-CaMKIIα transfected cells (n = 6, p > 0.05, two-tailed Wilcoxon Signed Rank Test). (D) Sample traces showing that myr-CN27 inhibited AMPAR EPSCs in control cells, but had no effect in CRISPR-CaMKIIα transfected cells. Black traces are control cell, green traces are transfected cell. Mean ± standard error of the mean (SEM). **p < 0.01. (E) A comparison of the reduction of synaptic transmission in field and whole cell recording. For the field recording (black, n = 8) after a 60-min stable baseline, 1 μM myr-CN27 was applied to the slice for 100 min. For the whole cell recording (blue, n = 7), the drug was applied after a 10-min stable baseline. In both, the response was reduced to 50% of baseline. Example traces for both are shown taken at the timepoints indicated by 1 and 2. The field EPSPs shown on the bottom right have scale bars vertical 0.1 mV and 5 ms horizontal. The whole cell recording shown bottom left have scale bars 50 pA vertical and 20 ms horizontal. Both recordings are done in WT slices with the whole cell recordings done in slice culture DIV3–14 while the fields were done in acute slices from P15–28 mice.
-
Figure 1—source data 1
Ca2+-calmodulin-dependent kinase II (CaMKII) inhibitor myr-CN27 inhibits AMPAR synaptic transmission through CaMKIIα.
In this dataset, the results of the effects of myr-CN27 on AMPAR EPSCs and NMDAR EPSCs are included.
- https://cdn.elifesciences.org/articles/60360/elife-60360-fig1-data1-v2.xlsx
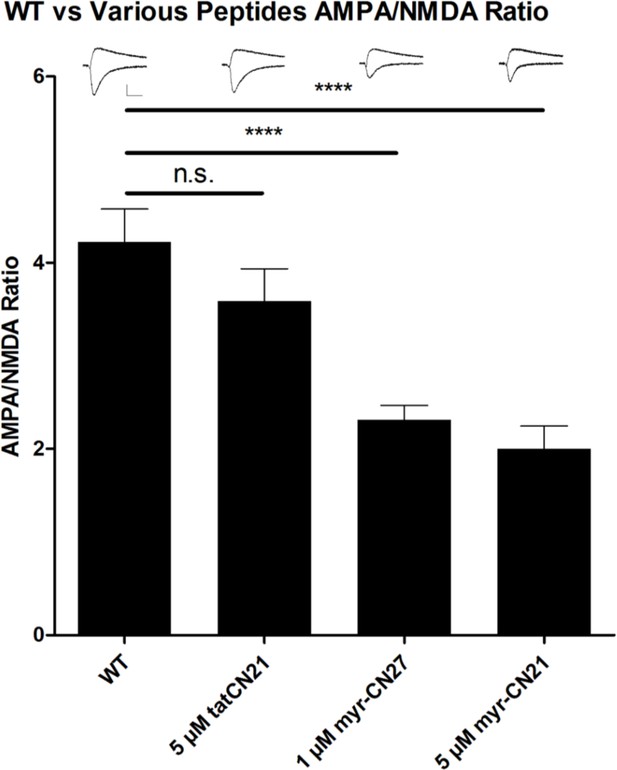
The effect of various Ca2+-calmodulin-dependent kinase II (CaMKII) peptide inhibitors on the AMPAR/NMDAR ratio.
AMPAR/NMDAR ratios recorded in acute slices made from wild-type (WT) mice incubated without inhibitor treatment (WT) (n = 34), with 5 μM tatCN21 (n = 10), with 1 μM myr-CN27 (n = 47), and with 5 μM myr-CN21 (n = 9). Sample traces are shown at top. Scale bar = 50 pA vertical and 30 ms horizontal p < 0.01, Mann–Whitney U test.
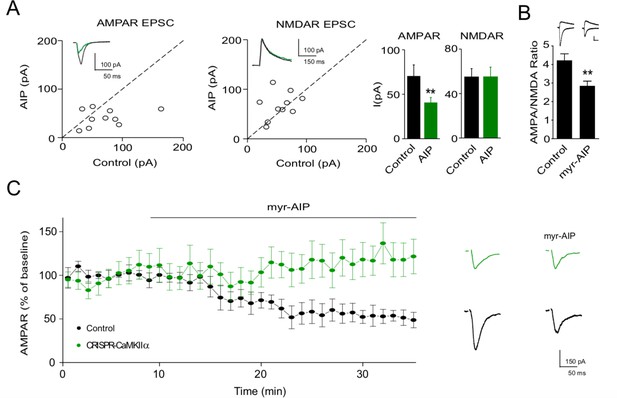
Ca2+-calmodulin-dependent kinase II (CaMKII) inhibitor AIP inhibits AMPAR synaptic transmission through CaMKIIα.
(A) In culture slices, transfection of AIP reduced AMPAR EPSCs (left, n = 10, p < 0.01, two-tailed Wilcoxon Signed Rank Test) but not NMDAR EPSCs (right, n = 10, p > 0.05, two-tailed Wilcoxon Signed Rank Test). Sample traces show the effects of AIP on AMPAR EPSCs and NMDAR EPSCs. (B) AMPA/NMDA ratios compared to wild-type (WT) (n = 34) are reduced after myr-AIP (20 µM) treatment (n = 12). Scale bar = 50 pA vertical and 30 ms horizontal. (C) Left, summary of the effect of myr-AIP (20 µM) on AMPAR EPSCs in wt cells (n = 6) and interleaved CRISPR-CaMKIIα transfected cells (n = 5) from culture slices, normalized to each cell’s baseline. The difference between control and CRISPR-CaMKIIα transfected cells at 30 min: p < 0.01, Mann–Whitney U test. Right, sample traces showing myr-AIP inhibition of AMPAR EPSC in control cells, but not in CRISPR-CaMKIIα transfected cells. Black traces are control cell, green are transfected cell. Mean ± standard error of the mean (SEM).
-
Figure 2—source data 1
Ca2+-calmodulin-dependent kinase II (CaMKII) inhibitor AIP inhibits AMPAR synaptic transmission through CaMKIIα.
In this dataset, the results of the effects of AIP on AMPAR EPSCs and NMDAR EPSCs are included.
- https://cdn.elifesciences.org/articles/60360/elife-60360-fig2-data1-v2.xlsx
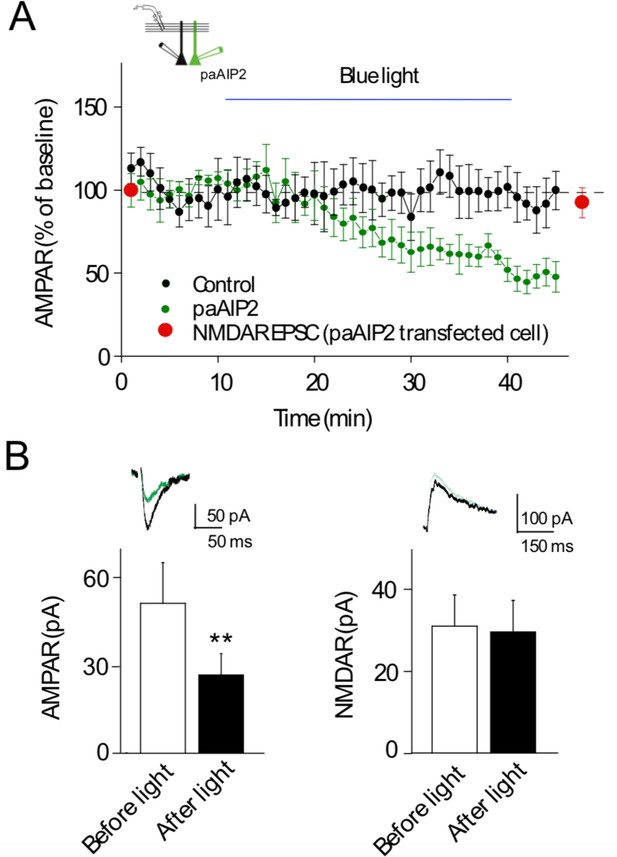
Light-activated Ca2+-calmodulin-dependent kinase II (CaMKII) inhibitor paAIP2 inhibits AMPAR synaptic transmission through CaMKIIα.
(A) Time course of paAIP2 effect on AMPAR and NMDAR synaptic transmission in culture slices. Blue light exposure inhibited AMPAR synaptic transmission in paAIP2 expressing cells (green circles), but not in simultaneously recorded control cells (black circles) or NMDAR synaptic transmission (red circles). (B) Summary data showing that paAIP2 inhibited AMPAR synaptic transmission (before light: 51 ± 13.8 pA; after light: 26.5 ± 7.2 pA; n = 12, p < 0.01, two-tailed Wilcoxon Signed Rank Test), but had no effect on NMDAR synaptic transmission (before light: 31.2 ± 7.6 pA; after light: 29.8 ± 7.7 pA; n = 5, p > 0.05, two-tailed Wilcoxon Signed Rank Test). Mean ± standard error of the mean (SEM). **p < 0.01.
-
Figure 3—source data 1
Light-activated Ca2+-calmodulin-dependent kinase II (CaMKII) inhibitor paAIP2 inhibits AMPAR synaptic transmission through CaMKIIα.
In this dataset, the results of the effects of paAIP2 on AMPAR EPSCs and NMDAR EPSCs are included.
- https://cdn.elifesciences.org/articles/60360/elife-60360-fig3-data1-v2.xlsx
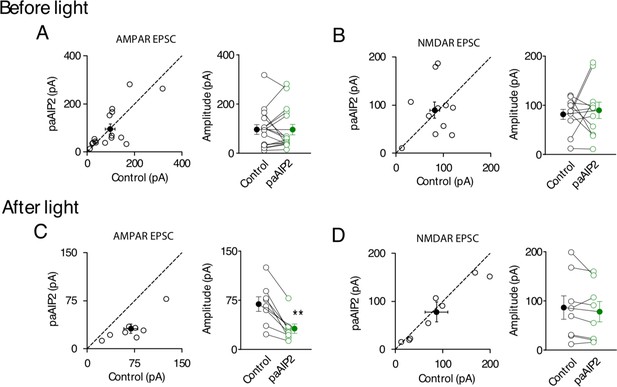
Characterization of synaptic transmission in control cells and paAIP2 transfected cells from culture slices, before and after blue light exposure.
(A, B) transfection of paAIP2 had no effect on AMPAR synaptic transmission (control, 96.4 ± 19.7 pA; paAIP2, 96.1 ± 21.3 pA; n = 16, p > 0.05, two-tailed Wilcoxon Signed Rank Test), or NMDAR synaptic transmission (control, 81.5 ± 10.1 pA; paAIP2, 89.7 ± 16.6 pA; n = 11, p > 0.05, two-tailed Wilcoxon Signed Rank Test). (C, D) blue light exposure reduced AMPAR synaptic transmission in paAIP2 transfected cells (control, 69.1 ± 11.1 pA; paAIP2, 31.7 ± 7.1 pA; n = 8, p < 0.01, two-tailed Wilcoxon Signed Rank Test), but have no effect on NMDAR synaptic transmission (control, 86.5 ± 23.7 pA; paAIP2, 78 ± 20.6 pA; n = 8, p > 0.05, two-tailed Wilcoxon Signed Rank Test). Mean ± standard error of the mean (SEM).
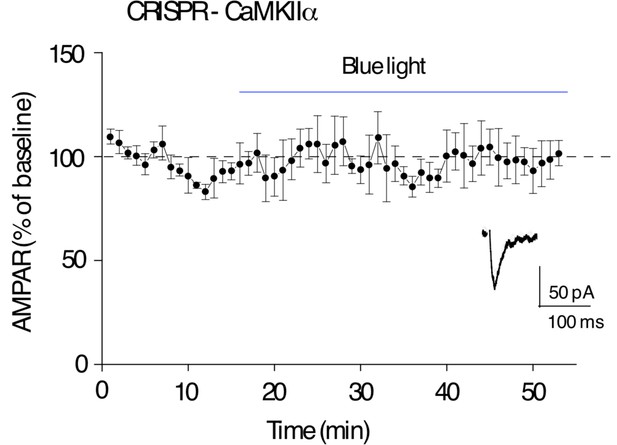
CRISPR deletion of Ca2+-calmodulin-dependent kinase II (CaMKIIα) prevents paAIP2 effect on AMPAR synaptic transmission in culture slices.
Blue light exposure on transfected cells (with coexpression of paAIP2 and CRISPR-CaMKIIα) did not inhibit AMPAR EPSCs (n = 5, p > 0.05, two-tailed Wilcoxon Signed Rank Test). Mean ± standard error of the mean (SEM).
The depression caused by transient inhibition of Ca2+-calmodulin-dependent kinase II (CaMKII) is long lasting and is absent in cells lacking NMDARs.
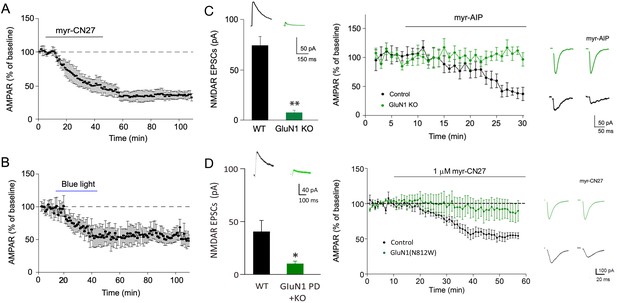
(A) In acute slices, time course showing that following myr-CN27 application AMPAR synaptic transmission remains depressed (the difference between before and after myr-CN27: n = 4, p < 0.01, two-tailed Wilcoxon Signed Rank Test). (B) In culture slices, time course showing that following blue light exposure on paAIP2 expressing cells AMPAR synaptic transmission remains depressed (the difference between before and after blue light exposure: n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test). (C) Left panel, paired recordings from acute slices of control cells and those expressing Cre in GluN1 floxed mice (n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test). Middle panel, time course of the myr-AIP effect on AMPAR synaptic transmission in control cells (n = 5) and interleaved cells expressing Cre in GluN1 floxed mice (n = 5). p < 0.01, Mann–Whitney U test. Right panel, sample traces showing myr-AIP inhibited AMPAR synaptic transmission in control cell (black traces), but not in a GluN1KO cell (green traces). Mean ± standard error of the mean (SEM). (D) Left panel, paired recordings from acute slices of control cells and those expressing Cre and N812W (pore dead, PD) in GluN1 floxed mice (n = 6, p < 0.05, two-tailed Wilcoxon Signed Rank Test). Middle panel, the action of myr-CN27 is not rescued by expressing a GluN1 pore dead mutant (n = 8). Right panel, sample traces showing myr-CN27 inhibited AMPAR synaptic transmission in control cell (black traces), but not in a GluN1KO + PD cell (green traces).
-
Figure 4—source data 1
The depression caused by transient inhibition of Ca2+-calmodulin-dependent kinase II (CaMKII) is long lasting and is absent in cells lacking NMDARs.
In this dataset, the results of the effects of myr-CN27 and paAIP2 on AMPAR EPSCs over time are included.
- https://cdn.elifesciences.org/articles/60360/elife-60360-fig4-data1-v2.xlsx
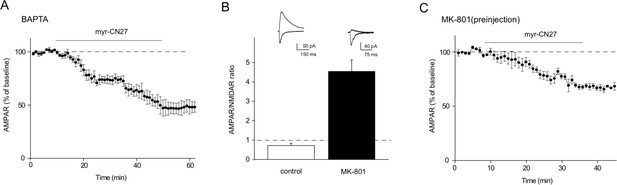
Origin of constitutive Ca2+-calmodulin-dependent kinase II (CaMKII) effect on synaptic transmission in acute slices.
(A) Loading cells with 15 mM BAPTA did not alter the CN-27-induced depression of AMPAR synaptic transmission (n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test). (B) Preinjection of MK801 (i.p.10 mg/kg body weight) 1 hr before slicing greatly depressed the NMDAR EPSC resulting in a large increase in the AMPAR/NMDAR ratio (control: 0.72 ± 0.11, n = 5; MK-801: 4.55 ± 0.58, n = 5; p < 0.01, Mann–Whitney U test). (C) With preinjection of MK-801 1 hr before slicing, and in the presence of MK-801 (100 µM), myr-CN27 inhibited AMPAR synaptic transmission (n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test).
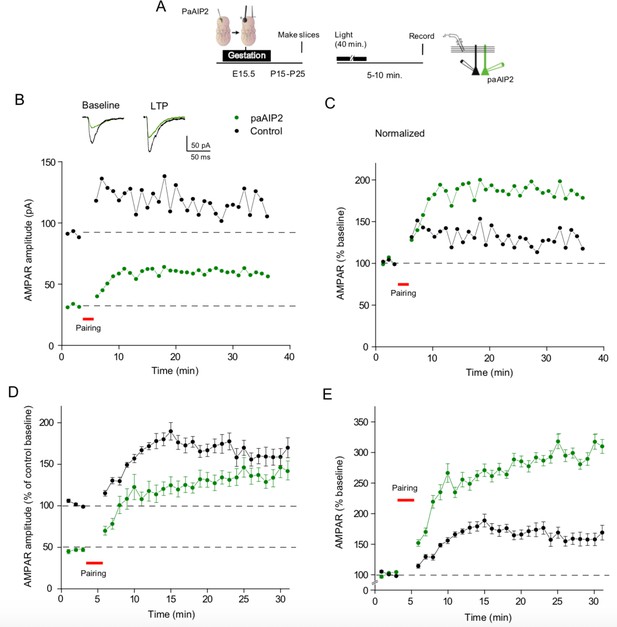
Transient inhibition of Ca2+-calmodulin-dependent kinase II (CaMKII) enhances subsequent long-term potentiation (LTP).
(A) Timeline of experimental procedure. Following in utero electroporation acute slices were prepared at P15–P25. Slices were then exposed to blue light for 40 min. Paired recordings were then made from a control cell (black circles) and a paAIP2 expressing cell (green circles). (B) One representative experiment showing that prior paAIP2 activation reduced baseline AMPAR EPSCs, but LTP was larger than that recorded in the neighboring control cell. (C) Normalized representative experiment showing that prior paAIP2 activation increased LTP. (D) Summary data showing that prior paAIP2 activation reduced baseline AMPAR EPSCs, but enhanced LTP (n = 5). (E) Normalized summary data showing that prior paAIP2 activation doubles the size of LTP. n = 5, p < 0.01, two-tailed Wilcoxon Signed Rank Test. Mean ± standard error of the mean (SEM).
-
Figure 5—source data 1
Transient inhibition of Ca2+-calmodulin-dependent kinase II (CaMKII) enhances subsequent long-term potentiation (LTP).
In this dataset, the results of the effects of LTP induction on control cells and preinhibition cells are included.
- https://cdn.elifesciences.org/articles/60360/elife-60360-fig5-data1-v2.xlsx
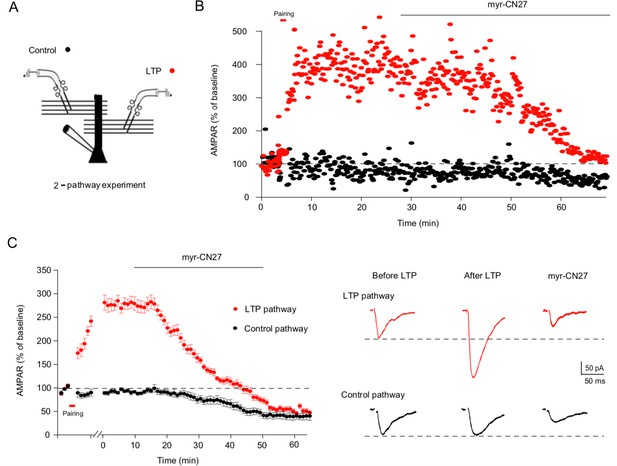
Myr-CN27 erases long-term potentiation (LTP) in acute slices.
(A) Cartoon diagram of two pathway experiment. To record the response from two independent pathways, two bipolar stimulating electrodes were positioned to either side of the recorded cell with a distance of around 100 μm. Stimuli were applied alternately every 20 s. (B) A sample experiment showing that myr-CN27 inhibited the LTP pathway more strongly than the control pathway. (C) Left, summary data showing that myr-CN27 reduced the control pathway (black circles) 50%, while completely reversing LTP (red circles) (the difference between control and LTP pathway at 60 min: n = 11, p > 0.05, two-tailed Wilcoxon Signed Rank Test). Responses are mean ± standard error of the mean (SEM). Right, sample traces showing the effect of myr-CN27 on AMPA EPSCs in control and LTP pathway. LTP is induced by 2 Hz stimulation for 90 s, while holding the cell at 0 mV.
-
Figure 6—source data 1
Myr-CN27 erases long-term potentiation (LTP) in acute slices.
In this dataset, the results of the effects of myr-CN27 on LTP and control pathways are included.
- https://cdn.elifesciences.org/articles/60360/elife-60360-fig6-data1-v2.xlsx
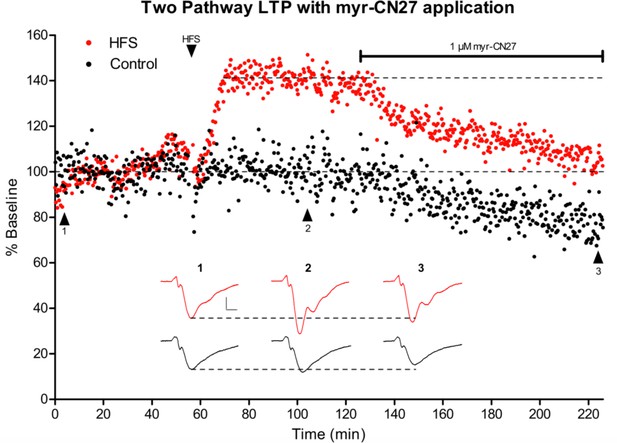
Reversal of long-term potentiation (LTP) and reduction of synaptic transmission with application of 1 μM myr-CN27.
After a 55-min stable baseline in both pathways (Control = black, LTP = red, both n = 1), a train of 100 Hz for 1-s stimuli was applied four times with an interval of 20 s. Following the stimulus, the LTP pathway exhibited a rapid increase in fEPSP slope that plateaued at ~140% of baseline. The control pathway did not exhibit this increase. After a stable baseline was achieved for the LTP pathway, 1 μM myr-CN27 was applied. Following application, both pathways exhibited a decrease in fEPSP slope with the LTP pathway exhibiting a greater decrease. Example traces for both pathways are displayed below the graph and taken at the indicated timepoints (1–3). Traces are colored according to the pathway they correspond to. The scale bars are vertical 0.1 mV and 5 ms horizontal. Recording done in WT acute slices from P15–28 mice.






