Loss of circadian protection against influenza infection in adult mice exposed to hyperoxia as neonates
Figures
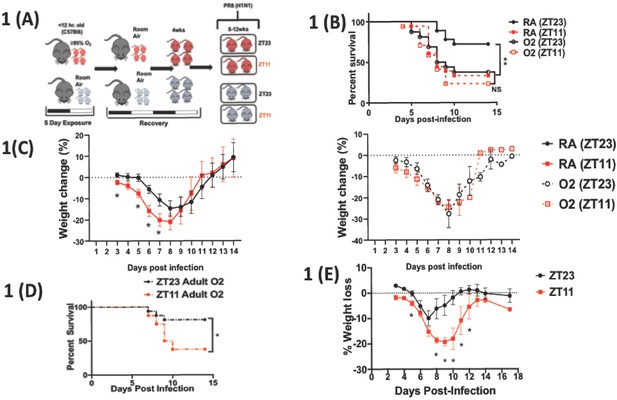
Overall experimental design and effects of neonatal hyperoxia on circadian regulation of IAV infection adulthood.
(A) Experimental model for neonatal hyperoxia followed by IAV infection: C57Bl6 pups aged <12 hr were exposed to either hyperoxia (>95% O2) or room air (21% O2) from PN0–5. Thereafter, the pups were recovered in room air to adulthood (8–12 weeks). As adults, the mice were place in reverse 12 hr light–dark cycles for 2 weeks before being infected with the influenza A virus (PR8). Experimental design: Adult mice were placed in 12 hr light–dark reverse cycles and infected with 25–40 PFU of PR8 at either ZT23 (dawn or just before the onset of rest phase) or at ZT11 (dusk or just before the onset of activity, since mice are nocturnal animals). (B) Survival (n = 17–18 per group, **p=0.0027 log-rank test test from three independent experiments) and (C) Percentage of body weight lost (n = 10–15 per group, *p<0.001 ANOVA for repeated measures) following IAV infection (25–40 PFU PR8) at either ZT23 (dawn) or at ZT11(dusk). (D) Survival of mice exposed to hyperoxia as adults and infected with IAV after 3–4 weeks of recovery (n = 8–16 per group, *p=0.0417 log-rank test from two independent experiments). (E) Percentage of body weight lost (n = 17–18 per group, *p=0.0091 for time of infection by REML mixed effects model for repeated measures) following IAV infection (25–40 PFU PR8) at either ZT23 (dawn or just before the onset of rest phase) or at ZT11 (dusk or just before the onset of activity, since mice are nocturnal animals).
-
Figure 1—source data 1
Source data for Figure 1.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig1-data1-v1.xlsx
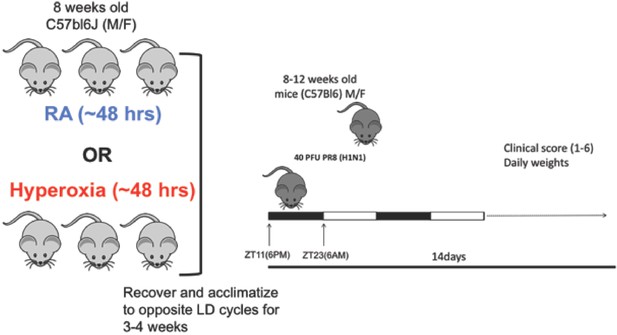
Experimental model for adult hyperoxia followed by IAV infection: C57Bl6/J mice at least 8 weeks old were exposed hyperoxia (>95% O2) for ~48 hr.
Thereafter, the animals were allowed to recover in room air and acclimatized to revere light–dark cycles for 3–4 weeks. Mice were infected with 25–40 PFU of PR8 at either ZT23 (dawn or just before the onset of rest phase) or at ZT11 (dusk or just before the onset of activity, since mice are nocturnal animals).
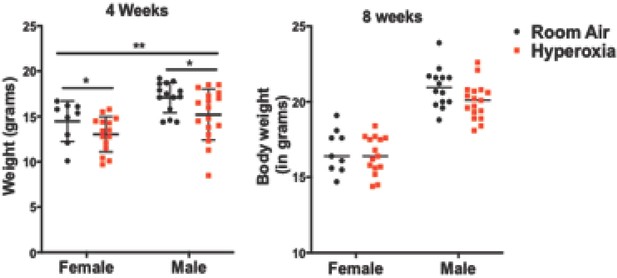
Body weights post-hyperoxia exposure.
After the 5 day neonatal hyperoxia exposure, body weight of both the hyperoxia-exposed mice and their room air littermates were measured at 4 weeks and at 8 weeks as a measure of overall well-being (n = 9–15 per group for females and n = 16–17 per group for males).
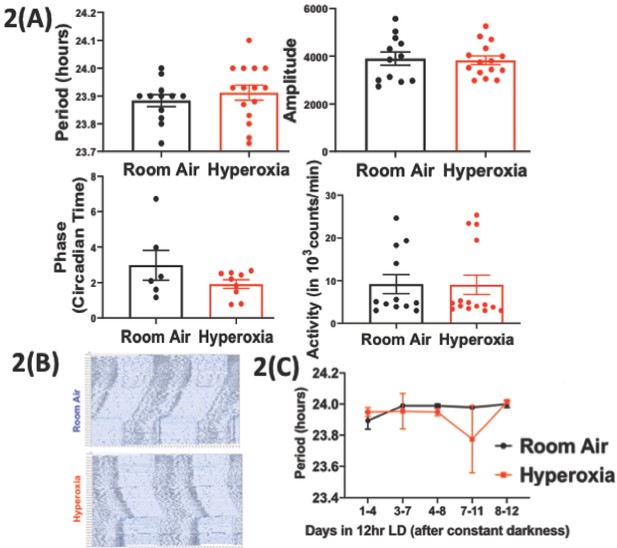
Loss of temporal gating of IAV infection in adults exposed to hyperoxia as neonates is not mediated through central circadian clock.
Mice pups <12 hr old were exposed to either hyperoxia or room air for 5 days and then recovered in RA into adulthood. As adults, effect on the central (locomotor activity) and peripheral (gene expression from lung and spleen, flow cytometry of lung resident immune cells, and bioluminescence of period two gene) clocks was measured. (A) Central effects were assessed by actigraphy measurements of rest and activity in constant darkness. Period, amplitude, activity counts, and phase of this locomotor activity in adult mice exposed to neonatal hyperoxia or room air (n = 6–15 per group). (B) Representative actigraph images taken from adult mice exposed to either neonatal hyperoxia or room air. This graph depicts the rest–activity behavior from a representative mouse where consecutive days are plotted on the y-axis, and time (in hours) is shown on the x-axis. The black bars represent the number of turns of the running wheel/movement sensed by the infrared motion sensors and indicates a time when the mouse of active. (C) Period length across days to acclimatize from constant darkness (DD) to 12 hr LD conditions adult mice exposed to neonatal hyperoxia or room air (n = 5–6 per group).
-
Figure 2—source data 1
Source data for Figure 2.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig2-data1-v1.xlsx
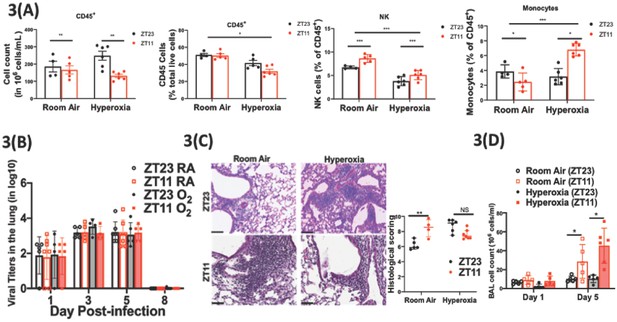
Exposure to hyperoxia as neonates has subtle effects of the circadian regulation of the immune response to influenza infection.
(A) Flow cytometric enumeration of the CD45+ cells from lungs of adult mice exposed to neonatal hyperoxia or room air (n = 5–7/group from three independent experiments). (B) Viral burden measure by hemagglutination inhibition assay (n = 5–7/group) by two-way ANOVA, p<0.001 for time post-infection, p=0.8855 for treatment group and interaction. (C) Left: Representative micrographs of H and E stained lung sections 8 days after IAV (40 PFU) treatment of adults exposed to neonatal hyperoxia or room air (photomicrograph bar = 100 µm). Right: Severity of lung injury quantified using an objective histopathological scoring system by a researcher blinded to study group (n = 4–8 mice/group; data as median, IQR; Wilcoxon rank sum test; **p<0.01; pooled data from two independent experiments). (D) Bronchoalveolar lavage (BAL) from animals infected at either ZT11 or ZT23 on day 1 and 5 p.i. (n = 5–6/group, p=0.0001 for time at infection, p<0.0001 time after infection, and p=0.0023 for interaction by two-way ANOVA).
-
Figure 3—source data 1
Criteria for scoring lung injury on histology.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig3-data1-v1.xlsx
-
Figure 3—source data 2
Source data for Figure 3.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig3-data2-v1.xlsx
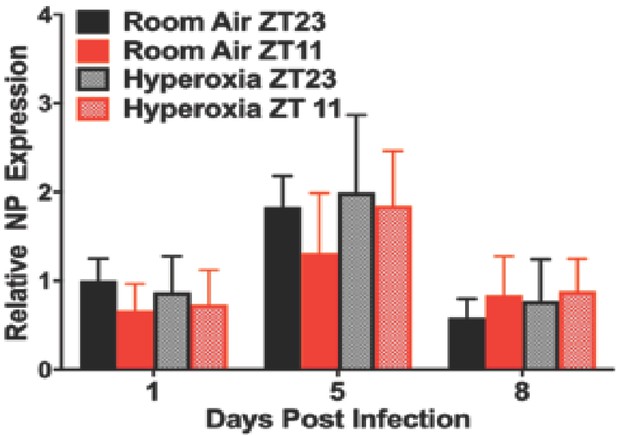
Viral nucleic acid measured by qPCR after IAV infection in adult animals exposed to hyperoxia as neonates (n = 6–8/group).
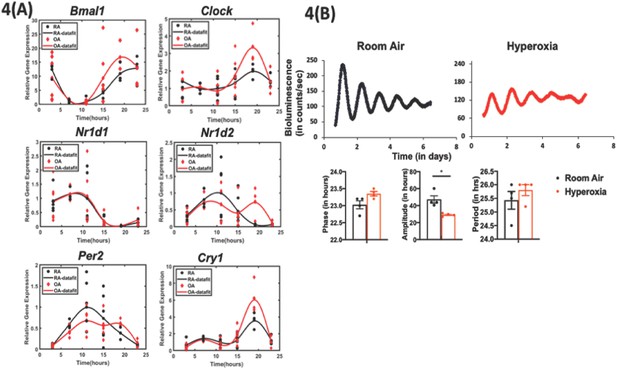
Exposure to hyperoxia as neonates reduce the amplitude of circadian oscillations in lung explants from adult animals.
(A) Gene expression of clock genes from whole lungs harvested at 6 hr intervals determined by qPCR (n = 4–6 per time point from three different experiments). (B) Representative bioluminescence tracings, period, amplitude, and phase from lung explants from adult Per2luc mice exposed to neonatal hyperoxia or room air (n = 4 from two independent experiments).
-
Figure 4—source data 1
Source data for Figure 4.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig4-data1-v1.xlsx
-
Figure 4—source data 2
qPCR primers used to generate data for Figure 4.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig4-data2-v1.xlsx
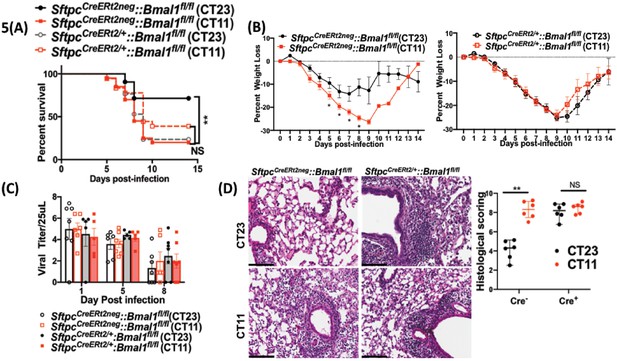
Disrupting the circadian clock in AT2 cells in adults recapitulates the phenotype seen in adult animals exposed to hyperoxia as neonates.
Experimental design: SftpcCreERt2/+::Bmal1fl/fl mice (mice lacking Bmal1 in AT2 cells of the lung epithelium) and their creneg littermates were treated with tamoxifen at 6–8 weeks of age and acclimatized to reverse cycles of 12 hr LD for 2 weeks. Thereafter, they were maintained in constant darkness for 2–4 days prior to administering IAV (PR8) at CT23 and CT11 and acclimatized to reverse cycles of 12 hr LD for 2 weeks. Thereafter, they were maintained in constant darkness for 2–3 days prior to administering IAV (PR8) at CT23 and CT11. CT23 and CT11 refer to the time corresponding to ZT23 and ZT11, respectively, when animals are maintained under constant darkness conditions. (A) Survival curves (n = 16–18 in SftpcCreERt2/+::Bmal1fl/fl groups and n = 20–21 in creneg, *p=0.0014 Mantel–Cox test; pooled data from four independent experiments). (B) Percentage of body weight lost (n = 16–18 in SftpcCreERt2/+::Bmal1fl/fl groups and n = 18–20 in creneg group from three independent experiments *p<0.01 ANOVA for repeated measures). (C) Viral burden measure by hemagglutination inhibition assay (n = 5–13/group) by two-way ANOVA, p<0.0001 for time post-infection, p=0.9981 for treatment group and interaction. (D) Left: Representative micrographs of H and E stained lung sections 8 days after IAV (40 PFU) treatment of SftpcCreERt2/+::Bmal1fl/fl mice and their cre− littermates (photomicrograph bar = 100 µm). Right: Severity of lung injury quantified using an objective histopathological scoring system by a researcher blinded to study group (n = 4–8 mice/group; data as median, IQR; Wilcoxon rank sum test; **p=0.0014, CT23 vs. CT11 for Cre+ refers to SftpcCreERt2/+::Bmal1fl/fl mice vs. Cre− which refers to SftpcCreERt2neg::Bmal1fl/fl; pooled data from three independent experiments).
-
Figure 5—source data 1
Source data for Figure 5.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig5-data1-v1.xlsx
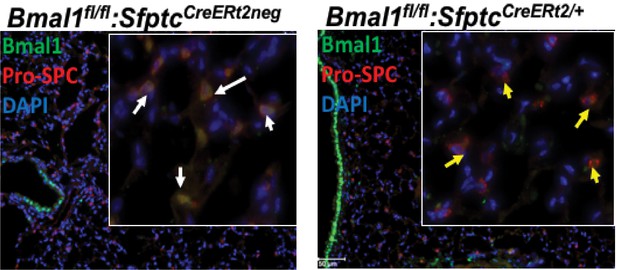
Immunofluorescence staining to demonstrate the AT2-specific loss of Bmal1 in SftpcCreERt2/+::Bmal1fl/fl mice, but not in SftpcCreERt2neg::Bmal1fl/fl littermates.
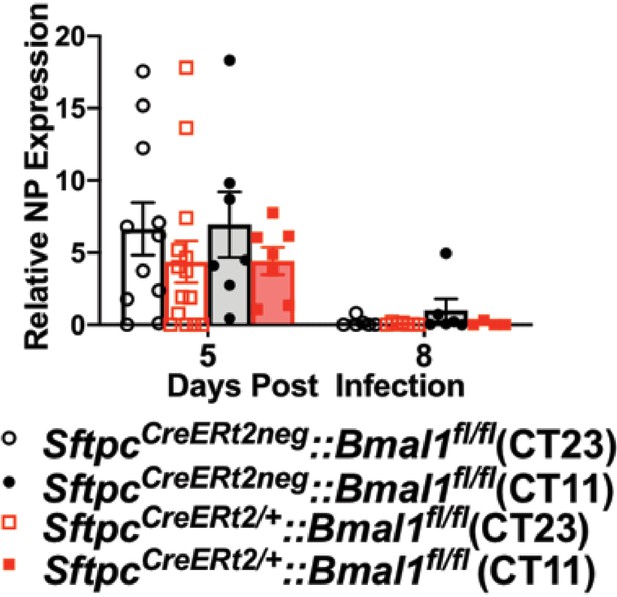
Viral nucleic acid measured by qPCR after IAV infection of SftpcCreERt2/+::Bmal1fl/fl mice and SftpcCreERt2neg::Bmal1fl/fl littermates.
(n = 5–8/group).
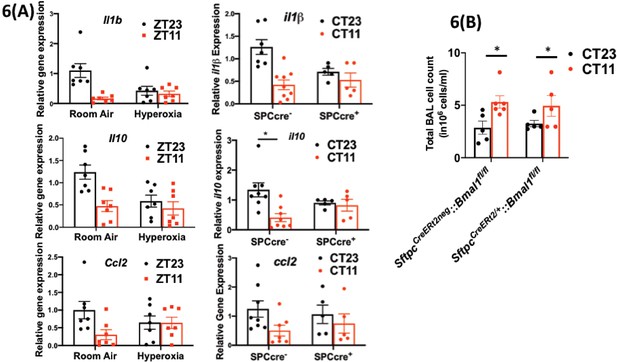
Disrupting the circadian clock in AT2 cells in adults recapitulates the gene expression pattern and BAL expression in adult animals exposed to hyperoxia.
(A) Whole lung gene expression measured by qPCR on day 5 post-infection in adults exposed to neonatal hyperoxia or room air (left panel) and SftpcCreERt2/+::Bmal1fl/fl mice (mice lacking Bmal1 in AT2 cells of the lung epithelium referred to in the figures as SPC-cre+) and their cre− littermates referred to as SPC-cre− (right panel). (B) Bronchoalveolar lavage (BAL) from animals infected at either CT11 or CT23 on day 5 p.i. (n = 4–6/group, p=0.0063 for time of day by two-way ANOVA).
-
Figure 6—source data 1
Source data for Figure 6.
- https://cdn.elifesciences.org/articles/61241/elife-61241-fig6-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti-Bmal1 (Rabbit monoclonal) | Abcam | ab230822 | IF(1:1000) |
| Antibody | anti-Pro-SPC (Rabbit polyclonal) | EMD Millipore | ab3786 | IF(1:2000) |
| Antibody | Nk1.1 (Mouse monoclonal) | Biolegend | 108716 | Flow cytometry (1:100) |
| Antibody | Ly6C (Mouse monoclonal) | Biolegend | 128024 | Flow cytometry (1:100) |
| Antibody | CD45 (Rat monoclonal) | Biolegend | 103114 | Flow cytometry (1:100) |
| Antibody | CD11c (Hamster monoclonal) | Biolegend | 117324 | Flow cytometry (1:100) |
| Antibody | CD11b (Mouse monoclonal) | EBiosciences | 11-0112-41, 47-0112-82 | Flow cytometry (1:100) |
| Antibody | CD3 (Rat monoclonal) | EBiosciences | 11-0032-82 | Flow cytometry (1:50) |
| Antibody | Ly6G (Rat monoclonal) | Biolegend | 127606, 127608, | Flow cytometry (1:100) |
| Antibody | Siglec F (Rat monoclonal) | BD Pharmingen | 552126 | Flow cytometry (1:100) |
| Software Algorithm | Clocklab | Actimetrics | Rest-activity analyses | |
| Other | DAPI stain | D1306 | (1 µg/mL) | |
| Chemicals strain, strain background (include species and sex here) | Tamoxifen | Sigma | T5648-1G | |
| Strain, strain background (include species and sex here) | C57BL/6J | Jax | Stock No: 000664 | B6 | Both genders and age at infection > 8 weeks |





