Reduced synchroneity of intra-islet Ca2+ oscillations in vivo in Robo-deficient β cells
Figures
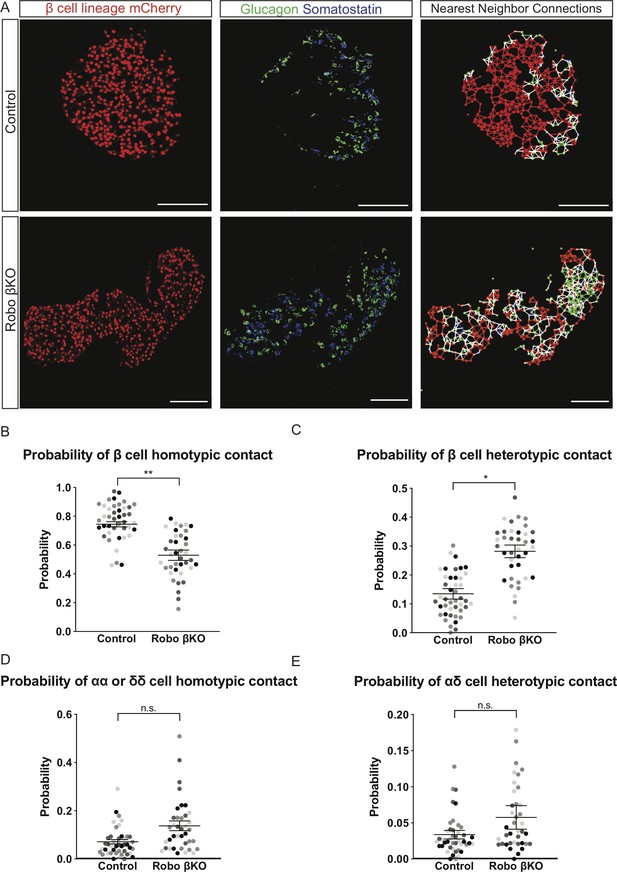
Robo βKO islets have a decreased ratio of homotypic nearest neighbors than controls.
(A) Immunofluorescence images (left and middle panels) and cell connectivity maps generated by nearest-neighbor analysis (right panels) of control and Robo βKO islets. β cells (red), α cells (green), and δ cells (blue) are denoted by nodes on the connectivity maps. A line the same color as both nodes it connects denotes a homotypic interaction of that corresponding cell type. A white line connecting two nodes denotes a heterotypic interaction between cell types. Scale bars are 100 µm. (B) Probability of β cell homotypic contact in Robo βKO islets vs. controls. n = 4 mice; control 0.74 ± 0.02 SEM, Robo βKO 0.53 ± 0.04 SEM, p<0.005 t-test. (C) Probability of β cell heterotypic contacts in Robo βKO islets vs. controls, n = 4 mice, control 0.13 ± 0.02 SEM, Robo βKO 0.28 ± 0.02 SEM, p<0.05 MW. (D) Probability of αα or δδ homotypic contacts in Robo βKO islets vs. controls, n = 4 mice; control 0.07 ± 0.01 S.EM, Robo βKO 0.13 ± 0.02 SEM, p=0.06 MW. (E) Probability of α-δ heterotypic contact in Robo βKO islets vs. controls, n = 4 mice; control 0.03% ± 0.01 SEM, Robo βKO 0.06% ± 0.02 SEM, p=0.22 t-test. (B–E) Similar shaded points in graphs indicate islets from the same mouse, while mean and error bars represent statistics performed on average values from each mouse. Error bars show SEM. 9–11 islets from an individual mouse were measured as technical replicates, and the average values per mouse were used as biological replicates. MW: Mann–Whitney; SEM: standard error of the mean.
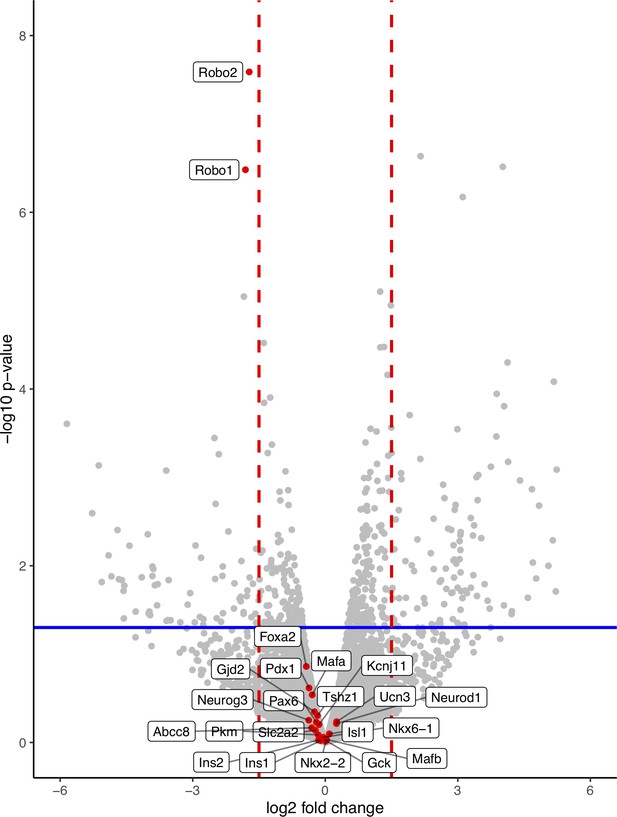
Robo βKO islets retain β cell differentiation and maturity, and Ca2+ regulatory genes.
Volcano plot of differential gene expression from bulk RNA sequencing on lineage-traced FACS-sorted β cells from Robo βKO and control mice showing no significant differential gene expression of markers (n = 2 mice from each group). Red lines denote a fold change of 1.5, and blue line denotes a p value of 0.05.
-
Figure 1—figure supplement 1—source data 1
RNA sequensing source data.
- https://cdn.elifesciences.org/articles/61308/elife-61308-fig1-figsupp1-data1-v2.xlsx
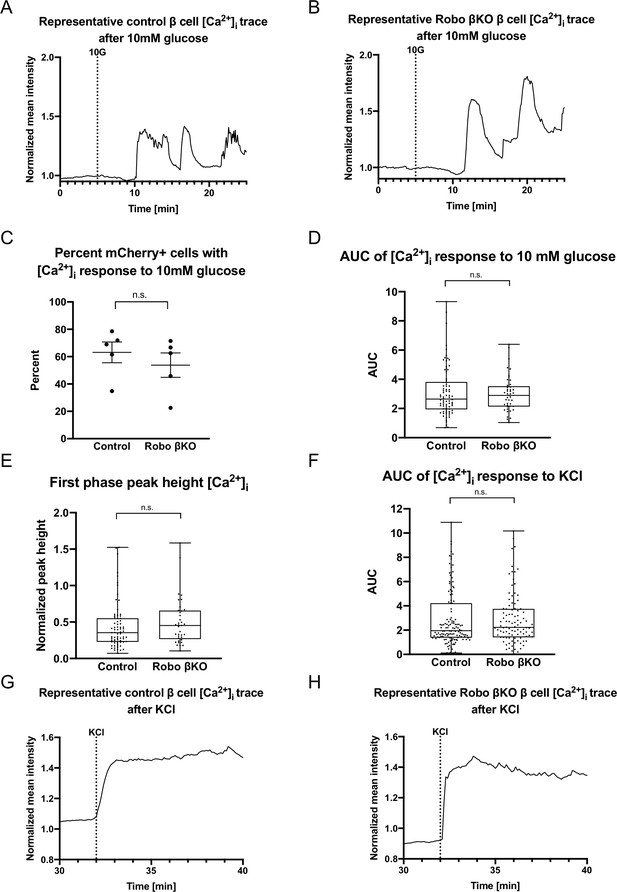
Dissociated Robo βKO β cells show no difference in glucose-stimulated Ca2+ oscillations.
(A) Representative [Ca2+]i trace (Fura2) of a dispersed β cell from a control islet. 10G line marks the addition of 10 mM glucose. (B) Representative [Ca2+]i trace (Fura2) of a dispersed β cell from a Robo βKO islet. 10G line marks the addition of 10 mM glucose. (C) Proportion of [Ca2+]i-responsive β cells in Robo βKO compared to controls; n = 5 mice from each genotype, control 63.15 ± 7.6%, Robo βKO 53.80 ± 8.9% SEM, p=0.45 t-test. Error bars shown are SEM. (D) Area under the curve (AUC) of [Ca2+]i (Fura2) from control and Robo βKO single dispersed β cells in response to 10 mM glucose. n = 78 β cells from five mice for control and n = 45 β cells from five mice for Robo βKO, control 3.1 ± 0.20 SEM, Robo βKO 3.0 ± 0.18 SEM, p=0.87 MW. (E) Peak height of [Ca2+]i corresponding to first phase insulin secretion from control and Robo βKO single dispersed β cells in response to 10 mM glucose. n = 71 β cells from five mice for control and n = 37 β cells from five mice for Robo βKO, control 0.44 ± 0.04 SEM, Robo βKO 0.50 ± 0.05 SEM, p=0.17 MW. (F) AUC of [Ca2+]i (Fura2) from control and Robo βKO single dispersed β cells in response to KCl. n = 132 β cells from five mice for controls and n = 98 β cells from five mice for Robo βKO, control 2.9 ± 0.2 SEM, Robo βKO 2.9 ± 0.2 SEM, p=0.65 MW. (G) Representative [Ca2+]i trace (Fura2) of a single dispersed β cell from a control islet. Line marks the addition of KCl. (H) Representative [Ca2+]i trace (Fura2) of a dispersed β cell from a Robo βKO islet. Line marks the addition of KCl. MW: Mann–Whitney; SEM: standard error of the mean.
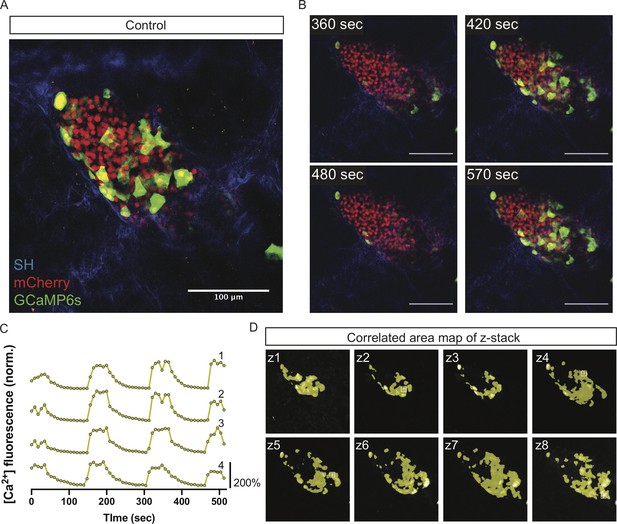
Control islets show highly synchronized whole-islet [Ca2+]i oscillations.
(A) Control islet in vivo in an AAV8-RIP-GCaMP6s-injected mouse showing GCaMP6s in green, nuclear mCherry β cell lineage tracing in red, and collagen (second harmonic) in blue. (B) Stills from a recording over one oscillation period from control islet in Video 1, when blood glucose level was >200 mg/dL following IP glucose injection. Video was recorded for 10 min with an acquisition speed of ~0.1 Hz. (C) Representative time courses of [Ca2+]i activity in four individual areas from control islet in Video 1 showing high correlation in activity over 97.2% of the active islet area. Time courses are normalized to average fluorescence of individual area over time. Similar color indicates that the time courses have a Pearson’s correlation coefficient of ≥0.70 and matches the region of coordination that is seen in (D). (D) False color map of top four largest coordinated areas across z-stack of control islet from analysis in (C). Areas used in time courses in (C) are labeled. Scale bars are 100 μm.
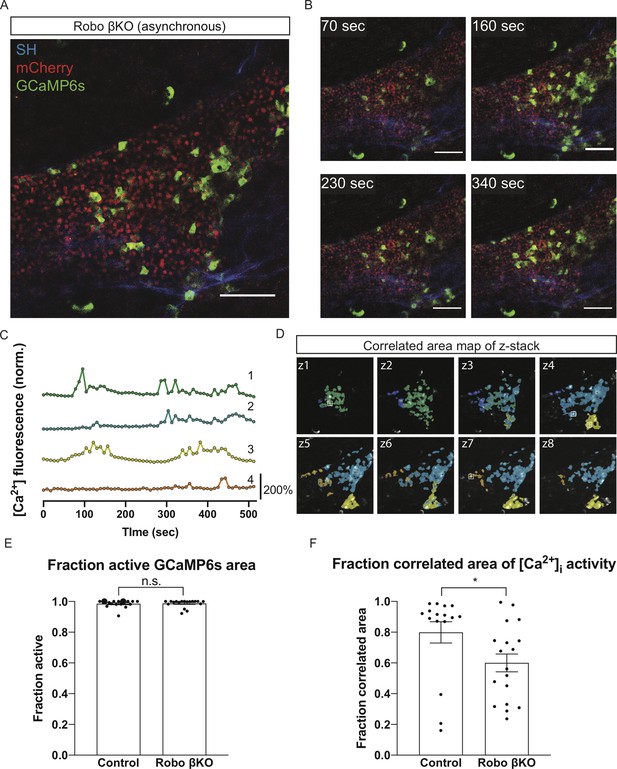
Robo βKO islets show decreased synchronization of whole-islet [Ca2+]i oscillations.
(A) Robo βKO islet in vivo in an AAV8-RIP-GCaMP6s-injected mouse showing GCaMP6s in green, nuclear mCherry β cell lineage tracing in red, and collagen (second harmonic) in blue. (B) Stills from a recording over one oscillation period from Robo βKO islet in Video 4, when blood glucose level was >200 mg/dL after IP glucose injection. Video was recorded for 10 min with an acquisition speed of ~0.1 Hz. (C) Representative time courses of [Ca2+]i activity in five individual areas from Robo βKO islet in Video 4, showing high correlation in activity over 56.1% of the active islet area. Time courses are normalized to average fluorescence of individual area over time. Similar color indicates that the time courses have a Pearson’s correlation coefficient of ≥0.70 and matches the region of coordination that is seen in (D). (D) False color map of top four largest coordinated areas across z-stack of Robo βKO islet from analysis in (C). Areas used In (C) for traces are labeled. (E) Fraction of active islet area showing elevated [Ca2+]i activity for control and Robo βKO islets. Control n = 16 islets collectively from seven mice, Robo βKO n = 18 islets collectively from nine mice, control 0.99 ± 0.006 SEM, Robo βKO 0.99 ± 0.006 SEM, p = 0.93 MW. (F) Largest fraction of area in islet exhibiting coordinated [Ca2+]i oscillations for control and Robo βKO islets. Control n = 16 islets collectively from seven mice, Robo βKO n = 18 islets collectively from nine mice, control 0.80 ± 0.07 SEM, Robo βKO 0.60 ± 0.06 SEM, p<0.05 MW. Each islet was treated as a biological replicate for intravital oscillation experiments. Oscillation data was collected from five separate experiments. Error bars shown are SEM. Scale bars are 100 μm. MW: Mann–Whitney; SEM: standard error of the mean.
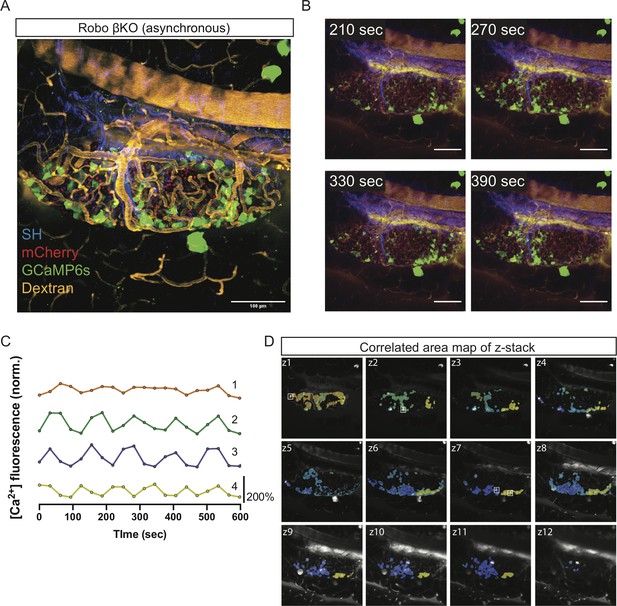
Robo βKO islets show uncoordinated whole-islet [Ca2+]i oscillations.
(A) Robo βKO islet in vivo in an AAV8-RIP-GCaMP6s-injected mouse showing GCaMP6s in green, nuclear mCherry β cell lineage tracing in red, and collagen (second harmonic) in blue. Scale bar is 100 μm. (B) Stills over one oscillation period from Robo βKO islet in Video 3, starting after blood glucose level reached >200 mg/dL from IP glucose injection. Video was recorded for 10 min with an acquisition speed of ~0.03 Hz. (C) Representative time courses of [Ca2+]i activity in four individual areas from Robo βKO islet in Video 3, showing correlation of 28.7% of the active islet area. Time courses are normalized to average fluorescence of individual area over time. Similar color indicates that the time courses have a Pearson’s correlation coefficient of ≥0.70 and matches the region of coordination that is seen in (D). (D) False color map of top four largest coordinated areas across z-stack of Robo βKO islet from analysis in (C). Areas used in time courses in (C) are labeled.
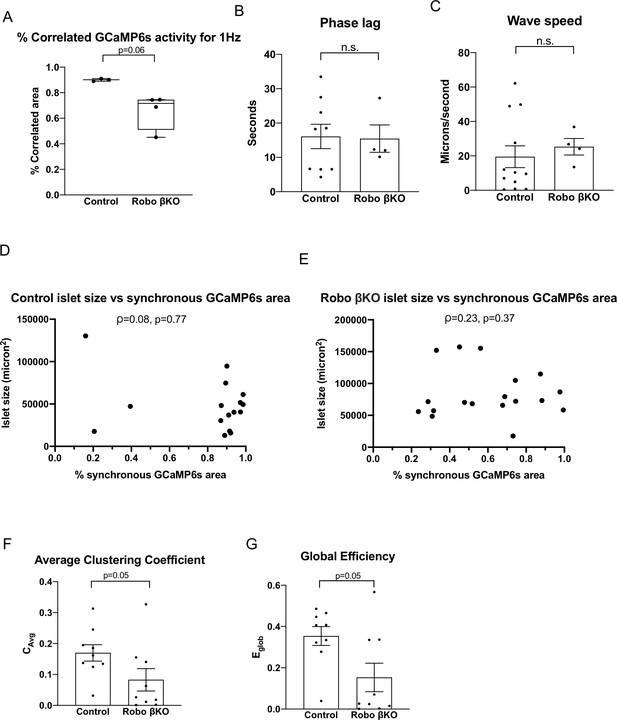
Robo βKO islets show uncoordinated whole-islet [Ca2+]i oscillations regardless of imaging speed or islet size while a subset display wildtype-like wave properties.
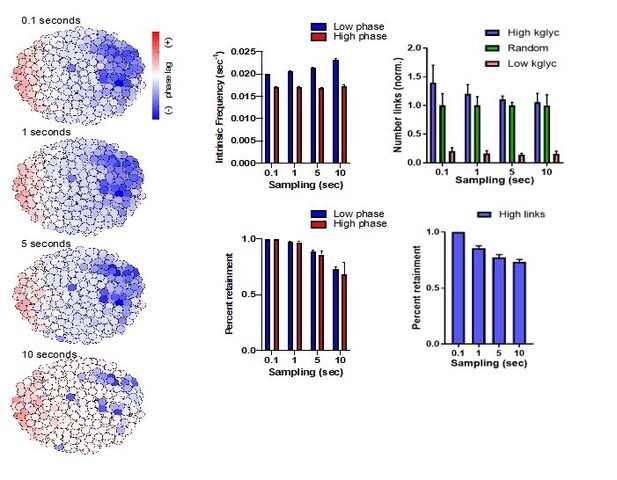
(A) Largest fraction of area in islet exhibiting coordinated [Ca2+]i oscillations for control and Robo βKO islets imaged at ~1 Hz. Control n = 3 islets collectively from two mice, Robo βKO n = 4 islets collectively from two mice, control 0.90 ± 0.01, Robo βKO 0.66 ± 0.07, p=0.06 MW. (B) Phase lag in islets with >80% correlated area in control and Robo βKO. Controls n = 9 islets collectively from six mice, Robo βKO n = 4 islets collectively from four mice, control 16.1 ± 3.5 s, Robo βKO 15.5 ± 4.0 s, p = 0.94 MW. (C) Wave speed in islets that showed >80% correlated area in control and Robo βKO. Control 19.5 ± 6.4 s, Robo βKO 25.3 ± 4.8 s, p = 0.32 MW. (D) Scatterplot of islet size vs. synchronous GCaMP6s area for control islets. ρ = 0.08 Spearman, p=0.77. (E) Scatterplot of islet size vs. synchronous GCaMP6s area for Robo βKO islets. ρ = 0.23 Spearman, p=0.37. (F) Average clustering coefficient in control and Robo βKO islet networks, control 0.17 ± 0.03, Robo βKO 0.08 ± 0.04, p=0.05 MW. (G) Global efficiency in control and Robo βKO islet networks, control 0.35 ± 0.05, Robo βKO 0.15 ± 0.07, p=0.05 MW. MW: Mann–Whitney.
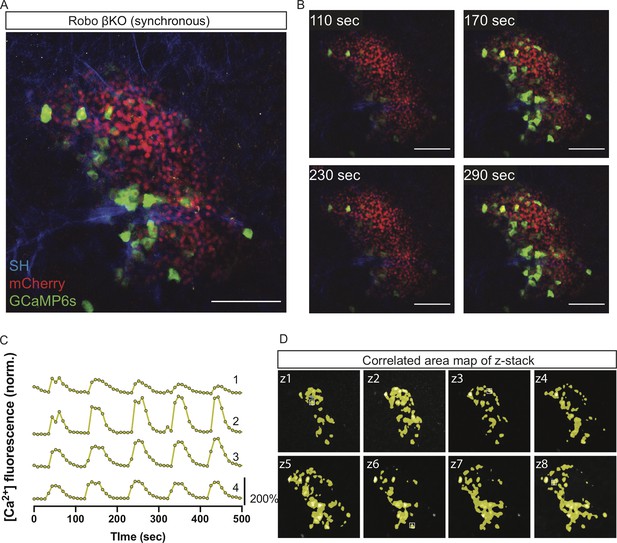
A subset of Robo βKO islets show coordinated whole-islet [Ca2+]i oscillations.
(A) Robo βKO islet in vivo in an AAV8-RIP-GCaMP6s-injected mouse showing GCaMP6s in green, nuclear mCherry β cell lineage tracing in red, and collagen (second harmonic) in blue. Scale bar is 100 µm. (B) Stills over one oscillation period from Robo βKO islet in Video 6, starting after blood glucose level reached >200 mg/dL from IP glucose injection. Video was recorded for 10 min with an acquisition speed of ~0.1 Hz. (C) Representative time courses of [Ca2+]i activity in four individual areas from Robo βKO islet in Video 6, showing correlation of 87.5% of the active islet area. Time courses are normalized to average fluorescence of individual area over time. Similar color indicates that the time courses have a Pearson’s correlation coefficient of ≥0.70 and matches the region of coordination that is seen in (D). (D) False color map of top four largest coordinated areas across z-stack of Robo βKO islet from analysis in (C). Areas used for traces in (C) are labeled.
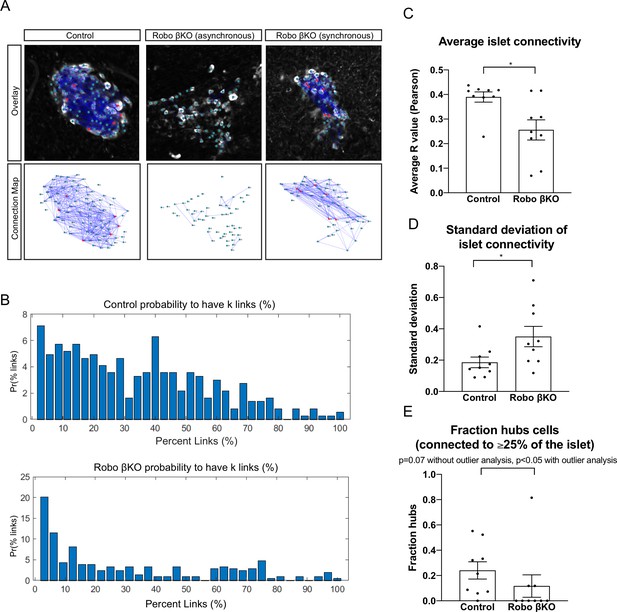
Network dynamics differ in Robo βKO islets compared to controls.
(A) Connectivity maps of control (left panel top and bottom), asynchronous Robo βKO (middle panel top and bottom), and synchronous Robo βKO (right panel top and bottom) islets. Nodes are connected if they have a correlation coefficient of R ≥ 0.95. Red nodes indicate cells that are connected to ≥25% of the islet. (B) Probability distributions of average percent links (%) per cell within control (top) and Robo βKO (bottom) islets. (C) Average islet connectivity as shown by average pairwise correlation coefficient in an islet, control Ravg = 0.40 ± 0.02 SEM, Robo βKO Ravg = 0.26 ± 0.04, p<0.05 MW. (D) Standard deviation of Ravg for each islet, control 0.19 ± 0.03, Robo βKO 0.35 ± 0.07, p<0.05 t-test. (E) Fraction of cells that were connected to ≥25% of the islet (hubs) in control and Robo βKO islets, without outlier analysis control 0.24 ± 0.07, Robo βKO 0.12 ± 0.09, p=0.07 MW; with outlier analysis (ROUT Q = 0.1%) control 0.24 ± 0.07, Robo βKO 0.03 ± 0.02, p<0.05 MW. n = 9 islets each from control and Robo βKO. Error bars shown are SEM. MW: Mann–Whitney; SEM: standard error of the mean.
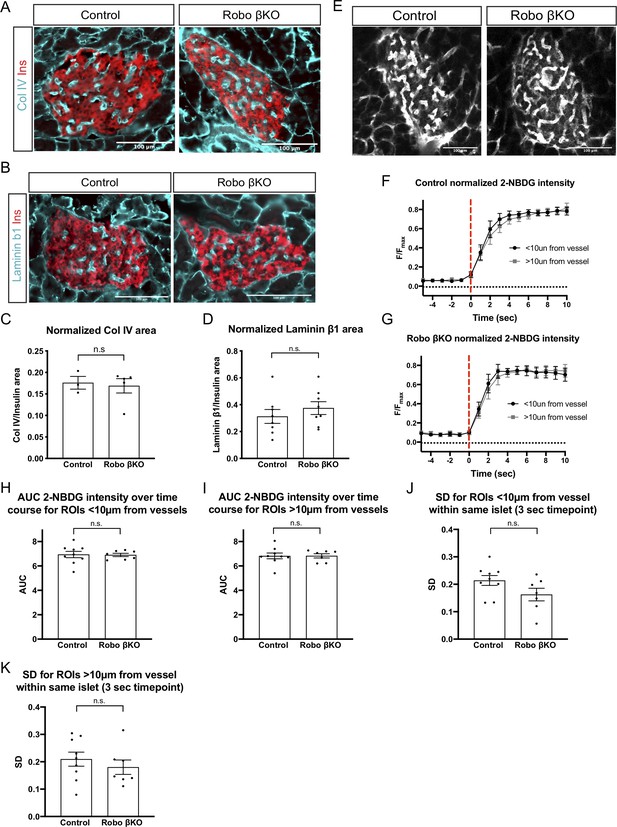
Amount and perfusion of vascularization remains unchanged in Robo βKO islets.
(A) Representative immunofluorescent staining of collagen IV marking vasculature showing similar amounts in Robo βKO and control islets. (B) Representative immunofluorescent staining of laminin marking vasculature showing similar amounts in Robo βKO and control islets. (C) Quantification of area of collagen IV staining normalized to islet area showing no difference in amounts of basement membrane marking blood vessels in Robo βKO compared to control islets. Control n = 3 mice, Robo βKO n = 5 mice, control 0.17 ± 0.02 SEM, Robo βKO 0.17 ± 0.02 SEM, p=0.99 MW. >12 islets from an individual mouse were measured as technical replicates, and the average values per mouse were used as biological replicates. (D) Quantification of area of laminin staining normalized to islet area showing no difference in amounts of basement membrane marking blood vessels in Robo βKO compared to control islets. Control n = 8 mice, Robo βKO n = 8 mice, control 0.31 ± 0.05 SEM, Robo βKO 0.38 ± 0.05 SEM, p=0.39 t-test. >12 islets from an individual mouse were measured as technical replicates, and the average values per mouse were used as biological replicates. (E) 2-NBDG perfused through the islet vasculature in a control and Robo βKO islet. (F) Average normalized 2-NBDG intensity (F/Fmax) over time in areas < and >10 µm from nearest vessel in control islets. (G) Average normalized 2-NBDG intensity (F/Fmax) over time in areas < and >10 µm from nearest vessel in Robo βKO islets. (H) Area under the curve (AUC) of 2-NBDG intensity over time in areas <10 µm from vessels in control and Robo βKO islets. Control 6.9 ± 0.26 SEM, Robo βKO 6.9 ± 0.14 SEM, p>0.90 t-test. (I) AUC of 2-NBDG intensity over time in areas >10 µm from vessels in control and Robo βKO islets. Control 6.8 ± 0.24 SEM, Robo βKO 6.8 ± 0.17 SEM, p>0.90 t-test. (J) Standard deviation of areas <10 µm from vessels within the same islet at the 3 s time point in control and Robo βKO islets. Control 0.21 ± 0.02 SEM, Robo βKO 0.16 ± 0.02 SEM, p=0.10 t-test. (K) Standard deviation of regions of interest >10 µm from vessels within the same islet at the 3 s time point in control and Robo βKO islets. Control 0.21 ± 0.03 SEM, Robo βKO 0.18 ± 0.03 SEM, p = 0.44 t-test. Error bars shown are SEM. MW: Mann–Whitney; SEM: standard error of the mean.
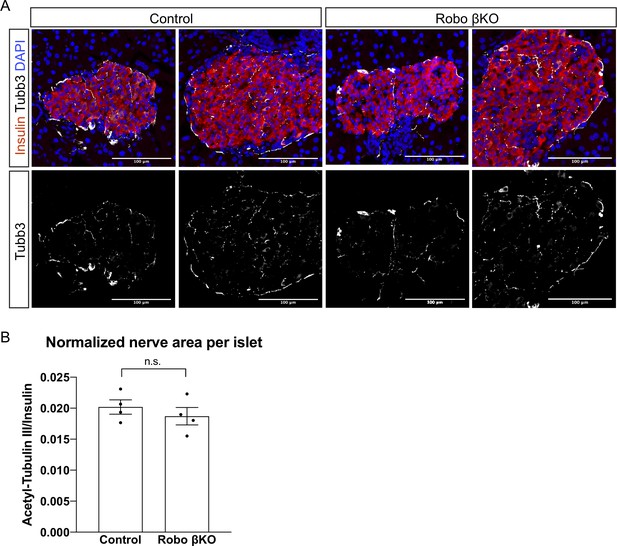
Robo βKO shows normal innervation within the islet.
(A) Immunofluorescent images of acetylated-tubulin3 (Tubb3) marking nerves in white, insulin in red, and DAPI in blue in islets from Robo βKO and control pancreatic tissue sections. (B) Quantification of area of Tubb3 staining normalized to insulin area showing no difference in amounts of innervation in Robo βKO compared to control islets. Control n = 4 mice, Robo βKO n = 4 mice, control 0.020 ± 0.001 SEM, Robo βKO 0.019 ± 0.001 SEM, p=0.45 t-test. 10–15 islets from an individual mouse were measured as technical replicates, and the average values per mouse were used as biological replicates. SEM: standard error of the mean.
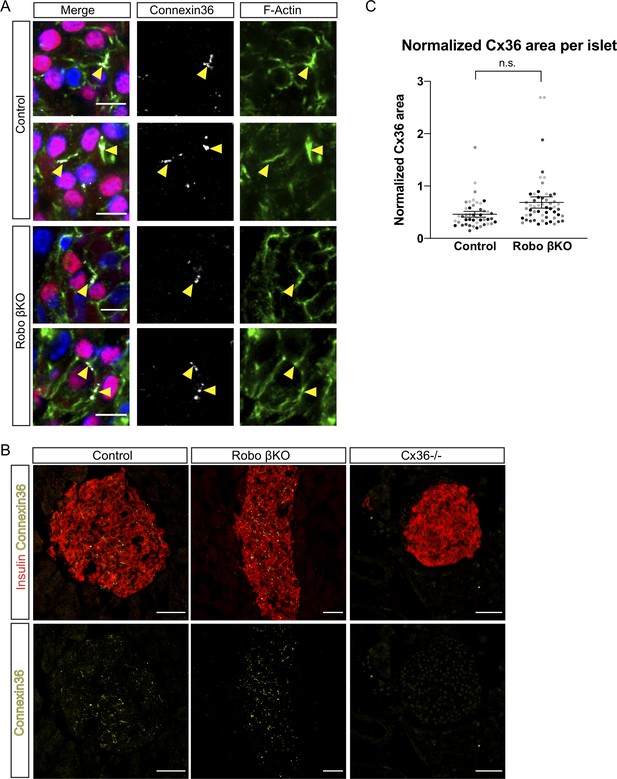
Amount of Cx36 gap junctions remains unchanged in Robo βKO.
(A) Immunofluorescent images showing DAPI in blue, histone H2B-mCherry β cell lineage trace in red, F-actin (phalloidin) in green, and Cx36 in gray demonstrating normal localization of Cx36 to plasma membrane of β cells in both control and Robo βKO islets. Arrowheads point to colocalized F-actin and Cx36. (B) Immunofluorescent images of Cx36 (yellow) and insulin (red) in islets from pancreatic sections of Control, Robo βKO, and Cx36 KO mice. (C) Quantification of area of Cx36 staining normalized to islet area in Robo βKO islets and controls showing no significant difference, n = 4 mice for each genotype, control 0.46 ± 0.06 SEM, Robo βKO 0.69 ± 0.11 SEM, p = 0.11 t-test. Similar shaded points in graphs indicate islets from the same mouse, while mean and error bars represent statistics performed on average values from each mouse. Error bars shown are SEM. 10–14 islets were measured per mouse as technical replicates, and the average for each mouse was considered a biological replicate. SEM: standard error of the mean.
Videos
Control islets show highly synchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a control β cell lineage-traced mouse infected with AAV8-Ins1-GcaMP6s. Lineage-traced β cells are marked by mCherry in red and GcaMP6s in green. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of eight slices each 8 µm apart were recorded at ~0.1 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
Control islets show highly synchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a control β cell lineage-traced mouse infected with AAV8-ins1-GCaMP6s. Lineage-traced β cells are marked by mCherry in red and GCaMP6s in green. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of three slices each 8 µm apart were recorded at ~0.2 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
Robo βKO islets show unsynchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a Robo βKO β cell lineage-traced mouse infected with AAV8-ins1-GCaMP6s and retroorbitally injected with rhodamine-dextran to mark vasculature. Lineage-traced β cells are marked by mCherry in red and GCaMP6s in green, and vasculature is shown in yellow. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of 12 slices each 8 µm apart were recorded at ~0.03 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
Robo βKO islets show unsynchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a Robo βKO β cell lineage-traced mouse infected with AAV8-ins1-GCaMP6s. Lineage-traced β cells are marked by mCherry in red and GCaMP6s in green. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of eight slices each 8 µm apart were recorded at ~0.1 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
Robo βKO islets show unsynchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a Robo βKO β cell lineage-traced mouse infected with AAV8-ins1-GCaMP6s. Lineage-traced β cells are marked by mCherry in red and GCaMP6s in green. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of three slices each 8 µm apart were recorded at ~0.2 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
A subset of Robo βKO islets retain synchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a Robo βKO β cell lineage-traced mouse infected with AAV8-ins1-GCaMP6s. Lineage-traced β cells are marked by mCherry in red and GCaMP6s in green. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of eight slices each 8 µm apart were recorded at ~0.1 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
A subset of Robo βKO islets retain synchronized [Ca2+]i oscillations.
Intravital time-course video of an islet within the in vivo pancreas of a Robo βKO β cell lineage-traced mouse infected with AAV8-ins1-GCaMP6s. Lineage-traced β cells are marked by mCherry in red and GCaMP6s in green. Mouse was injected IP with glucose, and video was recorded once blood glucose levels reached >200 mg/dL. Z-stacks of eight slices each 8 µm apart were recorded at ~0.2 Hz over 10 min. Scale bar is 100 µm. Time stamp shown in the upper-left corner shows time of image in min:s.
Control islet perfused with the fluorescent glucose analog 2-NBDG.
Intravital time-course video of an islet within the in vivo pancreas of a control mouse at 1 Hz imaging speed. Mouse was injected through tail vein IV with the fluorescent glucose analog 2-NBDG (gray) while imaging to visualize glucose analog perfusion throughout the islet. Time stamp shown in the upper-left corner shown in seconds.
Robo βKO islet perfused with the fluorescent glucose analog 2-NBDG.
Intravital time-course video of an islet within the in vivo pancreas of a Robo βKO mouse at 1 Hz imaging speed. Mouse was injected through tail vein IV with the fluorescent glucose analog 2-NBDG (gray) while imaging to visualize glucose analog perfusion throughout the islet. Time stamp shown in the upper-left corner shown in seconds.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | Robo1tm1Matl ;Robo2tm1Rilm | PMID:26743624 | RRID:MGI:3043177 RRID:MGI:5705332 | Linked alleles provided by Xin Sun |
| Genetic reagent (M. musculus) | Ins1tm1.1(cre)Thor | Jackson Laboratory | RRID:IMSR_JAX:026801 | |
| Genetic reagent (M. musculus) | B6.FVB(Cg)-Tg(Ucn3-cre)KF43Gsat/Mmucd | MMRRC | RRID:MMRRC_037417-UCD | |
| Genetic reagent (M. musculus) | R26H2BCherry | PMID:25233132 | R26H2BCherry | Generated as described in source |
| Genetic reagent (AAV8) | VB171020-1118pud | PMID:31186447 Generated by VectorBuilder | AAV8-INS-GcAMP6s | Sequence available in Supplementary materials of source |
| Antibody | Anti-insulin (guinea pig polyclonal) | Agilent | Cat#:IR002 RRID:AB_2800361 | (1:6) |
| Antibody | Anti-glucagon (mouse monoclonal) | Sigma | Cat#:G2654 RRID:AB_259852 | (1:500) |
| Antibody | Anti-glucagon (rabbit polyclonal) | Cell Signaling | Cat#:2760S RRID:AB_659831 | (1:200) |
| Antibody | Anti-somatostatin (rabbit polyclonal) | Phoenix | Cat#:G-060-03 RRID:AB_2890901 | (1:1000) |
| Antibody | Anti-Cx36 (rabbit polyclonal) | Invitrogen | Cat#:36-4600 RRID:AB_2314259 | (1:80) |
| Antibody | Anti-Col IV (rabbit polyclonal) | Abcam | Cat#:Ab6586 RRID:AB_305584 | (1:300) |
| Antibody | Anti-laminin β1 (rat monoclonal) | Invitrogen | Cat#:MA5-14657 RRID:AB_10981503 | (1:500) |
| Antibody | Anti-Tubb3 (rabbit polyclonal) | BioLegend | Cat#:poly18020 RRID:AB_2564645 | (1:4000) |
| Antibody | Anti-guinea pig 594 (donkey polyclonal) | Jackson | Cat#:706-585-148 RRID:AB_2340474 | (1:500) |
| Antibody | Anti-guinea pig 647 (donkey polyclonal) | Jackson | Cat#:706-605-148 RRID:AB_2340476 | (1:500) |
| Antibody | Anti-rabbit 594 (donkey polyclonal) | Invitrogen | Cat#:A21207 RRID:AB_141637 | (1:500) |
| Antibody | Anti-goat 647 (donkey polyclonal) | Invitrogen | Cat#:A21447 RRID:AB_141844 | (1:500) |
| Antibody | Anti-rat 488 (donkey polyclonal) | Invitrogen | Cat#:A21208 RRID:AB_141709 | 1:500 |
| Antibody | Anti-rabbit 488 (donkey polyclonal) | Invitrogen | Cat#:A21206 RRID:AB_2535792 | (1:500) |
| Antibody | Anti-mouse 488 (donkey polyclonal) | Jackson | Cat#:715-546-151 RRID:AB_2340850 | (1:500) |
| Antibody | Anti-biotin 488 (mouse polyclonal) | Jackson | Cat#:200-542-211 RRID:AB_2339040 | (1:500) |
| Chemical compound, drug | Phalloidin-647 | Invitrogen | A22247 | (1:400) |
| Chemical compound, drug | 2-NBDG | Invitrogen | N13195 | (5 mg/mL) |
| Chemical compound, drug | Fura2-AM | Thermo Fischer | F1201 | (5 μm) |
| Peptide, recombinant protein | Accutase | Thermo Fischer | A1110501 | |
| Peptide, recombinant protein | Geltrex | Thermo Fischer | A1413302 | |
| Software, algorithm | Prism | GraphPad | RRID:SCR_005375 | |
| Software, algorithm | MATLAB | MathWorks | RRID:SCR_001622 |
Additional files
-
Supplementary file 1
Differentially expressed transcripts linked to [beta] cell maturity and differeantion.
- https://cdn.elifesciences.org/articles/61308/elife-61308-supp1-v2.csv
-
Supplementary file 2
Robo βKO and control oscillation patterns.
- https://cdn.elifesciences.org/articles/61308/elife-61308-supp2-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/61308/elife-61308-transrepform-v2.docx