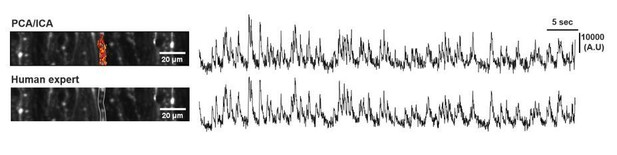
Direct translation of climbing fiber burst-mediated sensory coding into post-synaptic Purkinje cell dendritic calcium
Figures
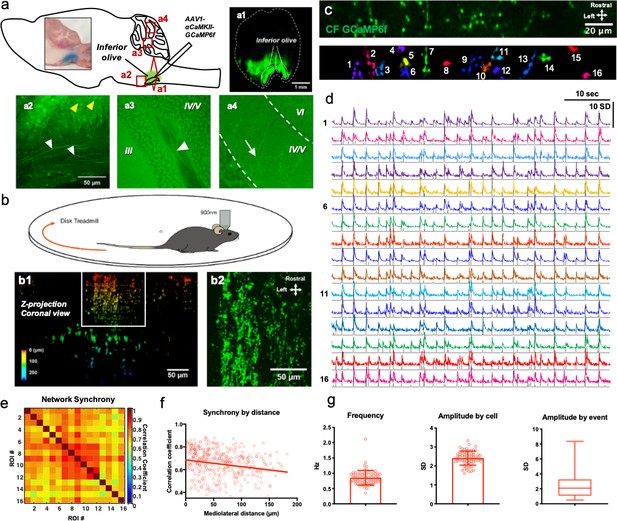
The CF Ca2+ activity is highly variable in awake mice in vivo.
(a) Schematic diagram of the IO viral injection. A coronal section view of the brain stem region of a brain in which GCaMP6f was expressed for 3 weeks (a1). GCaMP6f expressed soma and projecting axons (yellow and white arrowhead, respectively) in a brain stem region including the IO (a2). GCaMP6f-expressed axons (white arrowhead) within white matter (a3). GCaMP expressed CF varicosities (white arrow) within the molecular layer of lobules IV/V (a4). (b) A schematic diagram of two-photon microscopy of awake mice on a disk treadmill. A coronal view of the z-stack projection image of CFs expressing GCaMP6f in the cerebellar cortex (b1). A dorsal view of the z-stack image (maximum projection image) of white box regions of b1, which represent the molecular layer (b2). (c) An example of ROI detection of CF varicosities using the Suite2p. The field of view is 512 × 64 pixels. (d) Resting-state GCaMP6f intensity traces the 16 ROIs over 60 s with event detection plot (grey lines). Intensities were expressed as standard deviation as signals were z-score normalized. (e) An example matrix of correlation coefficients among every pair of the 16 ROIs, with the Pearson correlations described by colored intensity. Right: A scale bar for correlation coefficient. (f) The correlation coefficient among the CFs in terms of the mediolateral distance between each pair of all ROIs. n = 397 pairs of CFs, R2 = 0.034. (g) Average frequency (0.85 Hz ± 0.24 SEM, n = 69 varicosities), amplitudes by cell (2.41 SD ± 0.045 SEM, n = 69 cells), and amplitudes by event (2.349 SD ± 1.49 SD, n = 3481 events, N = 5 mice).
-
Figure 1—source data 1
Statistics for spontaneous Ca2+ activity.
- https://cdn.elifesciences.org/articles/61593/elife-61593-fig1-data1-v2.xlsx
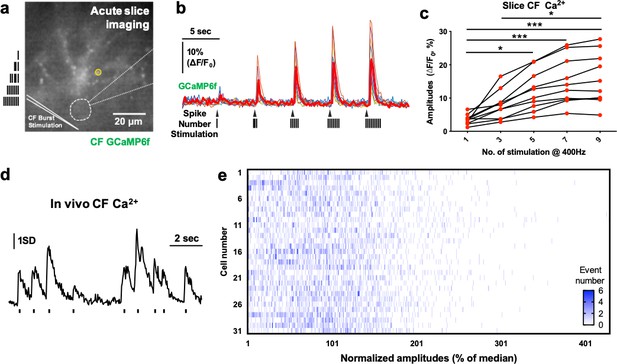
The variable CF Ca2+ activity encodes the spike number in the burst.
(a) The sagittal slice image of GCaMP6f-expressed CF in the cerebellar PC and molecular layer. Five responding axonal varicosities (<2 µm) were selected (as indicated by yellow circles) and averaged for each cell’s traces. The approximate PC soma was marked with a thick white dashed line. The patch pipette was depicted as a thin white dashed line. (b) Burst stimulation–evoked CF GCaMP6f intensity plots of the 30 s recordings of nine independent CFs from three mice. The thick red trace represents the averaged trace. (c) Quantification of the burst-evoked GCaMP6f amplitudes. One-way ANOVA followed by Bonferroni test: ***p<0.001, *p<0.05. (d) Example trace of in vivo CF Ca2+ imaging showing a sampling of events for amplitude distribution analysis in e. Only the first-peak amplitudes out of 0.5 s window were analyzed. The short lines below the trace represent the sampled events. (e) Amplitude distribution of 31 CFs from four independent 3 min recordings of 2-photon Ca2+ imaging in three animals. The first-peak amplitudes were normalized with the median values to display all data and were presented as a heat map histogram, in which x and y represent normalized amplitudes and cell numbers, respectively. The color map scale shows the number of events.
-
Figure 2—source data 1
The CF Ca2+ activity is highly variable in awake micein vivo.
- https://cdn.elifesciences.org/articles/61593/elife-61593-fig2-data1-v2.xlsx
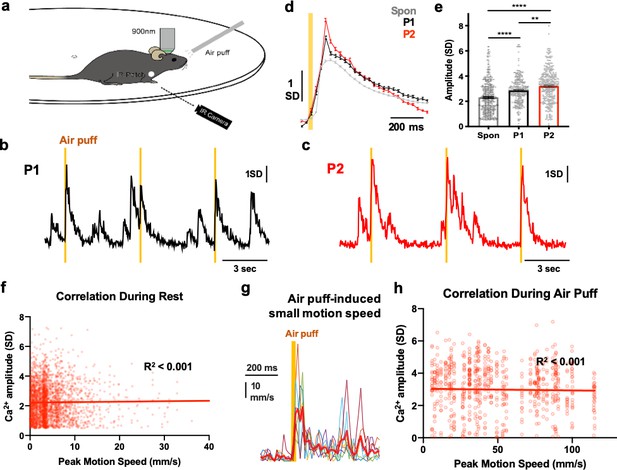
Sensory coding by CF Ca2+ signals.
(a) A schematic showing the 2-photon imaging with periocular air-puff stimulation and animal motion monitoring by IR camera. (b-c) Representative CF Ca2+ traces for spontaneous and 30 ms periocular air puffs (orange column) of pressure 1 (P1, b) and 2 (P2, c). (d-e) Averaged CF Ca2+ traces (d) and amplitudes (e) of spontaneous and air-puff responses of P1 and P2. n = 448, 283 and 346 CF Ca2+ events in 4 (P1) and 6 (P2) independent 1 min imaging sessions from four mice, respectively. One-way ANOVA followed by Tukey’s test: **p<0.01, ***p<0.001, **** p<0.0001. (f) Correlation analysis between CF Ca2+ amplitudes versus the peak speed of forepaw movement at rest. n = 2926 pairs from four recordings in two mice, R2 <0.001. (g) Representative traces of the speed of forepaw twitch-like movement during periocular air-puff stimulation. Air puff–to–motion onset time = 23.2 ± 2.9 SEM ms, air puff–to–peak motion speed time = 57.6 ± 5.8 SEM ms. (h) Correlation analysis between CF Ca2+ amplitudes versus the peak motion speed during air-puff stimulation. n = 760 pairs from four recordings in two mice. R2 <0.001.
-
Figure 3—source data 1
Sensory coding by CF Ca2+ signals.
- https://cdn.elifesciences.org/articles/61593/elife-61593-fig3-data1-v2.xlsx
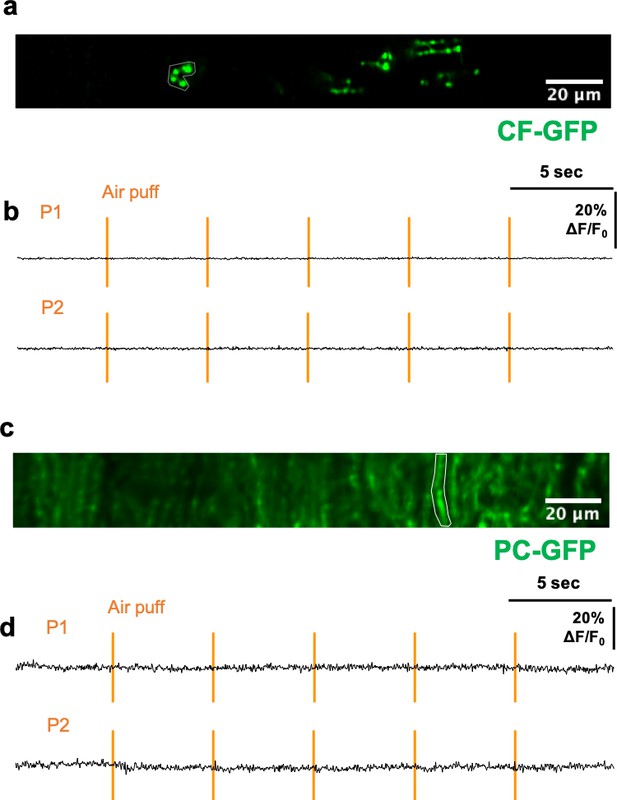
Minimal motion artifacts for imaging during air-puff stimulation.
(a) An averaged image of GFP expressed in CFs. GFP was expressed in IO neurons by injecting AAV-Camk2a-EGFP vector into the IO. (b) Traces of CF-GFP from the ROI in a. The ROI was selected after motion correction. The orange bars indicate the air-puff times for P1 and P2. (c) GFP was specifically expressed in the PC in Pcp2-cre mice using AAV-Flex-CAG-GCaMP6f. (d) Traces of PC-GFP from the ROI in c (white border). The ROI was selected after motion correction. The time when air puffs were given are indicated as orange bars. Mice were given P1 (above) and P2 (below) stimuli as indicated.
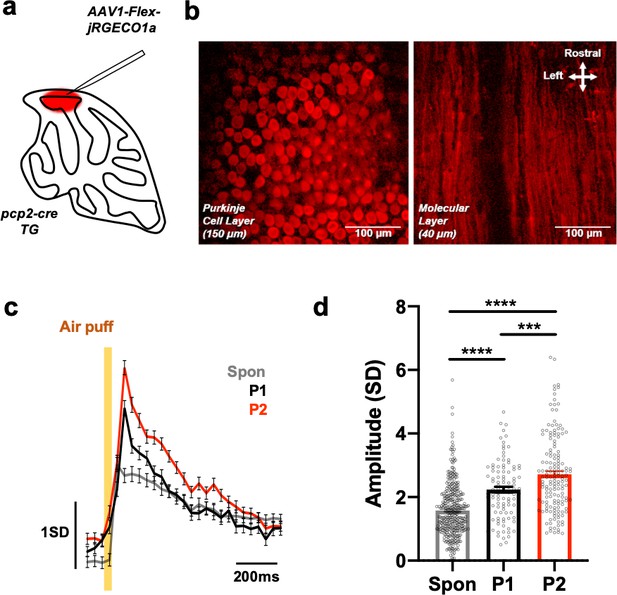
Sensory coding by Purkinje cell dendritic Ca2+ activity.
Related to Figure 3. (a) Specific expression of the red Ca2+ indicator jRGECO1a in PCs in Pcp2-cre TG mice. (b) Two-photon images in the PC and molecular layers. (c,d) Average traces (c) and the amplitudes of spontaneous and air puffevoked (P1 and P2) PC dendritic Ca2+ responses. The yellow bar indicates air-puff delivery. n = 213 (spon.), 168 (P1) and 186 (P2) events from two recordings for 1 min per condition performed in 27 dendrites from two mice. One-way ANOVA followed by post hoc multiple comparison Tukey tests: ***p<0.001.
-
Figure 3—figure supplement 2—source data 1
Sensory coding by Purkinje cell dendritic Ca2+ activity.
- https://cdn.elifesciences.org/articles/61593/elife-61593-fig3-figsupp2-data1-v2.xlsx
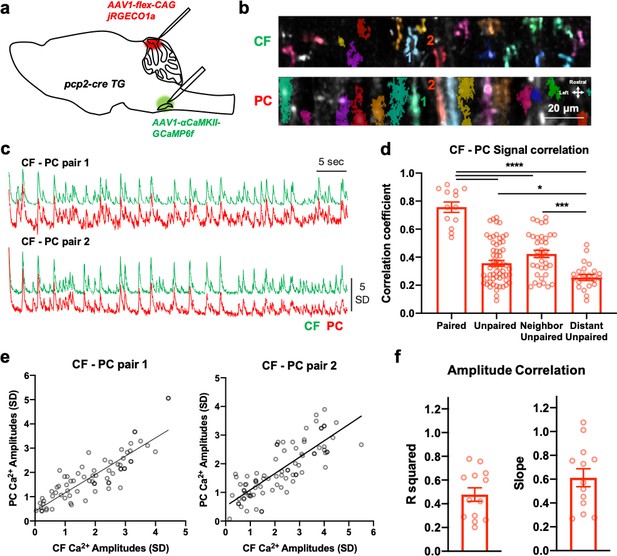
The direct translation of spike number for each CF burst by post-synaptic PC Ca2+ response.
(a) A schematic that shows the dual expression of Ca2+ indicator jRGECO1a and GCaMP6f in PC and CF, respectively. (b) ROI detection by Suite2p and average projection images of CF and PC dual-calcium imaging in the cerebellar cortex (50 µm from dura). Two examples of CF-PC pairs are indicated with number. (c) Traces of the two CF-PC pairs (1 and 2) from b. (d) Correlation coefficients of the signals are shown in four conditions which include ‘paired’, ‘unpaired’, ‘neighboring unpaired’ and ‘distant unpaired’. n = 13 (paired), 61 (unpaired), 37 (neighboring unpaired), and 24 (distant unpaired) CF-PC pairs. One-way ANOVA followed by post hoc Tukey multiple comparisons test. ****p<0.0001, ***p<0.001, *p<0.05. (e) A representative non-linear regression analysis of Ca2+ amplitudes in two pairs of CF-PC shown in c. n = 73 (pair 1) and 85 (pair 2) events. R2 = 0.756 (pair 1) and 0.658 (pair 2). (f) R2 values (left) and slope (right) for CF-PC pair Ca2+ amplitude-correlation analysis with 13 CF-PC pairs. Average R2 = 0.48 ± 0.06 SEM. Average slope = 0.61 ± 0.08 SEM. Data are from five independent recording sessions of three mice.
-
Figure 4—source data 1
The direct translation of spike number for each CF burst by post-synaptic PC Ca2+ response.
- https://cdn.elifesciences.org/articles/61593/elife-61593-fig4-data1-v2.xlsx
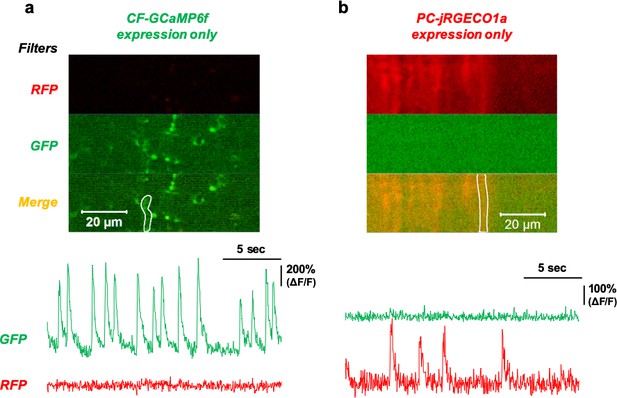
Limited signal bleed-through during simultaneous dual-color recording.
(a) Dual recording of the cerebellar cortex expressed only with GCaMP6f in the CF. Image of the imaging field (above) with the RFP and GFP filters set. The ROI selected for the representative traces is a white line. The intensity traces of the green and red signals are shown below. (b) Dual recording of the cerebellar cortex expressed only with jRGEGO1a in the PCs. The imaging field and ROI selection are shown above and traced below.
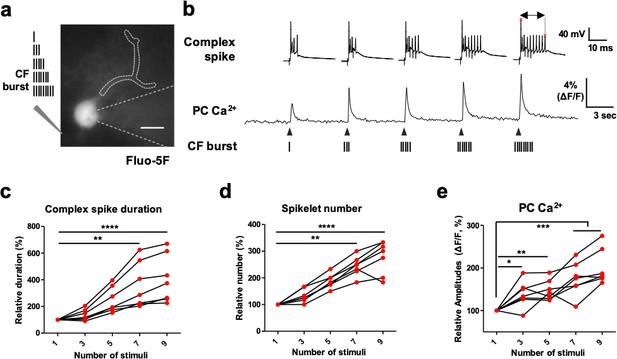
The CFs’ spike number-dependent Ca2+ influx in PC dendrites.
(a), Representative image of a PC filled with the low-affinity Ca2+ dye Fluo-5F taken with whole-cell recording. The schematics on the left describe the number of CF stimuli (1, 3, 5, 7, and 9) at 400 Hz. b, Representative aligned traces of CS recordings and PC Ca2+ traces measured by Fluo-5F in response to the indicated numbers of 400 Hz CF stimuli (1, 3, 5, 7, and 9). The length between the two red asterisks represents the duration of CS. c–e, CS duration (c), spikelet numbers, (d) and amplitudes of the post-synaptic PC Ca2+ transient (e) in response to different number of spikes in the CF burst stimuli. n = 7 recordings of seven independent cells from three mice. One-way ANOVA followed by Bonferroni test: *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
-
Figure 5—source data 1
The CFs’ spike number-dependent Ca2+ influx in PC dendrites.
- https://cdn.elifesciences.org/articles/61593/elife-61593-fig5-data1-v2.xlsx
Videos
CF-PC dual-calcium imaging.
60 s 32 Hz time-lapse movie for CF-PC dual imaging by two-photon microscopy using 1000 nm excitation. CF, PC, and merged images from the top to the bottom. Time scale is indicated in upper left and the scale bar is indicated lower right as 20 μm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background Mus musculus | B6.129-Tg(Pcp2-cre)2Mpin/J | Jackson Laboratory | RRID:IMSR_JAX:004146 | Stock no: 004146 |
| Strain, strain background M. | B6.Cg-Tg(Camk2a-cre)T29-1Stl/J | Jackson Laboratory | RRID:IMSR_JAX:005359 | Stock No: 005359 |
| Other | AAV1.Camk2a.GCaMP6f.WPRE.SV40 | Upenn Vector Core | ||
| Other | AAV1.CAG.FLEX.jRGECO1a.WPRE.SV40 | Upenn Vector Core | ||
| Other | AAV1.CAG.FLEX.GFP.WPRE.SV40 | Upenn Vector Core | ||
| Chemical compound, drug | Zoletil | Virvac | ||
| Chemical coumpound, drug | Rompun | Bayer | ||
| Chemical compound, drug | Dexamethasone | Samyang Phamaceutical | ||
| Chemical compound, drug | Meloxicam | Boehringer Ingelheim | ||
| Software, algorithm | MATLAB | Mathwroks Inc | RRID:SCR_002881 | |
| Software, algorithm | Python | https://www.python.org/ | RRID:SCR_008394 |