Pollen Tube Guidance: Growing straight through walls
In a flowering plant, reproduction begins when grains of pollen stick to cells called stigma papillae that are located at the top of the pistil, which is the female part of the flower. A cell called a pollen tube then delivers the sperm cells contained in the pollen grains to the female gametes for fertilization. This is a long journey that involves the pollen tube travelling from the stigma papillae at the top of the pistil to the ovules that contain the female gametes, which are at the bottom of the pistil.
So how does the plant ensure that the pollen tube – which is a single cell that grows longer over time – finds the ovules and does not get lost en route? Several molecules and nutrients secreted by the pistil direct the growth of the pollen tube (Higashiyama and Takeuchi, 2015). However, the identity of the cues that guide the pollen tube in the first stages of its journey have remained a mystery.
Most plant cells grow by increasing their surface area while remaining attached to neighboring cells: pollen tubes are different in that they are tip-growing cells that can grow through the walls of other cells to reach their target. When the pollen tube first enters the pistil, it remains within the cell wall of the stigma papillae (Figure 1; left): could the components of this cell wall, or the mechanical properties of these cells, influence the growth of the pollen tube?
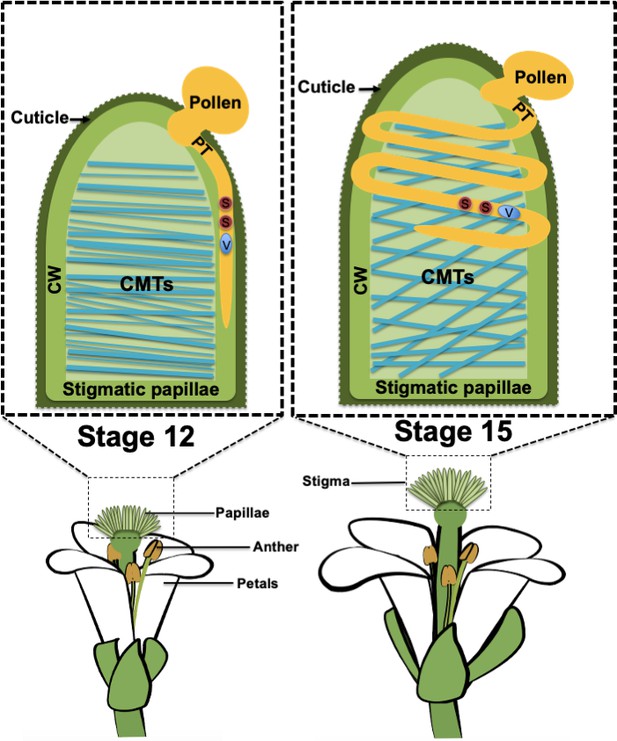
Pollen tube growth in stigma papillae.
When a grain of pollen (shown in mustard) lands on a papilla in the stigma (green) of a flowering plant, a pollen tube (PT; also shown in mustard) begins to grow through the cell wall (CW) of the papilla so that the sperm cells (S; red) in the pollen can be delivered to the female gametes, which are located in ovules deep inside the plant. In stage 12 flowers (left), the organization of the cortical microtubules (CMTs; blue lines) inside the papilla is highly anisotropic and the pollen tube grows in a straight line. In older stage 15 flowers (right), the organization of the microtubules is isotropic and the pollen tube forms a coil around the papilla as it grows. The vegetative cell (V) makes up the body of the pollen tube and encloses the sperm cells.
A number of studies have demonstrated how mechanical properties can influence a variety of cellular processes – including proliferation, differentiation, migration and cell signaling – in animal cells (Discher et al., 2005; Fu et al., 2010; Provenzano and Keely, 2011), and there is evidence that mechanical properties can also shape plant growth and development (Eng and Sampathkumar, 2018; Sampathkumar et al., 2019). For example, it is known that when a pollen tube penetrates the cell wall of a stigma papilla, it causes changes in the mechanical properties of the cell wall by exerting pressure (Zerzour et al., 2009; Sanati Nezhad and Geitmann, 2013).
However, the role of these mechanical properties in regulating the growth of pollen tube has not been explored in detail. Moreover, although the pollen tube is a good model for understanding the behavior of plant cells, and has been used in numerous in vitro studies of tip growth, it has proved challenging to study the directed growth of pollen tubes through the cell walls of stigma papillae in vivo. Now, in eLife, Thierry Gaude and co-workers at the Université de Lyon – including Lucie Riglet as first author – report the results of experiments on the model plant Arabidopsis thaliana that combine the power of microscopy, genetics, and chemical biology to provide new insights into the regulation of pollen tube growth (Riglet et al., 2020).
As stigmas age, they become less receptive to pollen (Gao et al., 2018), and the observation that pollen tubes tend to coil around papillae in aging stigmas forms the basis of this study. Riglet et al. found that aging was associated with changes in the organization of the cortical microtubules in the cytoskeleton: the orientations of these microtubules were more isotopic in older stigmas than in younger stigmas (Figure 1). To test the hypothesis that the organization of these microtubules has a role in directing pollen tube growth, the researchers examined plants that had a loss of function mutation in an enzyme called KATANIN (KTN1): this enzyme can sever microtubules, and thus allows microtubules to be re-oriented following mechanical stimulation (Sampathkumar et al., 2014). Riglet et al. found that pollen tubes coiled around the papillae in both young and old mutant plants: this indicates that the arrangement of the microtubules affects the ability of pollen tubes to grow straight through the cell walls and into the rest of the pistil.
Cortical microtubules are associated with cellulose synthesis, so the researchers tested whether the stiffness and composition of the cell wall in mutant and aging papillae was associated with pollen tube coiling. They found that softer cell walls and isotropic arrangements of cellulose microfibrils in mutant and aging papillae were associated with faster pollen tube growth and loss of directionality. Overall, the latest work supports the thesis that the mechanical properties and cell wall composition of the stigma papillae have an influence on pollen tube growth and help to guide it through the stigma. Moreover, by providing fundamental insights into the process of sexual reproduction in plants, the work is also relevant in the context of global food security as pollen-stigma interactions are critical for successful pollination and seed production in flowering plants.
Apart from pollen tubes, several types of plant, animal and fungal cells grow invasively, including root hairs, fibroblasts, cancer cells and fungal hyphae. In the future, it will be important to determine the contribution of mechanical forces to invasive growth. New technological advances such as lab-on-a-chip, MEMS (micro-electro-mechanical systems), deep-tissue imaging and computational tools will help researchers to measure the mechanical forces operating on and in cells (Nezhad et al., 2013). The pollen tube/pistil system will also make it possible to explore how chemical guidance cues work together with mechanical forces to regulate directional cell growth.
References
-
Getting into shape: the mechanics behind plant morphogenesisCurrent Opinion in Plant Biology 46:25–31.https://doi.org/10.1016/j.pbi.2018.07.002
-
The mechanism and key molecules involved in pollen tube guidanceAnnual Review of Plant Biology 66:393–413.https://doi.org/10.1146/annurev-arplant-043014-115635
-
Physical forces regulate plant development and morphogenesisCurrent Biology 24:R475–R483.https://doi.org/10.1016/j.cub.2014.03.014
-
The cellular mechanics of an invasive lifestyleJournal of Experimental Botany 64:4709–4728.https://doi.org/10.1093/jxb/ert254
Article and author information
Author details
Publication history
Copyright
© 2020, Sankaranarayanan and Kessler
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 4,059
- views
-
- 248
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Plant Biology
Obligate parasites often trigger significant changes in their hosts to facilitate transmission to new hosts. The molecular mechanisms behind these extended phenotypes - where genetic information of one organism is manifested as traits in another - remain largely unclear. This study explores the role of the virulence protein SAP54, produced by parasitic phytoplasmas, in attracting leafhopper vectors. SAP54 is responsible for the induction of leaf-like flowers in phytoplasma-infected plants. However, we previously demonstrated that the insects were attracted to leaves and the leaf-like flowers were not required. Here, we made the surprising discovery that leaf exposure to leafhopper males is required for the attraction phenotype, suggesting a leaf response that distinguishes leafhopper sex in the presence of SAP54. In contrast, this phytoplasma effector alongside leafhopper females discourages further female colonization. We demonstrate that SAP54 effectively suppresses biotic stress response pathways in leaves exposed to the males. Critically, the host plant MADS-box transcription factor short vegetative phase (SVP) emerges as a key element in the female leafhopper preference for plants exposed to males, with SAP54 promoting the degradation of SVP. This preference extends to female colonization of male-exposed svp null mutant plants over those not exposed to males. Our research underscores the dual role of the phytoplasma effector SAP54 in host development alteration and vector attraction - integral to the phytoplasma life cycle. Importantly, we clarify how SAP54, by targeting SVP, heightens leaf vulnerability to leafhopper males, thus facilitating female attraction and subsequent plant colonization by the insects. SAP54 essentially acts as a molecular ‘matchmaker’, helping male leafhoppers more easily locate mates by degrading SVP-containing complexes in leaves. This study not only provides insights into the long reach of single parasite genes in extended phenotypes, but also opens avenues for understanding how transcription factors that regulate plant developmental processes intersect with and influence plant-insect interactions.
-
- Microbiology and Infectious Disease
- Plant Biology
Programmed cell death occurring during plant development (dPCD) is a fundamental process integral for plant growth and reproduction. Here, we investigate the connection between developmentally controlled PCD and fungal accommodation in Arabidopsis thaliana roots, focusing on the root cap-specific transcription factor ANAC033/SOMBRERO (SMB) and the senescence-associated nuclease BFN1. Mutations of both dPCD regulators increase colonization by the beneficial fungus Serendipita indica, primarily in the differentiation zone. smb-3 mutants additionally exhibit hypercolonization around the meristematic zone and a delay of S. indica-induced root-growth promotion. This demonstrates that root cap dPCD and rapid post-mortem clearance of cellular corpses represent a physical defense mechanism restricting microbial invasion of the root. Additionally, reporter lines and transcriptional analysis revealed that BFN1 expression is downregulated during S. indica colonization in mature root epidermal cells, suggesting a transcriptional control mechanism that facilitates the accommodation of beneficial microbes in the roots.






