The effects of age and systemic metabolism on anti-tumor T cell responses
Figures
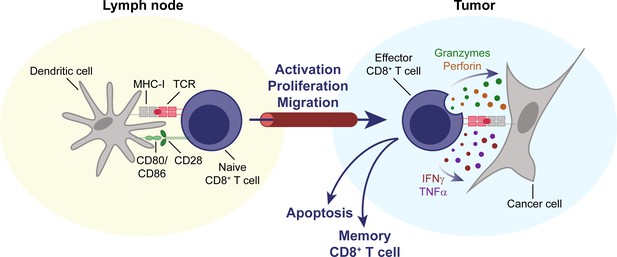
CD8+ T cells are key effectors of the anti-tumor immune response.
Naïve CD8+ T cells circulate through the body, until their cognate antigen is presented to them by an antigen-presenting cell, for example a dendritic cell, in a secondary lymphoid organ, for example a lymph node. T cells then become activated, proliferate, differentiate, and migrate to the tumor. Upon entering the TME, they can mediate an effective anti-tumor response through direct cytolytic activity, mediated by perforin and granzymes, and the secretion of cytokines like IFNγ and TNFα. IFNγ, interferon γ. MHC-I, major histocompatibility complex I. TCR, T cell receptor. TME, tumor microenvironment. TNFα, tumor necrosis factor α.
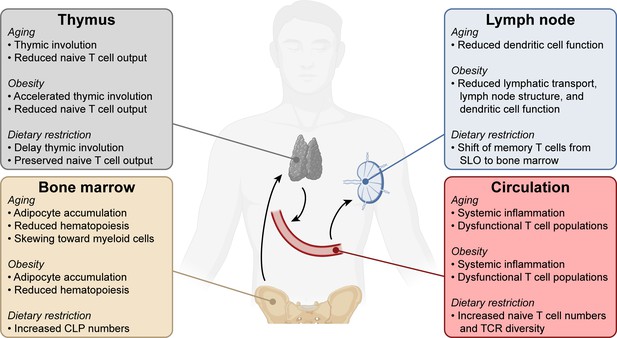
Systemic changes in the immune system with aging, obesity, and dietary restriction.
T cell progenitors arise in the bone marrow and travel to the thymus where they develop into mature naïve T lymphocytes. Naïve T cells circulate through the bloodstream and secondary lymphoid organs. Systemic conditions like aging, obesity, and dietary restriction affect the immune system at each of these levels, impacting both anti-tumor and other immune responses. Aging- and obesity-associated changes lead to reduced T cell immunity, while dietary restriction-associated changes promote T cell responses. CLP, common lymphoid progenitor. SLO, secondary lymphoid organs. TCR, T cell receptor.
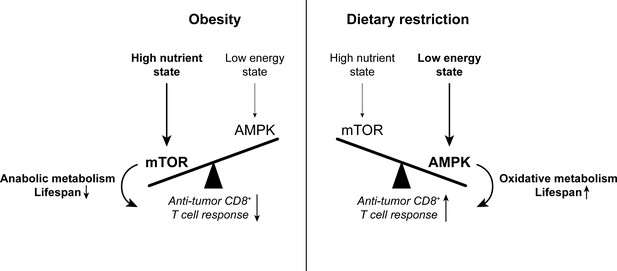
The balance between mTOR and AMPK signaling impacts anti-tumor immunity.
High nutrient states induce mTOR signaling, which promotes anabolic metabolism and reduces lifespan. Conversely, low energy states induce AMPK signaling, which promotes oxidative metabolism and extends lifespan. While mTOR signaling is important for effector T cell responses, there is evidence to suggest that memory T cell-like oxidative metabolism may be more beneficial for the anti-tumor T cell response. Since mTOR signaling is enhanced with obesity while AMPK signaling is promoted by dietary restriction, altering the mTOR-AMPK balance may one way by which systemic metabolic state can affect CD8+ T cell function in cancer. AMPK, AMP-activated protein kinase. mTOR, mammalian target of rapamycin.
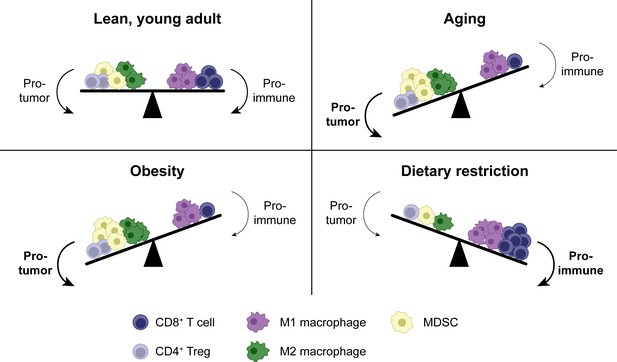
Shifts in the tumor immune infiltrate affect anti-tumor CD8+ T cell function in distinct systemic conditions.
In addition to CD8+ T cells, the TME contains other immune populations, some of which are immunostimulatory (e.g. M1-polarized macrophages), while others are suppressive (e.g. M2-polarized macrophages, MDSCs and Tregs). In aging, enhanced MDSC numbers and potentially M2 macrophages contribute to immunosuppression, resulting in a reduced anti-tumor CD8+ T cell response. Tregs may be either increased or decreased with aging. Similar shifts in cellular populations in the TME exist with obesity. In dietary restriction, Treg and MDSC numbers are reduced while macrophage polarization is shifted toward the M1 phenotype, resulting in an increased anti-tumor CD8+ T cell response. MDSC, myeloid-derived suppressor cell. TME, tumor microenvironment. Treg, regulatory T cell.
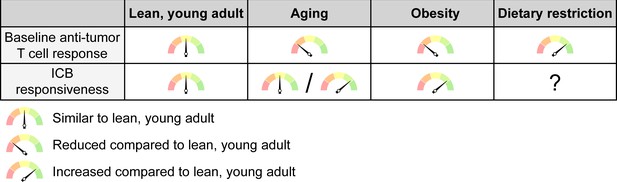
The net effects of systemic conditions on anti-tumor immunity and ICB responsiveness.
Aging and obesity lead to a reduced anti-tumor T cell response compared to a young, lean adult at baseline, i.e. without immunotherapy, while dietary restriction enhances the baseline anti-tumor immune response. However, the efficacy of ICB therapy is intact or even enhanced with aging and mostly increased with obesity, although this may not apply to all cancer types and patient subsets. ICB responsiveness with dietary restriction has not been sufficiently studied to know whether this is altered. ICB, immune checkpoint blockade.





