IL-2/JES6-1 mAb complexes dramatically increase sensitivity to LPS through IFN-γ production by CD25+Foxp3- T cells
Figures
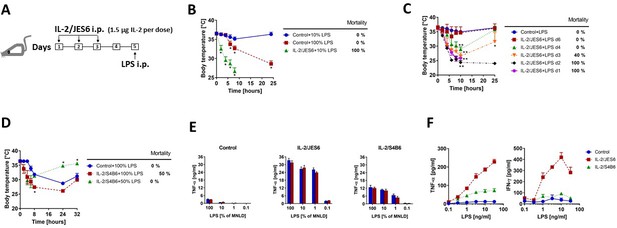
IL-2/JES6 dramatically increase sensitivity to LPS-induced shock and mortality.
(A) Schedule of sensitization of mice to LPS through administration of IL-2/JES6 used throughout the study, unless stated otherwise. (B) C56BL/6 mice were treated with IL-2/JES6 as shown in A. Control mice were treated with sterile PBS. The dose of injected LPS is shown in % of maximum non-lethal dose, henceforth (MNLD; 100% ~ 200 µg LPS/mice). (C) C56BL/6 mice were treated with IL-2/JES6 and LPS (10% of MNLD) was injected 1–6 d after the last dose of IL-2/JES6. (D) C56BL/6 mice were treated with complexes of IL-2 and S4B6 mAb (IL-2/S4B6) followed by LPS challenge (100 or 50% of MNLD) using the same schedule as in A. (E) C56BL/6 mice were treated as in A with PBS (Control), IL-2/JES6 or IL-2/S4B6 complexes. Mice were euthanized 90 min post administration of titrated doses of LPS and their individual sera were collected. Concentration of TNF-α in the serum was determined by ELISA. Each bar represents one individual mouse± SD (n = 3 technical replicates). (F) C56BL/6 mice were treated as in A with PBS (Control), IL-2/JES6 or IL-2/S4B6 complexes, but not challenged with LPS. Mice were euthanized 2 d after the last dose of complexes and their spleen cells were cultivated in titrated concentrations of LPS for 16 h in vitro. Concentrations of TNF-α and IFN-γ in the supernatant were determined by ELISA. Each point represents pool of three individual mouse ± SD (n = 3 technical replicates). All experiments were done at least twice with similar results; n = 4–7 technical replicates (B–D). Data were analysed using an unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05; ** p ≤ 0.01).
-
Figure 1—source data 1
Source data for Figure 1, panels B-F.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig1-data1-v1.xlsx
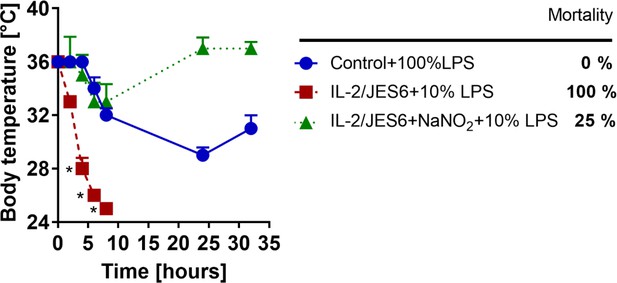
Nitrite significantly reduces toxicity of LPS in mice pretreated with IL-2/JES6.
C56BL/6 mice were treated with IL-2/JES6 and challenged with LPS (10% of MNLD) as shown in Figure 1A. One group of mice was i.p. injected with sodium nitrate (NaNO2; 1.5 mg/kg in 250 µl) 1 h rbefore LPS challenge. Control mice were challenged with LPS (100% of MNLD) only. Four mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05).
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig1-figsupp1-data1-v1.xlsx

IL-2/JES6 induce more severe lung oedema in comparison to IL-2/S4B6.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized 1 d after last dose of IL-2 complexes and their lungs were harvested. Pulmonary wet weight was determined by weighting lungs before and after lyophilization overnight at 58 °C under vacuum. Six mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05; ** p ≤ 0.01).
-
Figure 1—figure supplement 2—source data 1
Source data for Figure 1—figure supplement 2.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig1-figsupp2-data1-v1.xlsx
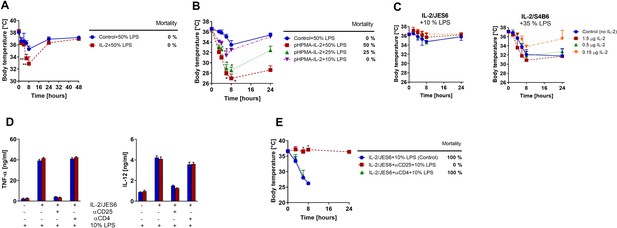
Strong sustained IL-2 signal and not solely IL-2/JES6, increases sensitivity to LPS; IL-2/JES6-mediated sensitivity to LPS could be completely blocked by αCD25 mAb.
(A) C56BL/6 mice were treated with rmIL-2 (35 µg/dose) according to the schedule shown in Figure 1A and challenged with LPS (50% of MNLD). (B) C56BL/6 mice were treated with IL-2 covalently bound to the polymeric carrier based on poly(HPMA) using the same schedule as in Figure 1A and subsequently challenged with titrated doses of LPS. (C) C56BL/6 mice were injected simultaneously with LPS and either IL-2/JES6 or IL-2/S4B6 complexes (1.5 or 0.5 or 0.15 µg IL-2/dose) in one i.p. injection. Dosage of LPS is shown in % of MNLD above each graph. Control mice were injected with the same dose of LPS only. (D) C56BL/6 mice were injected with IL-2/JES6 and then challenged with LPS (10% of MNLD) as shown in Figure 1A. Some mice were also injected with either αCD25 or αCD4 mAb (250 µg/mouse i.p.) 4 h prior to the first injection of IL-2/JES6 as shown below in the graphs. Mice were euthanized 4 h after the LPS challenge and concentrations of TNF-α and IL-12 were determined by ELISA. Each bar represents one individual mouse± SD (n = 3 technical replicates). (E) C56BL/6 mice were treated with IL-2/JES6 and subsequently challenged with LPS as shown in Figure 1A. Some mice were also injected with either αCD25 or αCD4 mAb (250 µg per mouse i.p.) 4 h prior to the first injection of IL-2/JES6. All experiments were done at least twice with similar results; n = 4–6 technical replicates. Data were analysed using an unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05; ** p ≤ 0.01).
-
Figure 2—source data 1
Source data for Figure 2, panels A-E.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig2-data1-v1.xlsx
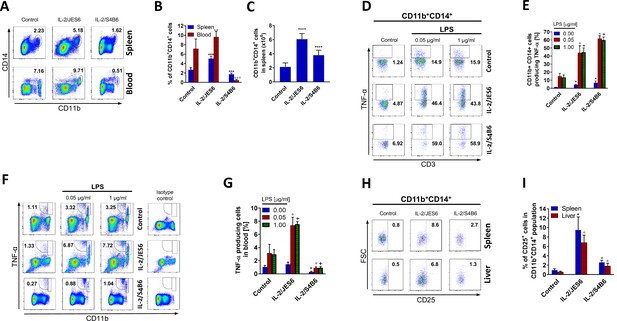
IL-2/JES6 increases counts of CD11b+CD14+ cells and their responsiveness to LPS in terms of TNF-α production.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. The relative number of CD11b+CD14+ cells was determined by flow cytometry in blood and spleen 2 d after the last dose of IL-2 complexes. Dot plots showing one representative mouse (A) and a bar graph showing the mean ± SD in the experimental groups (B) are shown. (C) Absolute numbers of CD11b+CD14+ cells in the spleen of mice from the same experiment as shown in A and B. C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Spleen cells were cultivated ex vivo with LPS for 2 h and TNF-α production in CD11b+CD14+ cells was determined by flow cytometry. Dot plots showing one representative mouse (D) and a bar graph showing the mean ± SD in experimental groups (E) are shown. A similar experiment to the one shown in D and E was done using the blood of C56BL/6 mice treated with IL-2/JES6 or IL-2/S4B6. TNF-α production in CD11b+ cells is shown in one representative mouse (F) and in a bar graph showing the mean ± SD in experimental groups (G). (H) C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Flow cytometry analysis of spleen and liver cells was used to evaluate CD25 expression in CD11b+CD14+ cells. Dot plots showing one representative mouse (H) and a bar graph showing mean ± SD in experimental groups (I) are presented. All experiments were done at least twice with similar results; n = 3–10 technical replicates. Data were analysed using an unpaired two-tailed Student’s t-test. Significant differences to control are shown (*,°,+ p ≤ 0.05; °° p ≤ 0.01; ***, °°° p ≤ 0.001).
-
Figure 3—source data 1
Source data for Figure 3, panels B, C, E, G, and I.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-data1-v1.xlsx
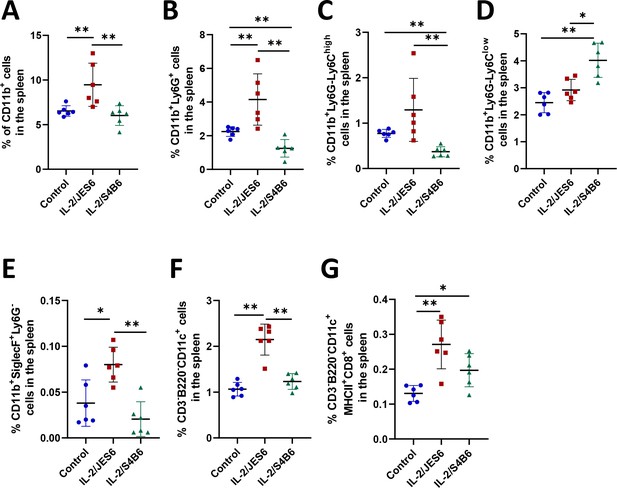
IL-2/JES6 expands various subsets of myeloid cells in the spleen.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized 2 d after last dose of IL-2 complexes and their spleens were harvested and analyzed by flow cytometry. Relative counts of myeloid cells in general (A), granulocytes (B), monocytes/macrophages (C, D), eosinophils (E) and dendritic cells (F, G) are shown. Six mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences are shown (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1, panels A-G.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp1-data1-v1.xlsx
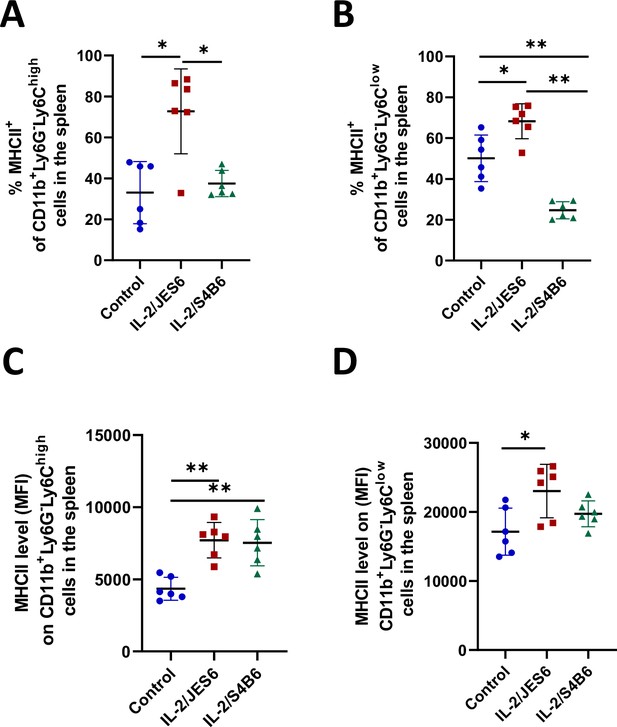
IL-2/JES6 increase MHC II expression in monocyte/macrophage population in the spleen.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized 2 d after last dose of IL-2 complexes and their spleens were harvested and analyzed by flow cytometry. Percentage of MHC II positive cells (A, B) and relative MHC II expression level (C, D) are shown. Six mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences are shown (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
-
Figure 3—figure supplement 2—source data 1
Source data for Figure 3—figure supplement 2, panels A-D.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp2-data1-v1.xlsx
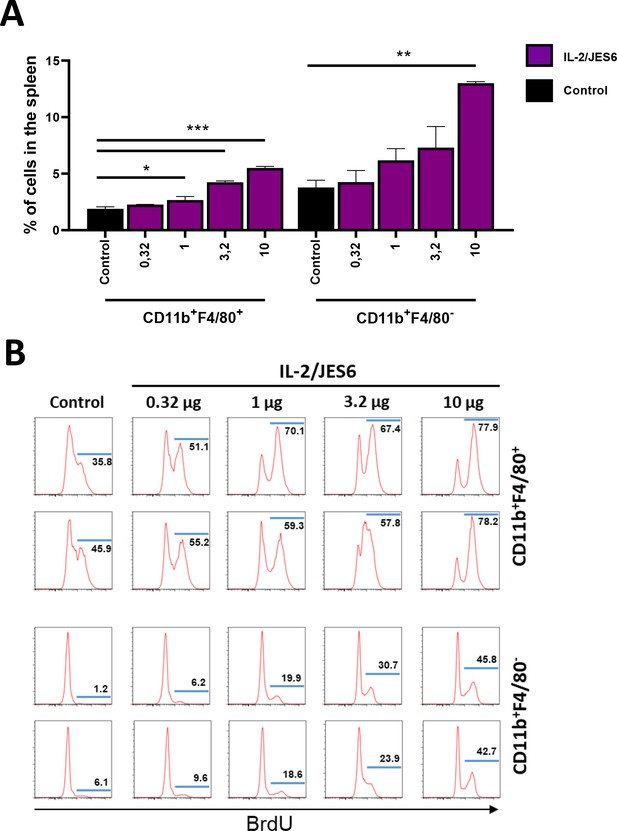
IL-2/JES6 promote proliferation and expansion of myeloid cells in dose-dependent manner in the spleen.
C56BL/6 mice were treated with titrated single dose of IL-2/JES6. Mice were i.p. injected with BrdU 4 h after administration of IL-2/JES and put on drinking water with BrdU. Mice were euthanized 2 d after administration of IL-2/JES6 and their spleens were harvested and analyzed by flow cytometry. Relative counts (A) and BrdU incorporation (B) of F4/80 positive and negative myeloid cells are shown. Two mice per group were used. Data were analysed using one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. Significant differences are shown (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
-
Figure 3—figure supplement 3—source data 1
Source data for Figure 3—figure supplement 3, panel A.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp3-data1-v1.xlsx

The expression of TLR4 in splenocytes of C57BL/6 mice is not affected by the treatment with IL-2/JES6 or IL-2/S4B6.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized on day five and mRNA was isolated from harvested spleen cells. Relative expression of Tlr4 was normalized to two reference genes (Casc3, H6pd). Data were analysed using unpaired two-tailed Student’s t-test. Data is presented as mean ± SD of six mice per group.
-
Figure 3—figure supplement 4—source data 1
Source data for Figure 3—figure supplement 4.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp4-data1-v1.xlsx
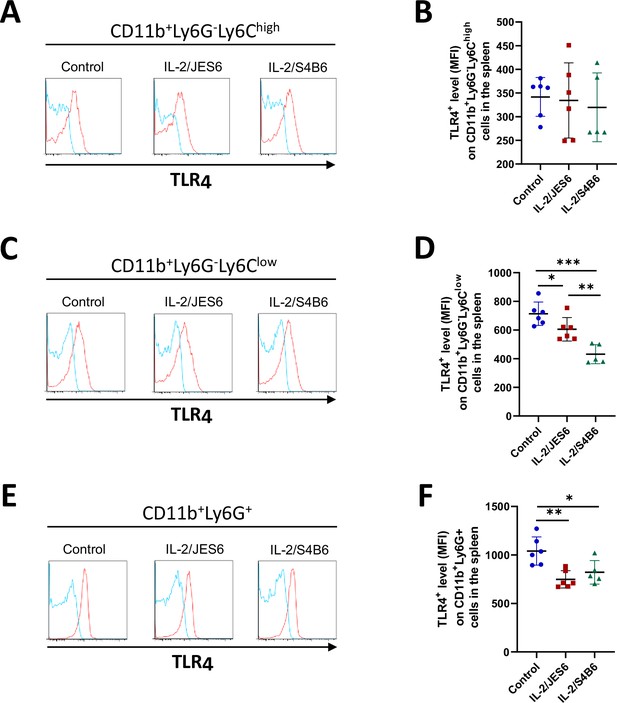
IL-2/JES6 do not increase the level of TLR4 in myeloid cells in the spleen.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized 2 d after last dose of IL-2 complexes and their spleens were harvested. Spleen cells were stained for surface markers (including TLR4), fixed, permeabilized, stained again for TLR4 and analyzed by flow cytometry. Histograms showing one representative mouse (A, C, and E; blue line: isotype control mAb, red line: anti-TLR4 mAb) and graphs showing average± SD in experimental groups (B, D and F) are presented. Six (control, IL-2/JES6) or five (IL-2/S4B6) mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences are shown (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
-
Figure 3—figure supplement 5—source data 1
Source data for Figure 3—figure supplement 5, panels B, D and F.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp5-data1-v1.xlsx
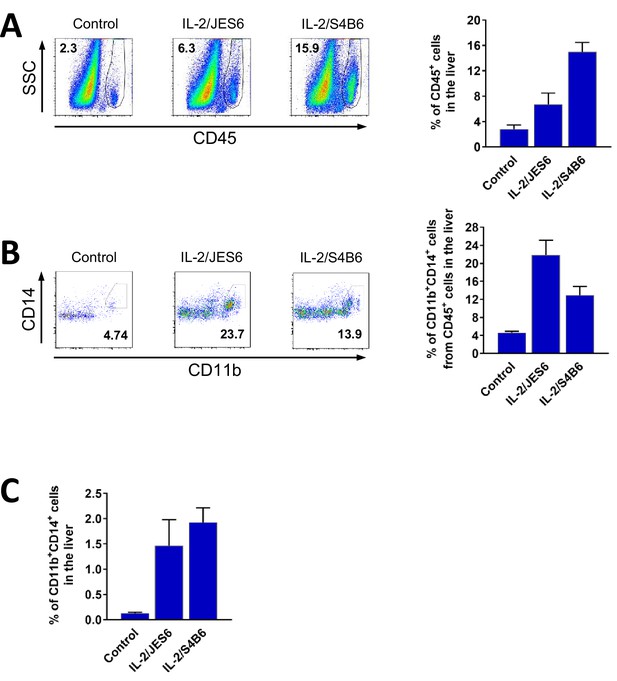
IL-2/JES6 increase counts of CD45+ and CD11b + CD14+ cells in the liver.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized 2 d after last dose of IL-2 complexes and their livers were harvested. Relative number of CD45+ cells in the liver (A), CD11b+ CD14+ cells within CD45+ cells in the liver (B) and CD11b+ CD14+ cells in the liver (C) were determined by flow cytometry. Dot plots showing one representative mouse (A and B) and a bar graph showing average± SD in experimental groups (B–C) are presented. Three mice per group were used.
-
Figure 3—figure supplement 6—source data 1
Source data for Figure 3—figure supplement 6, panels A-C.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp6-data1-v1.xlsx
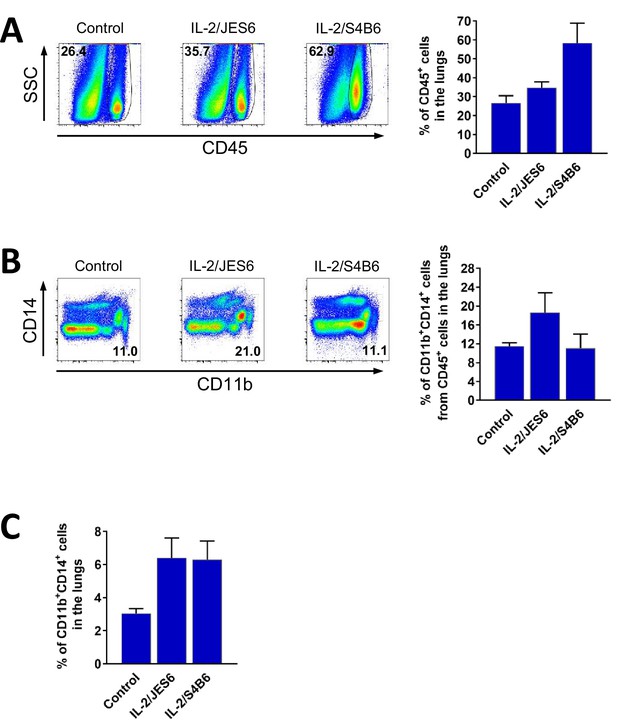
IL-2/JES6 increase counts of CD45+ and CD11b + CD14+ cells in the lungs.
C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were euthanized 2 d after last dose of IL-2 complexes and their lungs were harvested. Relative number of CD45+ cells in the lungs (A), CD11b+ CD14+ cells within CD45+ cells in the lungs (B) and CD11b+ CD14+ cells in the lungs (C) were determined by flow cytometry. Dot plots showing one representative mouse (A and B) and a bar graph showing average ± SD in experimental groups (B–C) are presented. Three mice per group were used.
-
Figure 3—figure supplement 7—source data 1
Source data for Figure 3—figure supplement 7, panels A-C.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig3-figsupp7-data1-v1.xlsx
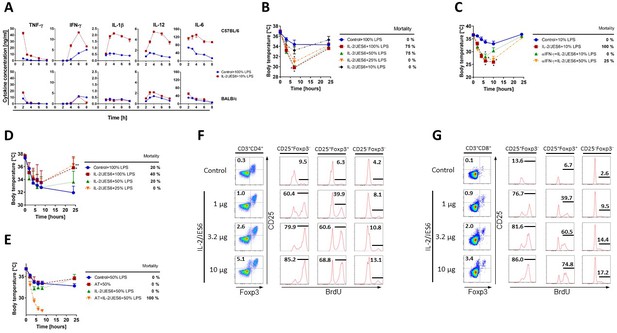
Sensitization to LPS via IL-2/JES6 requires endogenous IFN-γ production and is T cell-dependent.
(A) C56BL/6 and BALB/c mice were treated with IL-2/JES6 and challenged with LPS (10% of MNLD) as shown in Figure 1A. Control mice were treated with PBS and challenged with LPS (100% of MNLD). Mice were euthanized at selected time points and their individual sera were collected. Concentrations of cytokines in the serum were determined by ELISA. Each experimental point represents the mean of two mice ± SD (n = 2 technical replicates). (B) BALB/c mice were treated with IL-2/JES6 and challenged with titrated doses of LPS as shown in Figure 1A. (C) C56BL/6 mice were treated with IL-2/JES6 and challenged with LPS (10 or 50% of MNLD) as shown in Figure 1A. Some mice were also injected with anti-IFN-γ mAb (αIFN-γ; 250 µg/mice i.p.) 4 h prior to the first injection of IL-2/JES6. (D) Nu/Nu mice were treated with IL-2/JES6 and challenged with titrated doses of LPS as shown in Figure 1A. (E) Two groups of Nu/Nu mice were adoptively transferred (AT) with 2 × 106 CD4+CD25+ T cells from C56BL/6 mice pretreated with IL-2/JES6 as shown in Figure 1A. One group of AT mice and one group of normal Nu/Nu mice were treated with IL-2/JES6 as shown in Figure 1A. All groups including Nu/Nu mice without AT and IL-2/JES6 treatment (Control) were challenged with LPS (50% of MNLD). C56BL/6 mice were injected with one titrated dose of IL-2/JES6 or with PBS (Control). Mice were injected i.p. with BrdU 4 h after injection of IL-2/JES6 and put on drinking water with BrdU. Mice were euthanized 48 h after the injection of IL-2/JES6 and their CD4+ and CD8+ T cells (F and G, respectively) from the spleen were analysed by flow cytometry. One representative mouse out of two for each condition is shown. All experiments were done at least twice with similar results; n = 2–5 technical replicates (B–G). Data were analysed using an unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05; ** p ≤ 0.01).
-
Figure 4—source data 1
Source data for Figure 4, panels A-E.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig4-data1-v1.xlsx
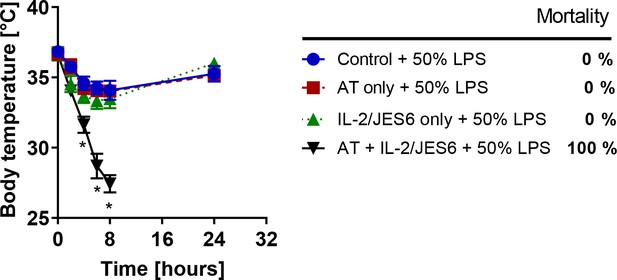
TLR4 signalling in T cells is dispensable for inducing LPS hypersensitivity by IL-2/JES6.
Two groups of Rag1-/- mice were adoptively transferred (AT) with 5.6 × 106 T cells (CD3+ cells sorted by MACS using negative selection) from MyD88-/- mice pretreated with IL-2/JES6 as shown in Figure 1A. One group of AT mice and one group of normal Rag1-/- mice were treated with IL-2/JES6 as shown in Figure 1A. All experimental groups including Rag1-/- mice without AT and IL-2/JES6 treatment (Control) were challenged with LPS (50% of MNLD). Four mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05).
-
Figure 4—figure supplement 1—source data 1
Source data for Figure 4—figure supplement 1.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig4-figsupp1-data1-v1.xlsx
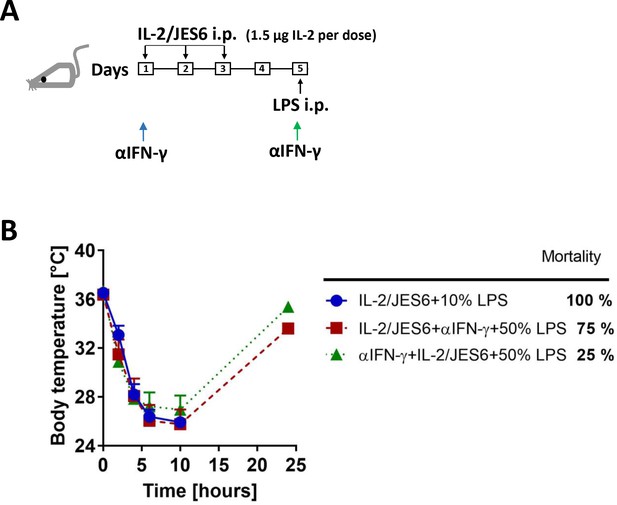
Anti-IFN-γ mAb protects from sensitization to LPS more effectively when administered before treatment with IL-2/JES6.
(A) Schedule of sensitization of mice to LPS by IL-2/JES6 and administration of anti-IFN-γ mAb (αIFN-γ; 250 µg/mice i.p.). Anti-IFN-γ mAb was injected either 4 h before first dose of IL-2/JES6 (blue arrow) or 4 h before LPS challenge (green arrow). (B) C56BL/6 mice were treated with IL-2/JES6 and challenged with LPS (10% and 50% of MNLD for control and mice injected with anti-IFN-γ mAb, respectively) as shown in A. Four mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test.
-
Figure 4—figure supplement 2—source data 1
Source data for Figure 4—figure supplement 2, panel B.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig4-figsupp2-data1-v1.xlsx
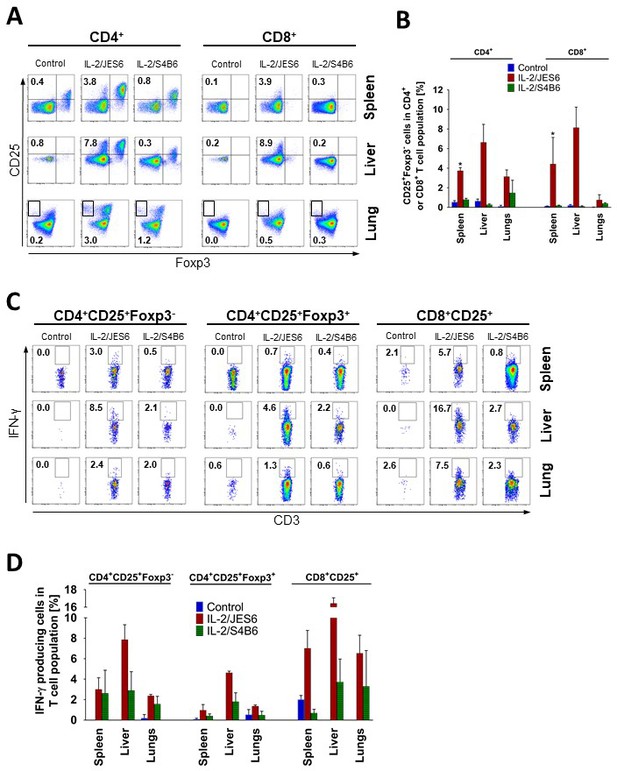
IL-2/JES6 expands CD25+Foxp3-T cells in both CD4+ and CD8+ subsets which produces IFN-γ in various tissues.
(A) C56BL/6 mice were treated with IL-2/JES6 or IL-2/S4B6 as shown in Figure 1A. Mice were i.p. injected with 150 µg/mouse of brefeldin A 2 h after the last dose of IL-2/JES6 and euthanized 12 h after the injection of brefeldin A. CD4+ and CD8+ T cells from spleen, liver, and lung were analysed by flow cytometry for CD25 and Foxp3 expression. Dot plots showing one representative mouse (A) and a bar graph showing mean ± SD in experimental groups (B) are presented. Intracellular production of IFN-γ in different subpopulations of T cells as shown in A, was determined. Dot plots showing one representative mouse (C) and a bar graph showing mean ± SD in experimental groups (D) are presented. Analysis of T cells from spleen and liver was carried out in the same experiment, but analysis of T cells from the lungs was done in a separate experiment. All experiments were done at least twice with similar results; n = 3 technical replicates. Data were analysed using an unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05).
-
Figure 5—source data 1
Source data for Figure 5, panels B and D.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig5-data1-v1.xlsx
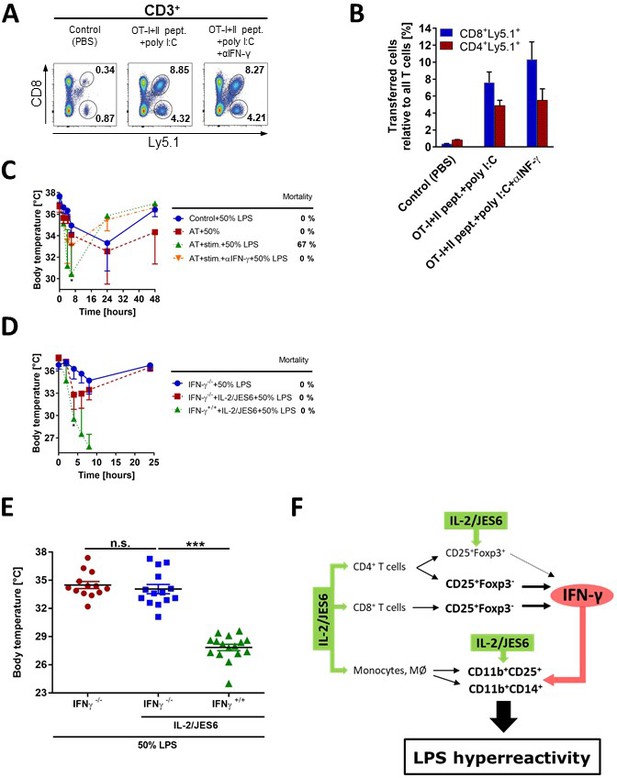
IFN-γ produced by activated antigen-specific T cells or by T cells stimulated with IL-2/JES6, is a crucial mediator of LPS hyperreactivity.
Purified CD8+ and CD4+ T cells from OT-I/Ly5.1 and OT-II/Ly5.1 mice (2 and 4 × 106 /mouse respectively), were adoptively transferred (AT) to C56BL/6 mice. One day later, mice were i.p. injected with PBS (Control), with both OT-I and OT-II specific peptides plus polyI:C (75 µg/mice) or with the latter plus αIFN-γ mAb (250 µg). Expansion of AT T cells was determined 3 d post stimulation by flow cytometry. Dot plots showing one representative mouse (A) out of two mice analysed by flow cytometry and a bar graph showing mean ± SD (B) are presented. (C) Three groups of C56BL/6 mice described above and one control group (no AT) were challenged with LPS (50% of MNLD). (D) IFN-γ-/- and normal C56BL/6 mice (IFN-γ+/+) were treated with IL-2/JES6 and challenged with LPS (50% of MNLD) as shown in Figure 1A. IFN-γ-/- C56BL/6 mice challenged with the same dose of LPS were used as the control. (E) Data pooled from three independent experiments (n = 13–16 technical replicates) described in D showing body temperature of mice 8 h after LPS challenge. (F) Scheme showing the proposed mechanism of how IL-2/JES6 induces LPS hyperreactivity. Experiments A-D were done at least twice with similar results; n = 2–6 technical replicates. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05; *** p ≤ 0.001).
-
Figure 6—source data 1
Source data for Figure 6, panels B-E.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig6-data1-v1.xlsx
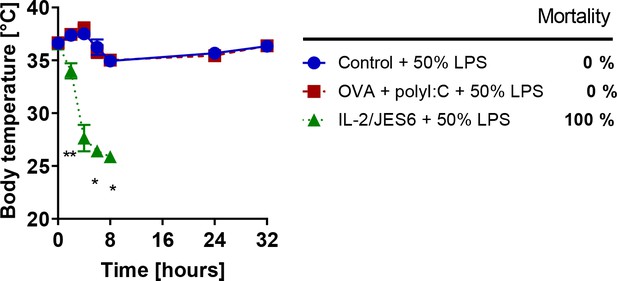
Immunization with ovalbumin plus poly I:C does not increase sensitivity to LPS.
C56BL/6 mice were treated with IL-2/JES6 and challenged with LPS (50% of MNLD) as shown in Figure 1A. Another group of C56BL/6 mice was immunized with ovalbumin (OVA; 0.5 mg/mice i.p) plus poly I:C (75 µg/mice i.p.) on day one and challenged with LPS (50% of MNLD) on day 5. Control group was challenged with the same dose of LPS only. Five mice per group were used. Data were analysed using unpaired two-tailed Student’s t-test. Significant differences to control are shown (* p ≤ 0.05; ** p ≤ 0.01).
-
Figure 6—figure supplement 1—source data 1
Source data for Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/62432/elife-62432-fig6-figsupp1-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Anti-mouse CD3-eFluor 450 (clone: 17A2; rat monoclonal) | eBioscience | Cat#: 48-0032-82; RRID:AB_1272193 | Flow Cytometry: (dilution 1:30) |
| Antibody | Anti-mouse CD8a-PerCP-Cyanine5.5 (clone: 53–6.7; rat monoclonal) | eBioscience | Cat#: 45-0081-82; RRID:AB_1107004 | Flow Cytometry: (dilution 1:100) |
| Antibody | Anti-mouse CD11b-Alexa Fluor 700 (clone: M1/70; rat monoclonal) | eBioscience | Cat#: 56-0112-82; RRID:AB_1107004 | Flow Cytometry: (dilution 1:300) |
| Antibody | Anti-mouse CD11b-eFluor450 (clone: M1/70; rat monoclonal) | eBioscience | Cat#: 48-0112-82; RRID:AB_1582236 | Flow Cytometry: (dilution 1:500) |
| Antibody | Anti-mouse CD14-FITC (clone: Sa2-8; rat monoclonal) | eBioscience | Cat#: 11-0141-85; RRID:AB_464950 | Flow Cytometry: (dilution 1:20) |
| Antibody | Anti-mouse CD25-APC (clone: PC61.5; rat monoclonal) | eBioscience | Cat#: 17-0251-82; RRID:AB_469366 | Flow Cytometry: (dilution 1:500) |
| Antibody | Anti-mouse CD25-eFluor 450 (clone: PC61.5; rat monoclonal) | eBioscience | Cat#: 48-0251-82; RRID:AB_10671550 | Flow Cytometry: (dilution 1:400) |
| Antibody | Anti-mouse CD45.1-APC (clone: A20; mouse monoclonal) | eBioscience | Cat#: 17-0453-82; RRID:AB_469398 | Flow Cytometry: (dilution 1:200) |
| Antibody | Anti-mouse Ly-6C-APC-eFluor 780 (clone: HK1.4; rat monoclonal) | eBioscience | Cat#: 47-5932-82; RRID:AB_2573992 | Flow Cytometry: (dilution 1:200) |
| Antibody | Anti-mouse MHC Class II (I-A/I-E)-FITC (clone: M5/114.15.2; rat monoclonal) | eBioscience | Cat#: 11-5321-82; RRID:AB_465232 | Flow Cytometry: (dilution 1:300) |
| Antibody | Anti-mouse CD45.1-eFluor 450 (clone: A20; mouse monoclonal) | eBioscience | Cat#: 48-0453-82; RRID:AB_1272189 | Flow Cytometry: (dilution 1:200) |
| Antibody | Anti-mouse CD45/B220-Horizont V500 (clone: RA3-6B2; rat monoclonal) | BD Biosciences | Cat#: 561226; RRID:AB_10563910 | Flow Cytometry: (dilution 1:100) |
| Antibody | Anti-mouse CD3e-Horizont V500 (clone: 500A2; Syrian hamster monoclonal) | BD Biosciences | Cat#: 560771; RRID:AB_1937314 | Flow Cytometry: (dilution 1:30) |
| Antibody | Anti-mouse CD4-Horizont V500 (clone: RM4.5; rat monoclonal) | BD Biosciences | Cat#: 560782; RRID:AB_1937327 | Flow Cytometry: (dilution 1:400) |
| Antibody | Anti-mouse CD4-PerCP (clone: RM4-5; rat monoclonal) | BD Biosciences | Cat#: 553052; RRID:AB_394587 | Flow Cytometry: (dilution 1:200) |
| Antibody | Anti-mouse CD8a-V500 (clone: 53–6.7; rat monoclonal) | BD Biosciences | Cat#: 560776; RRID:AB_1937317 | Flow Cytometry: (dilution 1:80) |
| Antibody | Anti-mouse Ly-6G-Alexa Fluor 700 (clone: 1A8; rat monoclonal) | BD Biosciences | Cat#: 561236; RRID:AB_10611860 | Flow Cytometry: (dilution 1:80) |
| Antibody | Anti-mouse Siglec-F-PE (clone: E50-2440; rat monoclonal) | BD Biosciences | Cat#: 562068; RRID:AB_394341 | Flow Cytometry: (dilution 1:100) |
| Antibody | Anti-mouse TLR4-APC (clone: SA15-21; rat monoclonal) | BioLegend | Cat#: 145406; RRID:AB_2562503 | Flow Cytometry: (dilution 1:300) |
| Antibody | Anti-mouse TNF alpha-PE (clone: MP6-XT22; rat monoclonal) | eBioscience | Cat#: 12-7321-82; RRID:AB_466199 | Flow Cytometry: (dilution 0.35 µL/test) |
| Antibody | Anti-FOXP3-PE (clone: FJK/16 s; rat monoclonal) | eBioscience | Cat#: 12-5773-82; RRID:AB_465936 | Flow Cytometry: (dilution 1 µL/test) |
| Antibody | Anti-mouse IFN gamma-PE (clone: XMG1.2; rat monoclonal) | eBioscience | Cat#: 12-7311-82; RRID:AB_466193 | Flow Cytometry: (dilution 0.35 µL/test) |
| Antibody | Anti-mouse CD16/32 (clone: 03; rat monoclonal) | eBioscience | Cat#: 14-0161-86; RRID:AB_467135 | Flow Cytometry: (dilution 0.5 µg/test) |
| Antibody | Anti-mouse IFN gamma (clone: XMG1.2; rat monoclonal) | BioXCell | Cat#: BE0055; RRID:AB_1107694 | In vivo IFN gamma neutralization (dose: 250 µg/mouse) |
| Antibody | Anti-mouse IL-2 (clone: JES6-1A12; rat monoclonal) | BioXCell | Cat#: BE0043; RRID:AB_1107702 | In vivo IL-2 receptor stimulation as a complex with IL-2 (dose: 1.5 µg /mouse) |
| Antibody | Anti-mouse IL-2 (clone: S4B6-1; rat monoclonal) | BioXCell | Cat#: BE0043-1; RRID:AB_1107705 | In vivo IL-2 receptor stimulation as a complex with IL-2 (dose: 1.5 µg /mouse) |
| Antibody | Anti-mouse CD4 (clone: GK1.5; rat monoclonal) | BioXCell | Cat#: BE0003-1; RRID:AB_1107636 | In vivo CD4+ T cell depletion (dose: 50 µg /mouse) |
| Antibody | Anti-mouse CD25 (clone: PC61.5; rat monoclonal) | BioXCell | Cat#: BE0012; RRID:AB_1107619 | In vivo regulatory T cell depletion (dose: 50 µg /mouse) |
| Sequence-based Reagent | Casc3for | Sigma-Aldrich | 5´-TTCGAGGTGTGCCTAACCA-3´ | |
| Sequence-based Reagent | Casc3rev | Sigma-Aldrich | 5´-GCTTAGCTCGACCACTCTGG-3´ | |
| Sequence-based Reagent | H6pdfor | Sigma-Aldrich | 5´-GGATTGTGTTTAAGAATCGGG-3´ | |
| Sequence-based Reagent | H6pdrev | Sigma-Aldrich | 5´-AGTAGGCGTCTTGCTC-3´ | |
| Sequence-based Reagent | Tlr4for | Sigma-Aldrich | 5´-GATCATGGCACTGTTCTTCTC-3´ | |
| Sequence-based Reagent | Tlr4rev | Sigma-Aldrich | 5´-CACACCTGGATAAATCCAGC-3´ | |
| Strain, Strain Background (Salmonella Typhymurium) | LT2 (S-strain) | Gift from Dr. H. Nikaido (University of California, Berkeley) | LPS isolation | |
| Strain, Strain Background (Mus musculus; Female) | BALB/c (H-2d) | Institute of Microbiology and Institute of Physiology of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus; Male) | C57BL/6 (H-2b) | Institute of Microbiology and Institute of Physiology of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus; Female) | CD1 nude mice (Nu/Nu) | Animal facility of Masaryk University, Czech Republic | ||
| Strain, Strain Background (Mus musculus, C57BL/6 J) | Transgenic OVA-specific T cells (OT-I) mice | Institute of Microbiology of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus, C57BL/6 J) | Transgenic OVA-specific T cells (OT-II) mice | Institute of Microbiology of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus; C57BL/6 J) | IFN gamma deficient (Ifng-/-) | Institute of Microbiology of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus; C57BL/6 J) | MyD88 deficient (Myd88-/-) | Institute of Molecular Genetics of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus; C57BL/6 J) | Rag1 deficient (Rag1-/-) | Institute of Molecular Genetics of the Czech Academy of Sciences | ||
| Strain, Strain Background (Mus musculus, C57BL/6 J) | B6.SJL (Ly5.1) | Institute of Microbiology of the Czech Academy of Sciences | ||
| Peptide, Recombinant Protein | Recombinant Murine IL-2 | PeproTech | Cat#: 212–12 | Purified from E. coli |
| Commercial Assay or Kit | Mouse TNF-alpha DuoSet ELISA | R&D Systems | Cat#: DY410 | |
| Commercial Assay or Kit | Mouse IFN-gamma DuoSet ELISA | R&D Systems | Cat#: DY485 | |
| Commercial Assay or Kit | Mouse IL-1 beta/IL-1F2 DuoSet ELISA | R&D Systems | Cat#: DY401 | |
| Commercial Assay or Kit | Mouse IL-12 p70 DuoSet ELISA | R&D Systems | Cat#: DY419 | |
| Commercial Assay or Kit | Mouse IL-6 DuoSet ELISA | R&D Systems | Cat#: DY406 | |
| Chemical Compound, Drug | Brefeldin A | Sigma-Aldrich | Cat#: B7651 | 150 µg/mouse |
| Software, Algorithm | FlowJo | Tree Star | RRID: SCR_008520 | |
| Software, Algorithm | GraphPad Prism | GraphPad Software | RRID: SCR_002798 | |
| Other | ACK Lysing Buffer | Thermo Fisher Scientific | Cat#: A1049201 | Red blood cell lysis |
| Other | Collagenase D | Sigma-Aldrich (Roche) | Cat#: 11088858001 | 1 mg/mL |
| Other | Foxp3/ Transcription Factor Fixation/Permeabilization Concentrate and Diluent | eBioscience | Cat#: 00-5521-00 | |
| Other | Polyinosinic-polycytidylic acid potassium salt (Poly I:C) | Sigma-Aldrich | Cat#: P9582 | 75 µg/mouse |
| Other | 5-Bromo-2′-deoxyuridine (BrdU) | Sigma-Aldrich (Roche) | Cat#: 10280879001 | 0.5 µg/mouse i.p., 0.8 mg/mL p.o. |
| Other | TRIzol Reagent | Thermo Fisher Scientific (Invitrogen) | Cat#: 15596026 | |
| Other | TURBO DNase | Thermo Fisher Scientific | Cat#: AM2238 | |
| Other | SuperScript IV Reverse Transcriptase | Thermo Fisher Scientific | Cat#: 18090010 |





