Regulation of RUVBL1-RUVBL2 AAA-ATPases by the nonsense-mediated mRNA decay factor DHX34, as evidenced by Cryo-EM
Figures
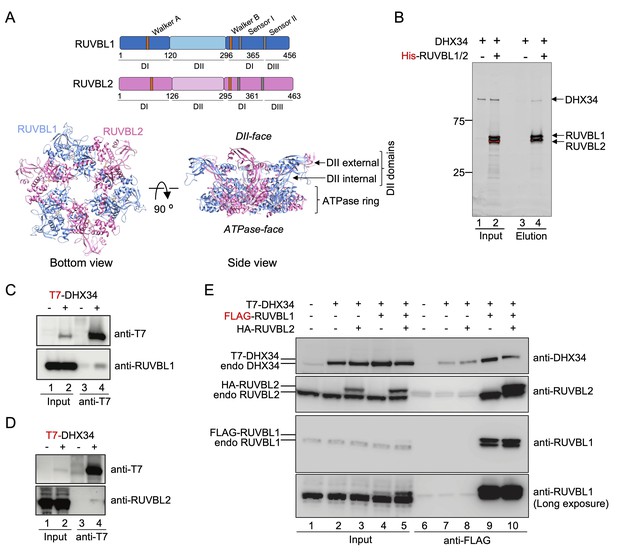
RUVBL1-RUVBL2 interacts with DHX34.
(A) Top panels: Schematic representation of RUVBL1 (blue) and RUVBL2 (pink) domains and catalytic motifs. Bottom panel: structure of the human RUVBL1-RUVBL2 hetero-hexameric ring with protruding domain II (DII), generated from atomic structures of RUVBL1 (PDB 2C9O) and RUVBL2 (PDB 6H7X) (Matias et al., 2006; Silva et al., 2018). Top and bottom views are shown with the color code from top panel. The ATPase-face and DII-face of the ring as well as the internal and external regions of DIIs are indicated. (B) Pull-down experiment testing the interaction of purified His-RUVBL1-RUVBL2 with DHX34, using His-tag affinity purification. Proteins bound to affinity beads were eluted and analyzed by SDS-PAGE and stained using Oriole Fluorescent Gel Stain (Bio-Rad). DHX34 was found to elute specifically only when His-RUVBL1-RUVBL2 was present. (C, D) Immunoprecipitation (IP) of transiently transfected HEK293T cells with T7-DHX34 from HEK293T cells was performed in the presence of RNase A. Inputs (0.5%) and anti-FLAG-IPs (20%) were subjected to western analysis using the indicated antibodies. Proteins bound to T7 tag affinity beads were eluted and analyzed by SDS-PAGE and western blot using antibodies against the T7 tag in DHX34 and RUVBL1 (C) or RUVBL2 (D). For Inputs (0.5%) and anti-T7 IP (20%) are shown. (E) IP experiment testing the interaction of FLAG-RUVBL1 and HA-RUVBL2 co-expressed in HEK293T cells with T7-DHX34. Inputs (0.5%) and anti-FLAG-IPs (20%) were analyzed by SDS-PAGE and western blot using antibodies against DHX34, RUVBL1 and RUVBL2. These antibodies detected both transfected and endogenous proteins and are indicated on the left site of the panel.
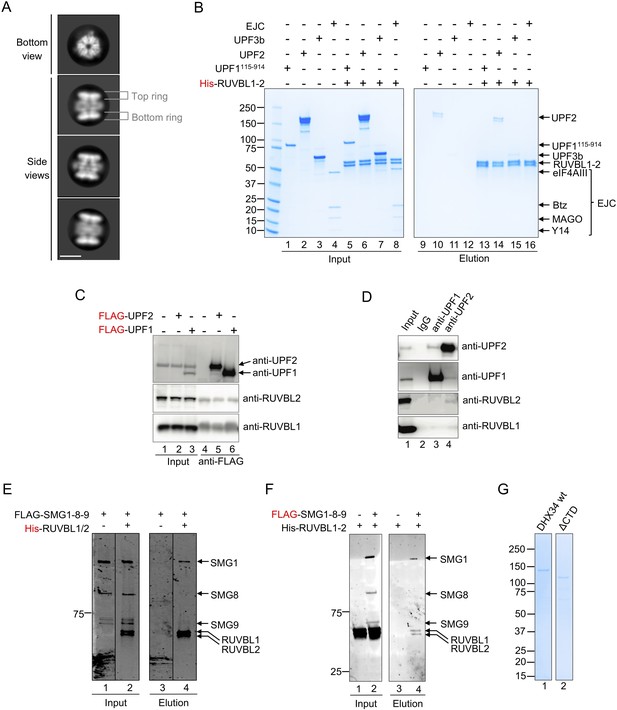
Testing the interaction between RUVBL1-RUVBL2 and NMD factors.
(A) Representative 2D averages of purified His-RUVBL1-RUVBL2 analyzed by cryo-EM. Scale bar, 10 nm. Features of the RUVBL1-RUVBL2 complex are indicated. (B) Pull-down experiment testing the interaction of purified His-RUVBL1-RUVBL2 with UPF1115-914, UPF2, UPF3b, and EJC using His-tag affinity purification. Proteins bound to affinity beads were eluted and analyzed by SDS-PAGE and stained using Quick Coomassie (Generon). None of these proteins interacted with RUVBL1-RUVBL2. Some elution detected for UPF2 (lane 14) corresponded to background binding by the beads since a similar elution was observed in the control experiment lacking His-RUVBL1-RUVBL2 (lane 10). (C) Immunoprecipitation assay testing the interaction of UPF1 and UPF2 with RUVBL1 and RUVBL2 using overexpressed FLAG-UPF1 and FLAG-UPF2 in cell extracts. Pulldowns show no increased signal for RUVBL1 and RUVBL2 compared to the negative control. (D) Immunoprecipitation experiment testing the interaction of endogenous UPF1 and UPF2 with RUVBL1 and RUVBL2 from cell extracts, using antibodies against endogenous UPF1 and UPF2. This experiment suggests that RUVBL1 and RUVBL2 do not interact with UPF1 or UPF2. Together with the lack of direct interaction detected in ‘C’ and when using purified proteins in ‘B’, these experiments were interpreted as indicating a lack of interaction between RUVBL1-RUVBL2 and either UPF1 or UPF2. (E) 4–15% SDS-PAGE showing a Ni-NTA agarose resin pull-down experiment of His-RUVBL1-RUVBL2 incubated with FLAG-SMG1-SMG8-SMG9 (FLAG-SMG1-8-9), stained with Oriole Fluorescent Gel Stain (Bio-Rad). Lanes 1 and 3 correspond to the input and elution of an experiment containing only FLAG-SMG1-SMG8-SMG9. Lanes 2 and 4 correspond to the input and elution of an experiment containing FLAG-SMG1-SMG8-SMG9 incubated with His-RUVBL1-RUVBL2. (F) SDS-PAGE of pull-down experiment as in (B) but using the FLAG tag in SMG1 as bait. Lanes 1 and 3 correspond to the input and elution of an experiment containing FLAG-SMG1-SMG8-SMG9 and His-RUVBL1-RUVBL2. Lanes 2 and 4 corresponds to input and elution of an experiment containing only His-RUVBL1-RUVBL2. (G) 4–15% SDS-PAGE and Quick Coomassie (Generon) staining of purified DHX34 constructs used: wild-type (wt) DHX34 and the DHX34 mutant lacking the CTD domain (ΔCTD) used in Figure 4—figure supplement 1D.
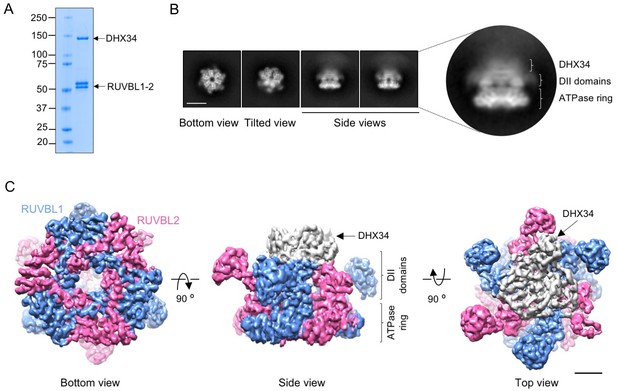
Cryo-EM of the RUVBL1-RUVBL2-DHX34 complex.
(A) Purified RUVBL1-RUVBL2-DHX34 complex used for structural studies in a 4–15% SDS-PAGE stained with Quick Coomassie (Generon). (B) Representative reference-free 2D averages from cryo-EM images of the complex. Side views clearly show the projection of one ring with some density attached to the DII face (close-up right panel). Scale bar represents 10 nm. (C) Several views of the cryo-EM density obtained for RUVBL1-RUVBL2-DHX34 (RUVBL1 in blue, RUVBL2 in pink, and DHX34 in gray). ATPase core and DII domains in RUVBL1-RUVBL2 are indicated. Scale bar represents 25 Å.
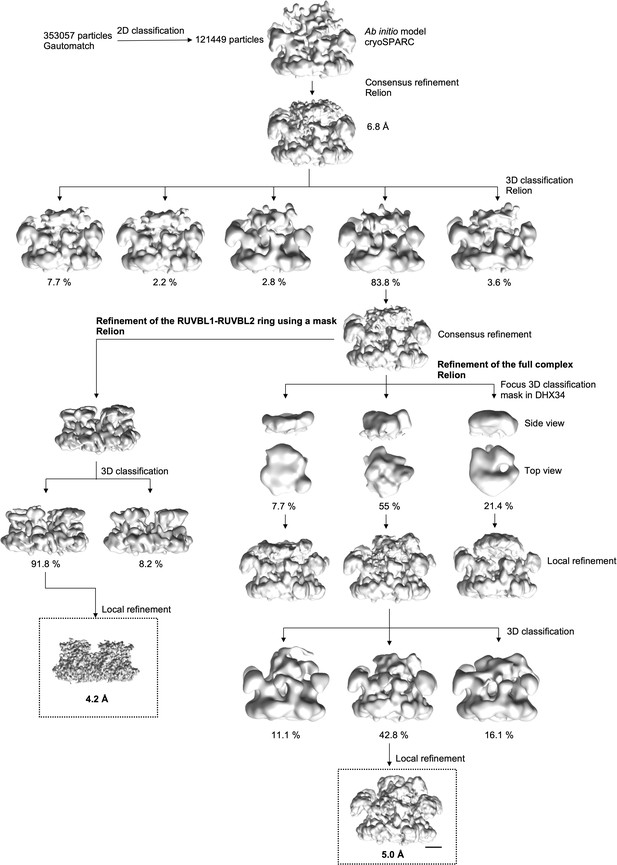
Image processing of the cryo-EM images of the RUVBL1-RUVBL2-DHX34 complex workflow of the image processing strategy followed in this work.
After some initial steps of image ‘cleaning’ and classification, a consensus refinement for 83.8% of the particles was obtained. From these initial stages, image processing was divided in two branches. To resolve the structure of the RUVBL1-RUVBL2 without the effect of DHX34 and the OB-fold domains, we removed the influence of the density of these flexible regions by using a mask, and refinements and classifications were focused only in the ring. In parallel, we refined the structure of the full complex. For this, particles were first classified according to the quality of the DHX34 density, removing those particles were the density was too small to accommodate DHX34. Then, these particles were classified and refined using the information of the whole complex. Scale bar represents 25 Å.
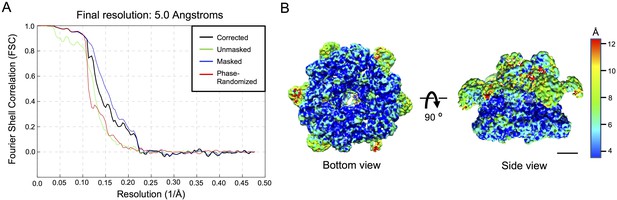
Resolution estimation of the cryo-EM for the RUVBL1-RUVBL2-DHX34 complex.
(A) Fourier Shell Correlation (FSC) curves estimating the average resolution of the cryo-EM volume of the RUVBL1-RUVBL2-DHX34 complex after refinement using the gold standard defined in RELION-3 (Zivanov et al., 2018). (B) Local resolution estimates map of RUVBL1-RUVBL2-DHX34 complex as provided by RELION. Bottom and side views of the complex are shown using the color scale shown on the right. Scale bar, 25 Å.
Cryo-EM structure of the RUVBL1-RUVBL2-DHX34 complex.
Several views of the cryo-EM density of the RUVBL1-RUVBL2-DHX34 complex, colored as in Figure 2.
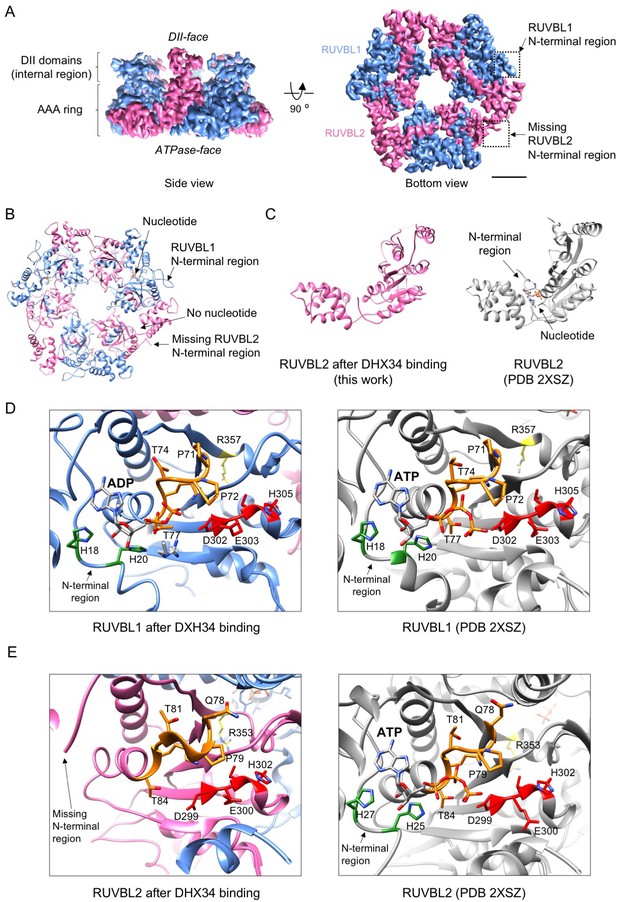
DHX34 induces large conformational changes in RUVBL2.
(A) Side and bottom views of the RUVBL1-RUVBL2 ring obtained after refinement without the influence of DHX34 and the OB-fold domains. Squares highlight N-terminal segments of RUVBL1 (blue) and RUVBL2 (pink). Scale bar represents 25 Å. The presence and absence of RUVBL1 and RUVBL2 N-terminal regions is indicated only in one copy of each subunit, but it applied to all the subunits in the complex. (B) Bottom view of the atomic structure of RUVBL1-RUVBL2 ring modeled from the cryo-EM density. Color codes are as in (A). (C) Right panel: a view of the nucleotide binding region in RUVBL2 from the crystal structure of RUVBL1-RUVBL2 (PDB 2SXZ) in gray color; left panel: similar view of RUVBL2 in RUVBL1-RUVBL2 after DHX34 binding (this work, pink). (D) Close-up view of the nucleotide-binding regions in RUVBL1, comparing the structure after DHX34 binding (left panel) and the crystal structure of the RUVBL1-RUVBL2 complex (PDB 2SXZ) (right panel) in gray. N-terminal histidines (H18 and H20) are indicated in gray, Walker A residues in orange, Walker B in red, and the Arg finger in yellow. (E) As in (D) but for the RUVBL2 subunit. Color codes for relevant and catalytic motifs are represented as in (D).
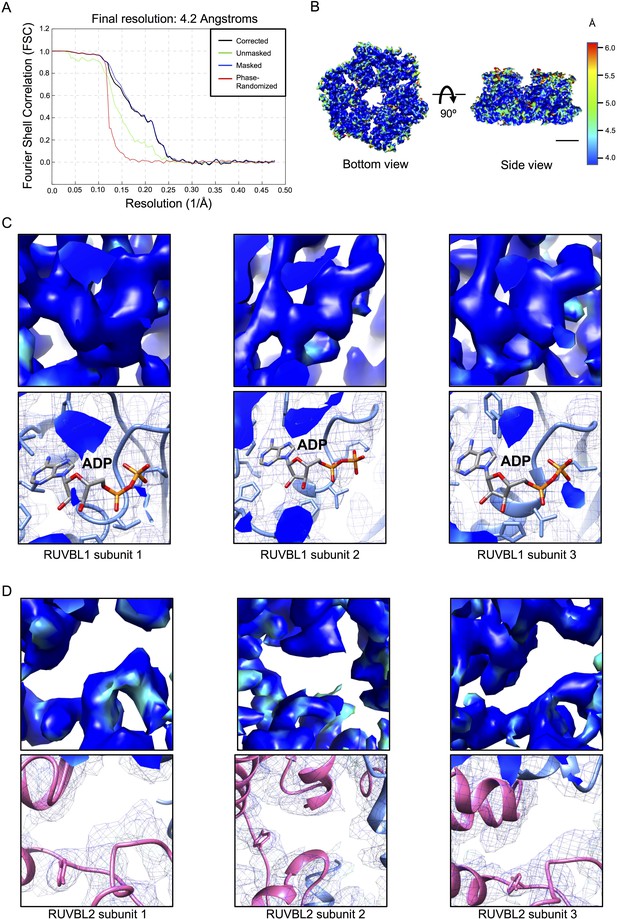
Resolution estimation of the cryo-EM for RUVBL1-RUVBL2 after DHX34 binding.
(A) Fourier Shell Correlation (FSC) curves for the hexameric ring after refinement removing the influence of DHX34 and the OB-fold domains. (B) Local resolution estimates for the hexameric ring of RUVBL1-RUVBL2 as provided by RELION. Bottom and side views of the map are shown using the color scale shown on the right. Scale bar, 25 Å. (C) Close-up of the local resolution estimates centered in the nucleotide binding pockets of RUVBL1, showing all three subunits. Top panels show the density of the local resolution map using the same color code as in (B). Bottom panels show the same region but as a mesh with the atomic model fitted. (D) As ‘C’, but for each RUVBL2 subunit.
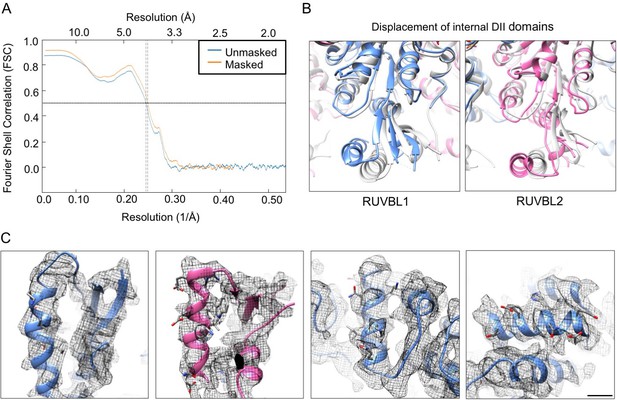
High-resolution features in cryo-EM map.
(A) Fourier Shell Correlation (FSC) curves for the atomic model versus the cryo-EM density of the RUVBL1-RUVBL2 ATPase core using for modeling. (B) Close-up views of RUVBL1 (left) and RUVBL2 (right) internal DII domains superimposed with the structure of unliganded RUVBL1-RUVBL2 (PDB 2SXZ) in gray color. (C) Selected areas showing high-resolution features with side chain of some residues shown. Scale bar, 5 Å.
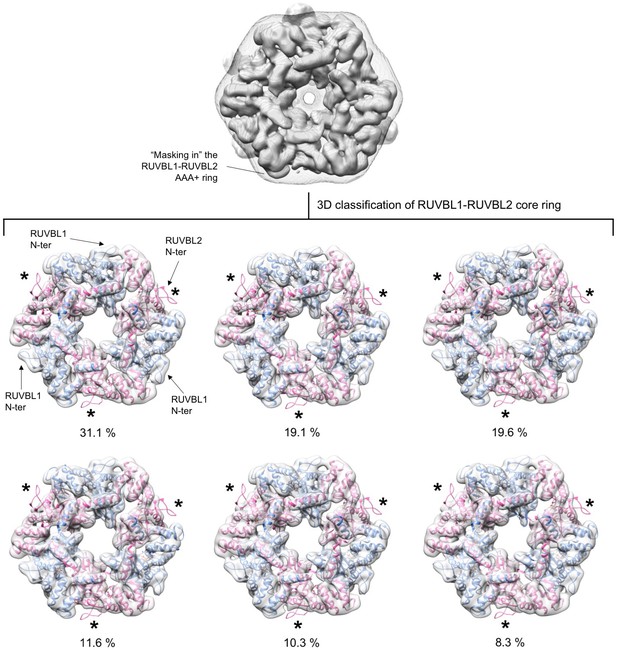
Analysis of conformational changes in each RUVBL2 subunit.
Experiment 1. RUVBL1-RUVBL2-DHX34 particles were classified in six groups with a mask that removed the influence of DHX34 and the flexible DII domains from the analysis. Each sub-group was analyzed by fitting the atomic structure of the RUVBL1-RUVBL2 core domains (PDB 2XSZ). Cryo-EM density for RUVBL1-RUVBL2 is shown as a white transparency, RUVBL1 is shown in blue color and RUVBL2 in pink color. The percentage of particles in each subgroup is indicated. All groups showed density for the N-termini of RUVBL1. The N-termini of RUVBL2 present in the crystal structure is not present in the cryo-EM density of any subunit in any of the groups. The positions of the N-termini of RUVBL2 are indicated with asterisks.
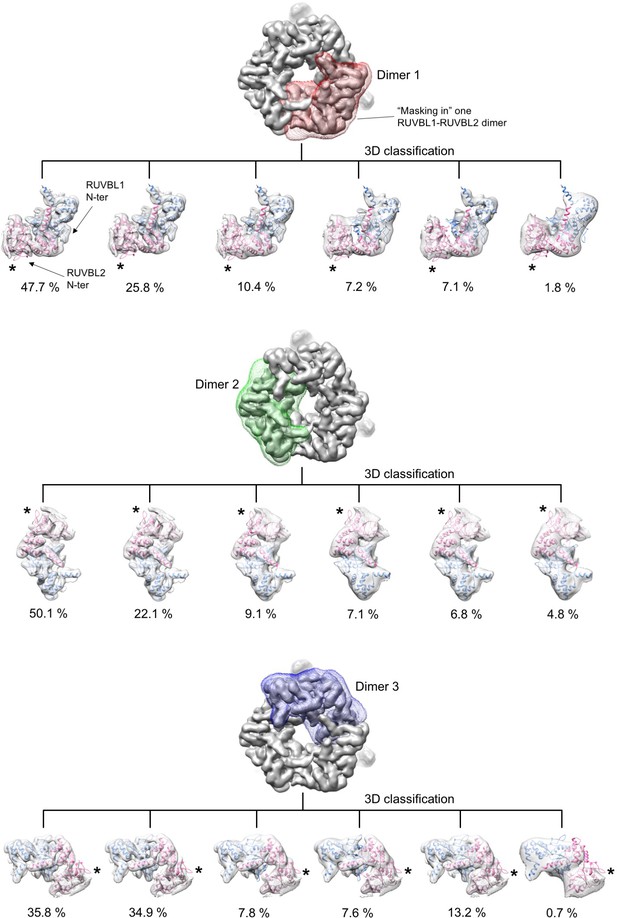
Analysis of conformational changes in each RUVBL2 subunit.
Experiment 2. RUVBL1-RUVBL2-DHX34 particles were classified in six groups with a mask centered in only one RUVBL1-RUVBL2 dimer at a time. Each sub-group was analyzed by fitting the atomic structure of the RUVBL1-RUVBL2 core domains (PDB 2XSZ). Cryo-EM density for RUVBL1-RUVBL2 is shown as a white transparency, RUVBL1 is shown in blue color and RUVBL2 in pink color. The percentage of particles in each subgroup is indicated. All groups showed density for the N-termini of RUVBL1. The N-termini of RUVBL2 present in the crystal structure is not present in the cryo-EM density of any subunit in any of the groups. The positions of the N-termini of RUVBL2 are indicated with asterisks.
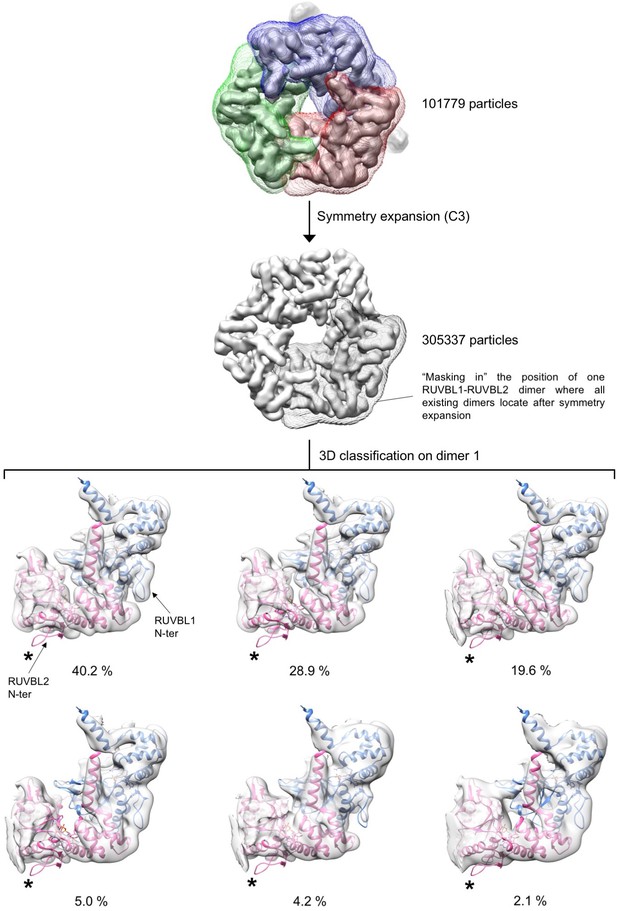
Analysis of conformational changes in each RUVBL2 subunit.
Experiment 3. The conformation of each RUVBL1-RUVBL2 dimer was analyzed using a symmetry expansion strategy. Each particle was rotated twice along its longitudinal axis so that each of the three RUVBL1-RUVBL2 dimers in each particle locates in the same position. After the expansion the data set is triplicated. Then, particles were locally classified in six groups using a mask focused in only one dimer. Each sub-group was analyzed by fitting the atomic structure of the RUVBL1-RUVBL2 core domains (PDB 2XSZ). Cryo-EM density for RUVBL1-RUVBL2 is shown as a white transparency, RUVBL1 is shown in blue color and RUVBL2 in pink color. The percentage of particles in each subgroup is indicated. All groups showed density for the N-termini of RUVBL1. The N-termini of RUVBL2 present in the crystal structure is not present in the cryo-EM density of any subunit in any of the groups. The positions of the N-termini of RUVBL2 are indicated with asterisks. A small percentage of particles (2.1%) showed a partial loss of density for RUVBL2 N-termini, which can be due in most part to the lower resolution of this subset.
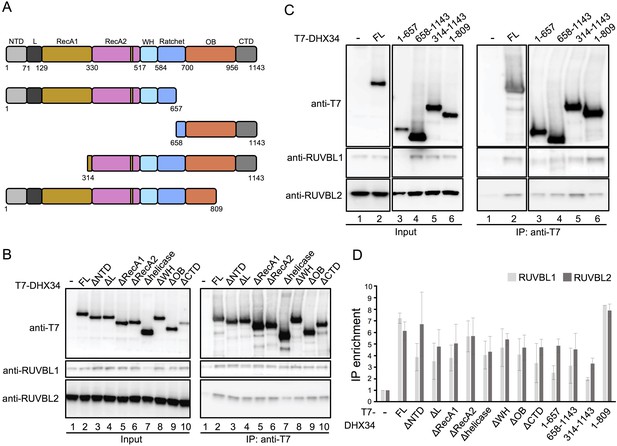
Mapping the interaction between DHX34 and RUVBL1-RUVBL2 in cells.
(A) Cartoon depicting the functional domains of DHX34, showing the residue numbers that define their boundaries. Names of the domains are: N-terminal (NTD), L, RecA1, RecA2, winged-helix (WH), Ratchet, OB-fold and C-terminal (CTD). (B–C) Effect of domain deletions of DHX34 (B) and larger truncation including several domains (C) on the interaction with RUVBL1 and RUVBL2 using cell extracts. The Immunoprecipitation (IP) of T7-tagged versions of DHX34 was analyzed by western blot using antibodies against the T7 tag, RUVBL1 and RUVBL2. (D) Quantification of the experiments shown in ‘B’ and ‘C’. The protein levels in the IP were quantified and normalized to the levels in the Input. Binding is expressed as IP enrichment compared to the empty vector control (-). For each expressed polypeptide, at least two independent experiments were analyzed.
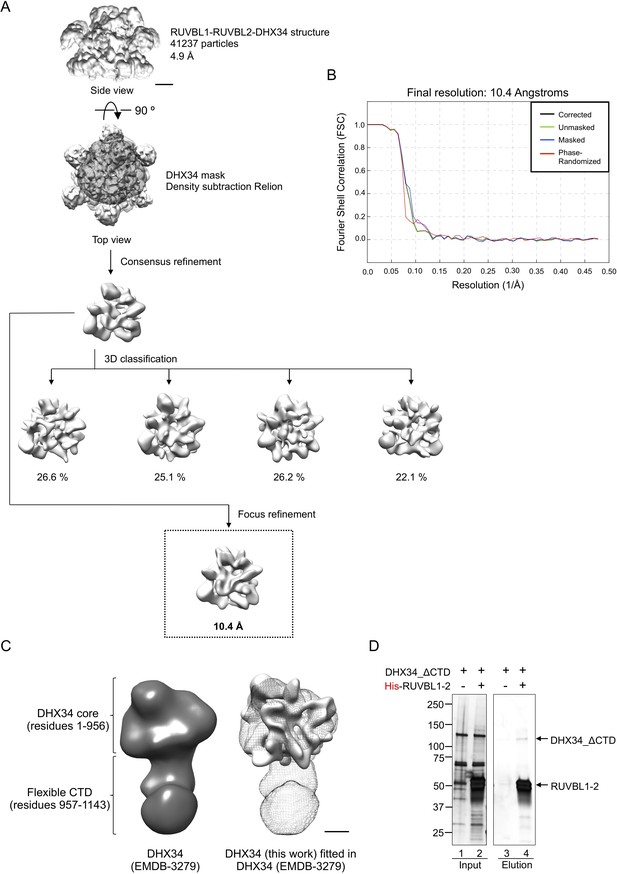
Analysis of the images of DHX34 in complex with RUVBL1-RUVBL2.
(A) Workflow for the image processing of DHX34 extracted from the images of the RUVBL1-RUVBL2-DHX34 complex. Scale bar, 25 Å. (B) Fourier Shell Correlation (FSC) curves for the DHX34 structure after refinement removing the influence of RUVBL1-RUVBL2 ring using density subtraction protocols. (C) Comparison between the structure of isolated DHX34 (Melero et al., 2014) and the DHX34 in complex with RUVBL1-RUVBL2 resolved at low-resolution structure. Left panel, structure of isolated DHX34 as a solid density (Melero et al., 2014). Right panel, structure of isolated DHX34 as mesh fitted with the low-resolution DHX34 from this work. Scale bar, 25 Å. (D) Effect of the truncation of the C-terminal domain (CTD) of DHX34 in the formation of a complex with RUVBL1-RUVBL2. The experiment was performed by pull-down of the His-tag in RUVBL1 in RUVBL1-RUVBL2 after incubation with DHX34-ΔCTD using purified proteins. SDS-PAGE was stained using Oriole Fluorescent Gel Stain (Bio-Rad).
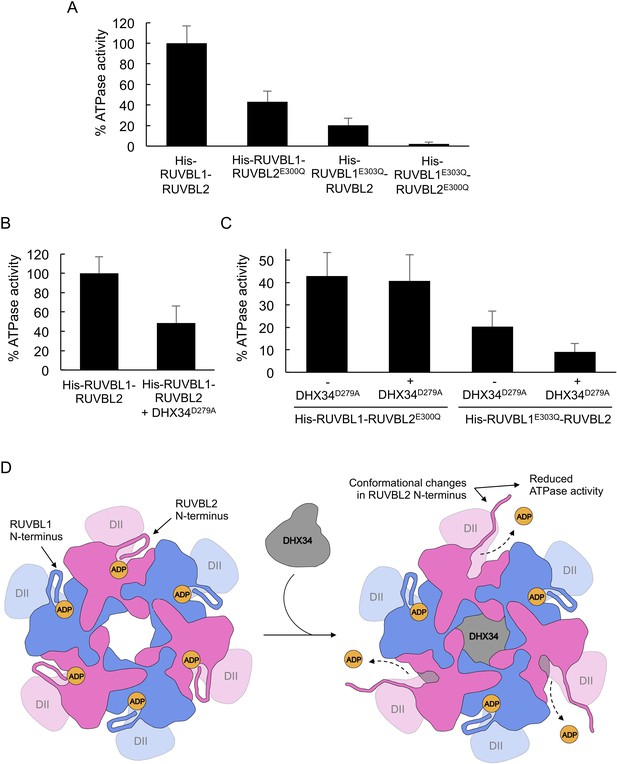
DHX34 down-regulates RUVBL2 ATPase activity.
(A) Graph comparing the ATPase activity for His-RUVBL1-RUVBL2, His-RUVBL1E303Q-RUVBL2, His-RUVBL1-RUVBL2E300Q and His-RUVBL1E303Q-RUVBL2E300Q shown as percentage of the rate measured for wild-type RUVBL1-RUVBL2. Standard deviations from three independent experiments are indicated. (B) Graph comparing the ATP activity of His-RUVBL1-RUVBL2 in the presence and absence of DHX34D279A, indicated as percentage of ATPase activity, assuming 100% activity for His-RUVBL1-RUVBL2. Standard deviations from four independent experiments are indicated. Sample His-RUVBL1-RUVBL2 used in these experiments contains a His-tag at the N-terminus of RUVBL1. (C) Graph showing the ATP activity for His-RUVBL1-RUVBL2 complexes where either RUVBL1 or RUVBL2 contains a mutation that abolished ATP hydrolysis (RUVBL1303Q or RUVBL1E300Q) and the effect after incubation with DHX34D279A. In all the experiments the ATPase activity for His-RUVBL1-RUVBL2 shown in ‘B’ is considered as 100%. Standard deviations from three independent experiments are indicated. (D) Model for the regulation of the ATPase activity of RUVBL1-RUVBL2 by DHX34. N-terminal regions in RUVBL1 (blue color) and RUVBL2 (pink color) subunits contribute interactions with the nucleotides in the nucleotide-binding pocket. DHX34 binds to the DII-face of the RUVBL1-RUVBL2 ring and induces large conformational changes in the N-termini of all RUVBL2 subunits promoting the loss of nucleotide and a down-regulation of the ATPase activity. Source files containing the data used for the time course measurements for ATP consumption in Figure 5 and the figure supplement are available in Figure 5—source data 1.
-
Figure 5—source data 1
Source data for the ATPase activity assays shown in Figure 5, Figure 5—figure supplement 1, Figure 5—figure supplement 2 and Figure 5—figure supplement 3' and the caption to 'The file includes 15 sheets, each one for 1 sample, containing the replicas done for the sample.
In each sheet is included: Name of sample, time (min), absorbance at 340 nm for the replicas, equation of the linear regression trendline for each replica used for data analysis, and R2 value of the linear regression trendline for each replica.
- https://cdn.elifesciences.org/articles/63042/elife-63042-fig5-data1-v2.zip
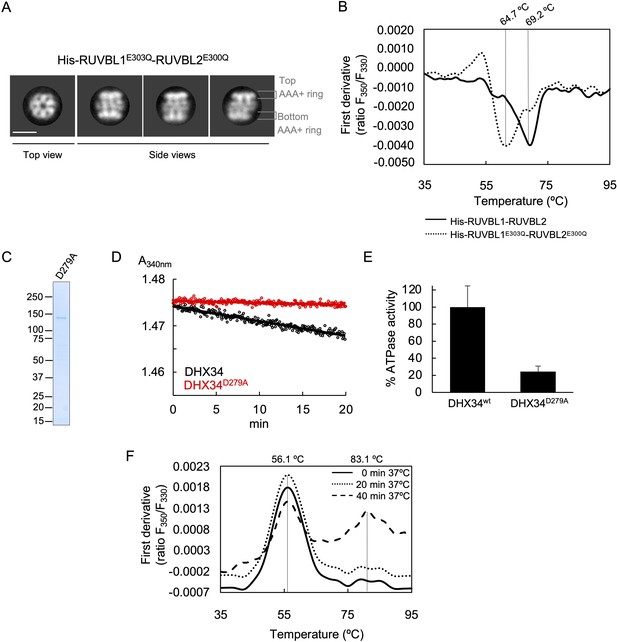
Oligomerization and stability of RUVBL1-RUVBL2 and DHX34 mutants.
(A) The oligomeric state of His-RUVBL1E303Q-RUVBL2E300Q mutant was monitored by using negative stain electron microscopy. His-RUVBL1E303Q-RUVBL2E300Q mutant assembled complexes similar to the wild-type complex when observed in the electron microscope (Figure 1—figure supplement 1). (B) The stability of the His-RUVBL1-RUVBL2 mutants was analyzed using nano-scanning fluorimetry. Graph represents the first derivative of the fluorescence ratios (F350/F330) along the temperature for His-RUVBL1-RUVBL2 (solid line), and His-RUVBL1E303Q-RUVBL2E300Q (dot line). Inflection temperatures (Ti) are indicated on top. (C) 4–15% SDS-PAGE and Quick Coomassie (Generon) staining of purified DHX34D279A. (D) Time course measurements for ATP consumption monitored as absorbance at 340 nm using the pyruvate kinase-lactate dehydrogenase-coupled assay to measure the ATPase activity of DHX34 and ATP-hydrolysis-dead mutant DHX34D279A. (E) Graph comparing the ATP activity in ‘D’ indicated as a percentage of wild-type DHX34. ATP-hydrolysis-dead mutant DHX34D279A mutant shows 24.2% of the ATPase activity measured for wild-type DHX34. Standard deviations from three independent experiments are indicated. (F) The stability of DHX34 after incubation at 37°C was analyzed by nano scanning fluorimetry. These experiments revealed that the protein was not affected after 20 min at 37°C (dot line) but it was significantly affected after 40 min (dashed line), where a second unfolding process is observed at a higher Ti. Source files containing the data used for the time course measurements for ATP consumption in Figure 5 and the figure supplement are available in Figure 5—source data 1.
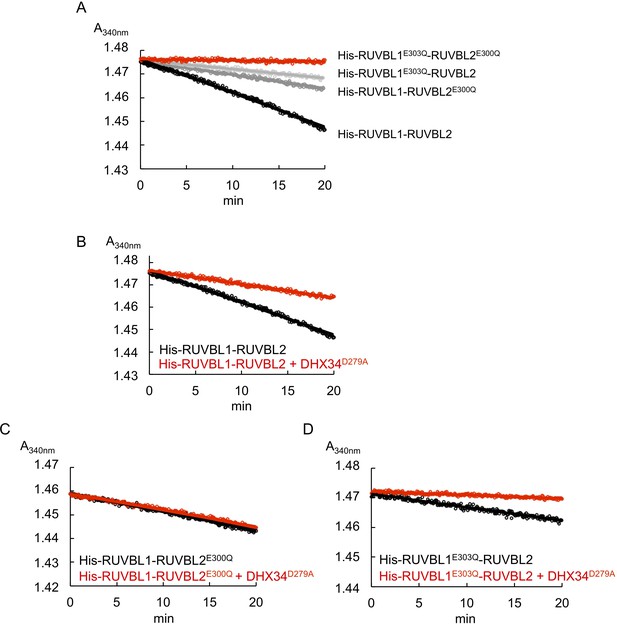
ATPase measurements and time courses for His-RUVBL1-RUVBL2 and several ATPase-dead mutants in the presence and absence of DHX34D279A.
(A) Time course measurements for ATP consumption monitored as absorbance at 340 nm using the pyruvate kinase-lactate dehydrogenase-coupled assay for His-RUVBL1-RUVBL2 (black line) and ATPase-dead mutants of His-RUVBL1E303Q-RUVBL2, His-RUVBL1-RUVBL2E300Q, and His-RUVBL1E303Q-RUVBL2E300Q. (B–D) Time course measurements were performed as in (A) in experiments measuring the ATPase activity of each mutant in the absence or presence of DHX34D279A. Source files containing the data used for the time course measurements for ATP consumption in Figure 5 and the figure supplement are available in Figure 5—source data 1.
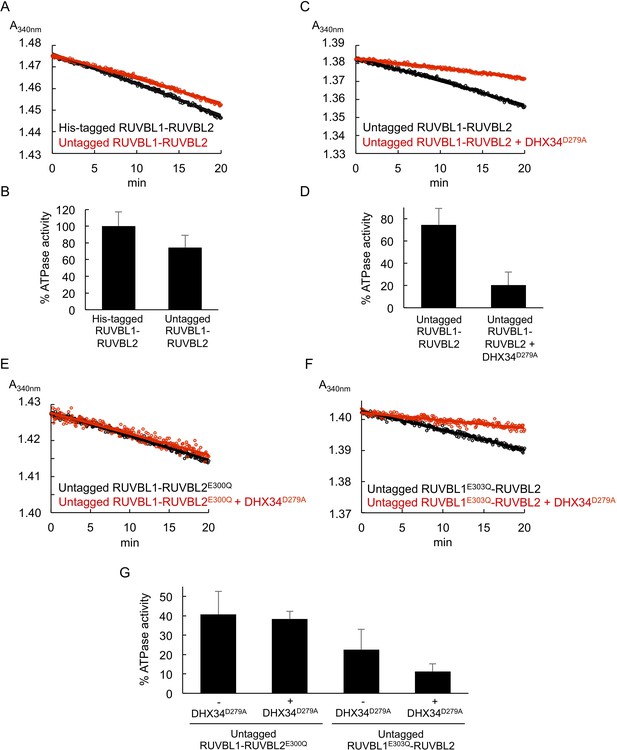
DHX34D279A interferes with RUVBL1-RUVBL2 ATPase activity also after the His-tag in RUVBL1 was removed.
(A) Time course measurements for ATP activity monitored as absorbance at 340 nm for untagged RUVBL1-RUVBL2 (red line) compared to His-RUVBL1-RUVBL2 (black line). (B) Graph representing the ATPase activity for the experiments in (A) using the values of His-RUVBL1-RUVBL2 as the 100%. (C, D) As in ‘A’ and ‘B’ respectively but after adding DHX34D279A. (E, F) As in ‘A’ and ‘B’ but using two ATPase-dead mutants RUVBL1-RUVBL2E300Q and RUVBL1E303Q-RUVBL2. (G) Graph summarizing the ATPase activity of RUVBL1-RUVBL2 and several mutants in the absence or presence of DHX34D279A. This graph is similar to the one shown in Figure 4 but using untagged RUVBL1-RUVBL2 complex. Source files containing the data used for the time course measurements for ATP consumption in Figure 5 and the figure supplement are available in Figure 5—source data 1.
Videos
Atomic structure of the RUVBL1-RUVBL2 ATPase ring.
Several views of the atomic model for RUVBL1-RUVBL2 to show the conformational changes in the hexameric ring after the interaction with DHX34. During the video, the structure is superimposed to the atomic structure of the RUVBL1-RUVBL2 ATPase ring (PDB 2SXZ) in gray color, to highlight the differences in RUVBL2 and RUVBL1, and the loss of nucleotide in RUVBL2. In the final part of the video, the morphing between the crystal structure of the RUVBL1-RUVBL2 ATPase ring (PDB 2SXZ) and the structure described is this work highlights the conformational changes induced by DHX34 in the polypeptide chains. During morphing, nucleotides in both subunits and both conformations have been removed, as well as the N-terminal region of RUVBL2, which is only present in the crystal structure and it cannot be modeled in the DHX34-bound structure.
Tables
Cryo-EM data collection and parameters.
| Data collection and processing | ||
|---|---|---|
| Structure | RUVBL1-RUVBL2-DHX34 (EMD-11788) | RUVBL1-RUVBL2 ATPase core (EMD-11789) (PDB ID 7AHO) |
| Microscope | FEI Titan Krios | FEI Titan Krios |
| Detector | Gatan K2 (counting mode) | Gatan K2 (counting mode) |
| Magnification | 47756 | 47756 |
| Voltage (kV) | 300 | 300 |
| Electron exposure (e–/Å2) | 48.1 (40 fractions) | 48.1 (40 fractions) |
| Defocus range (μm) | −1.5 to −3.0 | −1.5 to −3.0 |
| Pixel size (Å) | 1.047 | 1.047 |
| Symmetry imposed | C1 | C1 |
| Initial particle images (no.) | 353 057 | 353 057 |
| Final particle images (no.) | 41237 | 101774 |
| Map resolution (Å) FSC threshold | 4.97 0.143 | 4.18 0.143 |
| Map resolution range (Å) | 4.0–12.0 | 3.8–6.0 |
Validation statistics for the atomic model of RUVBL1-RUVBL2 ATPase core.
| Refinement RUVBL1-RUVBL2 ATPase core | |
|---|---|
| Software | phenix.real_space_refine Coot |
| Initial model used (PDB code) | 2XSZ |
| Model resolution (Å) | 4.1 |
| FSC threshold | 0.5 |
| Map sharpening B factor (Å2) | −222.98 |
| Model composition | 13521 |
| Non-hydrogen atoms | 1748 |
| Model composition | |
| Non-hydrogen atoms | 13521 |
| Protein residues | 1748 |
| Ligands | ADP (3) |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.006 |
| Bond angles (°) | 0.911 |
| Validation | |
| MolProbity score | 1.70 |
| Clashscore | 6.95 |
| Poor rotamers (%) | |
| Ramachandran plot (%) | |
| Favored | 95.39 |
| Allowed | 4.61 |
| Disallowed | 0.00 |
| Rotamer outliers (%) | 0.21 |
Correlation Coefficients (CC) of the atomic after model refinement.
| Correlation Coefficients (CC) after model refinement of RUVBL1-RUVBL2_core map | |
|---|---|
| CC (mask) | 0.77 |
| CC (box) | 0.72 |
| CC (peaks) | 0.62 |
| CC (volume) | 0.76 |
| Mean CC for ligands | 0.84 |
Oligonucleotides used for cloning.
| Construct | Name | Sequence 5´- 3´ |
|---|---|---|
| pET21b-RUVBL2_H10 | pET21b_FW | CACCACCACCACCACCACTG |
| RUVBL2_H10_FW | ATGGCAACCGTTACAGCCACTGTTTAACTTTAAGAAGGAGATATACAT | |
| RUVBL2_H10_RV | GGAGGTGTCCATGGTCTCGCGTGGTGGTGGTGGTGATGGTGATGGTGAGGTCCCTGGAACAGCACCTCCAG | |
| pET21b_RV | ATGTATATCTCCTTCTTAAAGTTAAACAAAATT | |
| pETEV15b-RUVBL1_E303Q | R1_E303Q_FW | AGGTCCACATGCTGG |
| R1_E303Q_RV | CATGTGGACCTGATCAACAAACAGCACACC | |
| pCDFDuet-1-RUVBL2_E300Q | R2_E300Q_FW | AGGTCCACATGCTGGAC |
| R2_E300Q_RV | GCATGTGGACCTGGTCGATGAACAGCACTCC |





