Simultaneous recording of multiple cellular signaling events by frequency- and spectrally-tuned multiplexing of fluorescent probes
Figures
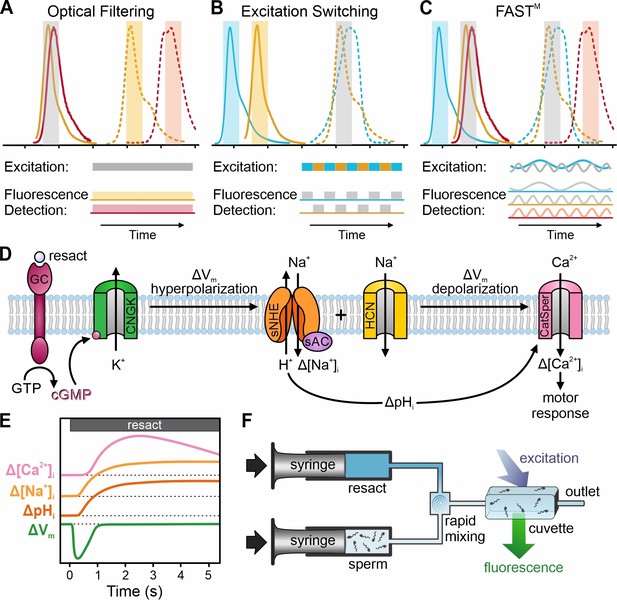
Strategies for multiplexing of fluorescent probes, and outline of the chemosensory signal transduction in the flagellum of sea urchin sperm.
(A) Spectrally separable emission spectra (dashed) of probes allow their simultaneous recording using optical filtering. (B) Spectrally separable excitation spectra (outlined) allow quasi-simultaneous recording of probes using excitation-switching. (C) Frequency-tagging and phase-sensitive detection of fluorescence combined with optical filtering using frequency- and spectrally-tuned multiplexing (FASTM) allow simultaneous recording of probes based on separable excitation and/or emission spectra. (D) Schematic of the chemosensory signaling pathway and (E) illustration of the time course of the signaling events in sea urchin sperm (reviewed in Strünker et al., 2015). Resact, the chemoattractant peptide released by the egg, triggers the synthesis of cGMP by activating a receptor guanylyl cyclase (GC). The rise in cGMP elicits a pulse-like Vm hyperpolarization mediated by a cyclic nucleotide-gated K+ channel (CNGK). The hyperpolarization activates a voltage-gated Na+/H+ exchanger (sNHE) and a hyperpolarization‐activated and cyclic nucleotide‐gated (HCN) channel. The Na+/H+ exchange increases [Na+]i and pHi. In turn, the increase in pHi primes pHi-controlled CatSper Ca2+ channels to open during the recovery from hyperpolarization driven by HCN channels. The resulting Ca2+ influx drives chemotactic steering towards the egg. (F) Schematic of the stopped-flow setup: one syringe is filled with a suspension of probe-loaded sperm, and a second syringe is filled with a solution of resact. The syringe pistons move synchronously to rapidly mix sperm with resact in a micromixer and subsequently push this mixture into an observation cuvette, where spectroscopic measurements are performed (see Hamzeh et al., 2019).
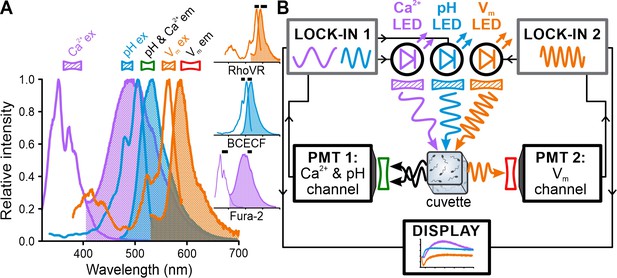
Experimental configuration for frequency- and spectrally-tuned multiplexing (FASTM) of Fura-2, BCECF, and RhoVR in a stopped-flow device.
(A) Superposition of excitation (outlined) and emission (filled) spectra of fluorescent probes for Ca2+ (Fura-2), pH (BCECF), and Vm (RhoVR). Bandpass filters used for excitation (filled) and emission (outlined) are shown above the spectra. Inset: excitation and emission spectra depicted individually with respective filters (black bars). (B) Schematic of FASTM: each probe is excited by an LED modulated at a different frequency. The modulated emission is optically filtered and collected by two photomultipliers (PMTs). The PMT signals are demodulated by lock-in amplifiers in a phase-sensitive fashion to recover in real time [Ca2+]i, pHi, and Vm signals.
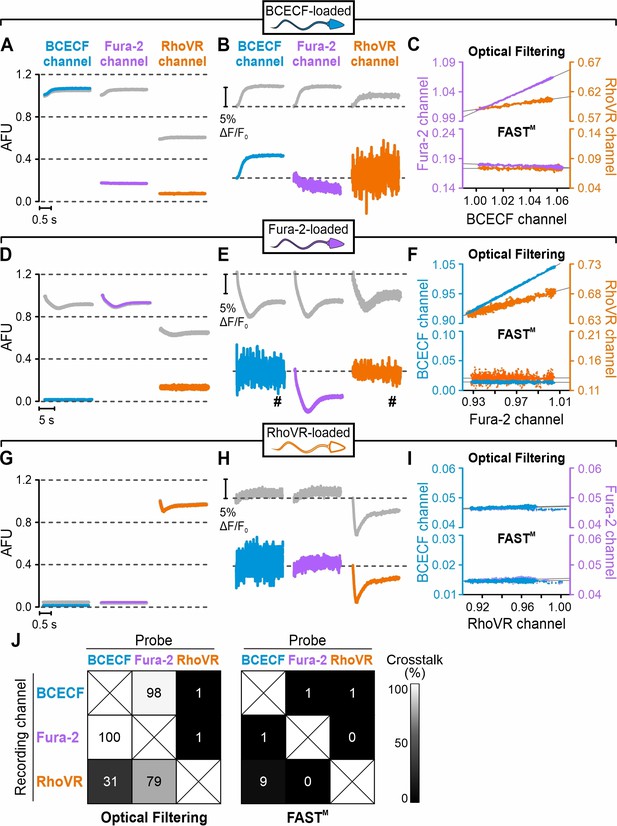
Resact-induced pHi, [Ca2+]i, and Vm signals recorded in sperm loaded with BCECF, Fura-2, or RhoVR using optical filtering alone or frequency- and spectrally-tuned multiplexing (FASTM).
Time course of the fluorescence signals recorded from BCECF- (A–C), Fura-2- (D–F), or RhoVR-loaded sperm (G–I) after mixing with resact (50 pM). Fluorescence was recorded in the BCECF, Fura-2, and RhoVR channels using optical filtering alone (gray traces) or FASTM (colored traces). (A, D, G) Fluorescence signals in arbitrary fluorescence units (AFU); to ease the comparison, signals in (A), (D), and (G) were normalized (set to 1) to the baseline fluorescence (F0) in the BCECF, the Fura-2, and the RhoVR channel, respectively, recorded immediately after mixing with resact. (B, E, H) Resact-evoked change in fluorescence (ΔF) with respect to the baseline fluorescence (F0), that is, ΔF/F0 (%); #signals smoothed with a sliding average of 80 ms. (C, F, I) First 2 s of the fluorescence signal recorded in the BCECF channel plotted against that recorded in the Fura-2 or the RhoVR channel using either optical filtering (top panel) or FASTM (bottom panel). Gray line: linear fit of the plots to quantify the crosstalk between the channels (see explanation in the text). (J) Percent crosstalk between the channels according to the analysis shown in (C), (F), and (I).
-
Figure 3—source data 1
Fluorescence signals in arbitrary fluorescence units.
- https://cdn.elifesciences.org/articles/63129/elife-63129-fig3-data1-v2.xlsx
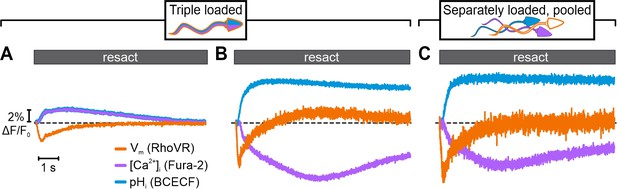
Simultaneous recording of resact-evoked pHi, [Ca2+]i, and Vm signals in sperm loaded with BCECF, Fura-2, and RhoVR.
Relative changes in fluorescence ∆F/F0 evoked by 50 pM resact. The respective control signal evoked by mixing with artificial sea water (ASW) was subtracted, setting the control-signal level to ΔF/F0 (%) = 0 (dotted line). Signals were recorded using optical filtering alone (A) or frequency- and spectrally-tuned multiplexing (FASTM) (B). (C) Simultaneous FASTM recording of resact-evoked signals from pooled sperm loaded separately with either BCECF, Fura-2, or RhoVR.
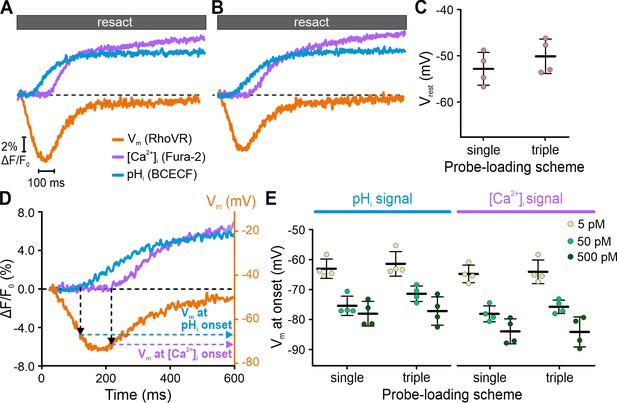
Interrogating putative probe-related perturbations of signaling.
Resact-evoked Vm, pHi, and [Ca2+]i signals recorded individually from different sperm samples loaded with one probe only (A) or recorded simultaneously from triple-loaded sperm (B); to facilitate direct comparison, Fura-2 fluorescence was multiplied by –1 to depict an increase of [Ca2+]i as an increasing signal. (C) Comparison of Vrest of sperm loaded with RhoVR (single-loaded) or RhoVR, BCECF, and Fura-2 (triple-loaded). (D) Calibrated resact-induced (50 pM) Vm response and accompanying pHi and [Ca2+]i signals. The artificial sea water (ASW) control was subtracted, and the dotted black line indicates ΔF/F0 = 0 and Vrest. The Vm at the onset of the pHi and [Ca2+]i signals was deduced from the signal latencies. (E) Vm at the onset of pHi and [Ca2+]i signals in single- versus triple-loaded sperm. With increasing resact concentrations, the rise in pHi and [Ca2+]i commenced at increasingly negative Vm (Seifert et al., 2015).
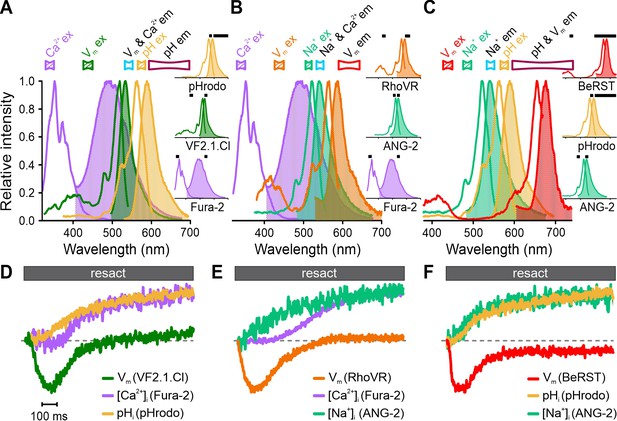
Simultaneous recording of resact-induced signaling events in sperm loaded with various triple combinations of probes for Ca2+, pH, Na+, and Vm using frequency- and spectrally-tuned multiplexing (FASTM).
Superposition of excitation (outlined) and emission (filled) spectra of (A) Fura-2, VF2.1.Cl, and pHrodo; (B) Fura-2, RhoVR, and ANG-2; (C) BeRST, pHrodo, and ANG-2. Bandpass filters used for excitation (filled) and emission (outlined) are depicted above the spectra. Inset: individual excitation and emission spectra with respective filters (black bars). (D–F) Signals (∆F/F0) evoked by 500 pM resact corrected for the artificial sea water (ASW) control and normalized to their respective peak values (set to 1) for easier illustration.
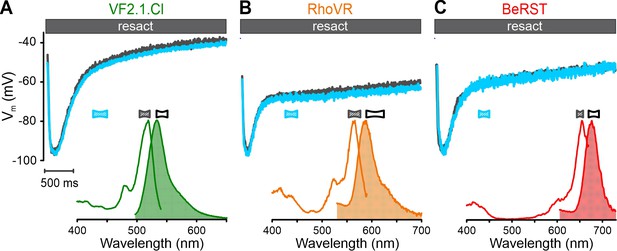
Spectral utility of voltage-sensitive probes.
Calibrated resact-evoked (500 pM) Vm response in sea urchin sperm reported by VF2.1.Cl (A), RhoVR (B), and BeRST (C) upon excitation at the principal peak (gray) versus excitation at the secondary peak (blue). Inset: excitation and emission spectra of probes in sea urchin sperm. Bandpass filters used for excitation (filled) and emission (outlined) are depicted above.
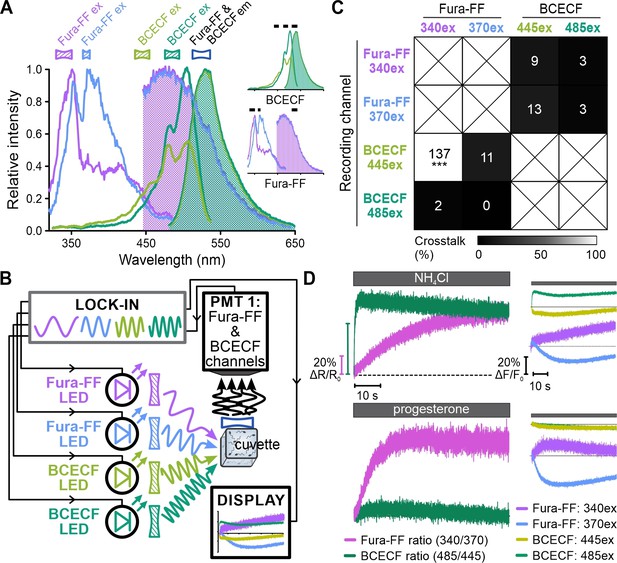
Simultaneous ratiometric recording of [Ca2+]i and pHi signals in human sperm.
(A) Superimposed excitation (outlined) and emission (filled) spectra of Fura-FF and BCECF. Inset: individual spectra with respective filters (black bars). (B) Schematic of frequency- and spectrally-tuned multiplexing (FASTM) configuration for simultaneous ratiometric dual-excitation recording of Fura-FF and BCECF in human sperm. (C) Crosstalk among channels based on the analysis shown in Figure 7—figure supplement 1. #Under these particular conditions, the approach to quantify crosstalk yielded an erroneously inflated value (for details, see Figure 7—figure supplement 1). (D) Left panels: ratiometric [Ca2+]i and pHi signals (ΔR/R0) in human sperm evoked by NH4Cl (10 mM) or progesterone (100 nM) corrected for the buffer control. Right panel: fluorescence signals in the individual Fura-FF and BCECF channels underlying the ratio.
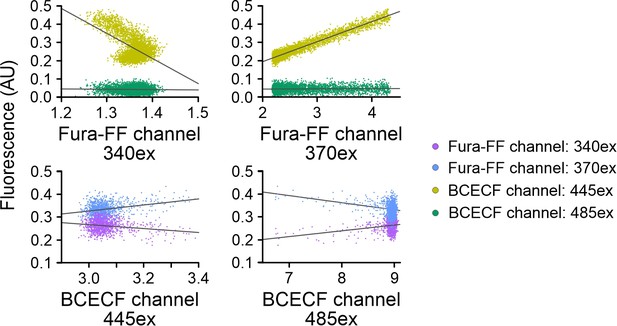
Analysis of crosstalk between BCECF and Fura-FF channels recorded with frequency- and spectrally-tuned multiplexing (FASTM).
Human sperm loaded with BCECF or Fura-FF were mixed with either NH4Cl or progesterone, respectively. The first 5 s (BCECF-loaded sperm) and 20 s (Fura-FF-loaded sperm) of the fluorescence signals recorded in the different channels were plotted against each other and crosstalk was evaluated by linear fitting of the data. The steepness of the linear fit (gray line) is a measure of the crosstalk between channels. Of note, Fura-FF fluorescence upon excitation at the isosbestic point at 340 nm does not change with [Ca2+]i. Fura-FF is also excited by 445 nm, and the emission changes with [Ca2+]i. Consequently, the linear fit of the plot of fluorescence signals at 445 vs. 340 nm excitation indicates severe crosstalk among channels. The absolute fluorescence values are, however, an order of magnitude lower compared to that recorded in BCECF-loaded sperm and do, thus, not affect simultaneous recording of Fura-FF and BCECF.
-
Figure 7—figure supplement 1—source data 1
Fluorescence signals for the analysis of crosstalk between BCECF and Fura-FF channels.
- https://cdn.elifesciences.org/articles/63129/elife-63129-fig7-figsupp1-data1-v2.xlsx
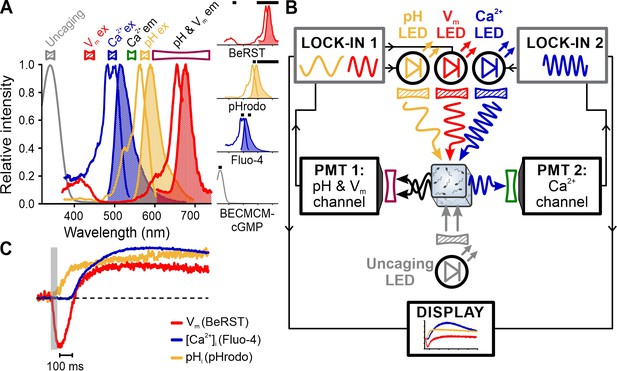
Simultaneous recording of [Ca2+]i, pHi, and Vm signals in sea urchin sperm evoked by flash photolysis of caged cGMP.
(A) Superimposed absorbance spectrum of BECMCM-cGMP and excitation (outlined) and emission (filled) spectra of Fluo-4, pHrodo, and BeRST. Bandpass filters used for excitation (filled) and emission (outlined) are depicted above the spectra. Inset: individual spectra and respective filters (black bars). (B) Schematic of frequency- and spectrally-tuned multiplexing (FASTM) configuration for uncaging experiments. (C) Vm, pHi, and [Ca2+]i signals evoked by uncaging intracellular cGMP with a 50 ms UV-flash (gray bar).
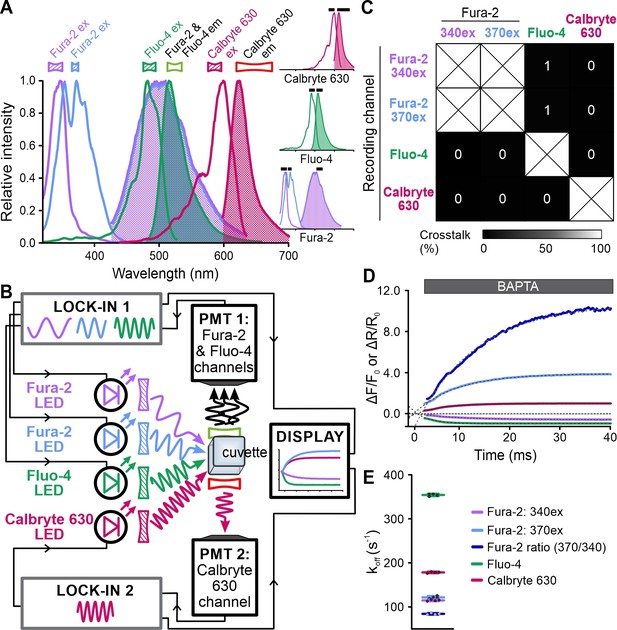
Simultaneous recording of the kinetics of Ca2+ dissociation from Fura-2, Fluo-4, and Calbryte 630.
(A) Superimposed excitation (outlined) and emission (filled) spectra of Fura-2, Fluo-4, and Calbryte 630. Inset: individual spectra depicted with respective filters (black bars). (B) Schematic of the frequency- and spectrally-tuned multiplexing (FASTM) configuration for simultaneous recording of Ca2+ dissociation from Fura-2, Fluo-4, and Calbryte 630. (C) Crosstalk between channels according to Figure 9—figure supplement 1. (D) Changes in Fura-2, Fluo-4, and Calbryte 630 fluorescence and 370 nm/340 nm emission ratio of Fura-2 upon mixing of the Ca2+-bound probes with the Ca2+ chelator BAPTA. (E) Koff values determined by exponential fitting of the individual fluorescence traces and the ratio of Fura-2 (370/340 nm): Fura-2, 340ex: 115 ± 2 s–1; Fura-2, 370ex: 122 ± 3 s–1; Fura-2 ratio: 84 ± 2 s–1; Fluo-4: 354 ± 3 s–1; Calbryte 630: 178 ± 2 s–1 (n = 4).
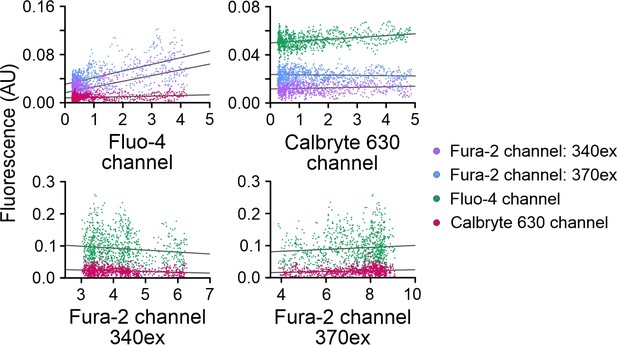
Analysis of crosstalk between Fura-2, Fluo-4, and Calbryte 630 channels simultaneously recorded with FASTM.
The first 18 ms of the fluorescence signals recorded in the different channels were plotted against each other, and crosstalk was evaluated by a linear fit to the data. The steepness of the linear fit (gray line) is a measure of the crosstalk between channels.
-
Figure 9—figure supplement 1—source data 1
Fluorescence signals for the analysis of crosstalk between Fura-2, Fluo-4, and Calbryte 630 channels.
- https://cdn.elifesciences.org/articles/63129/elife-63129-fig9-figsupp1-data1-v2.xlsx
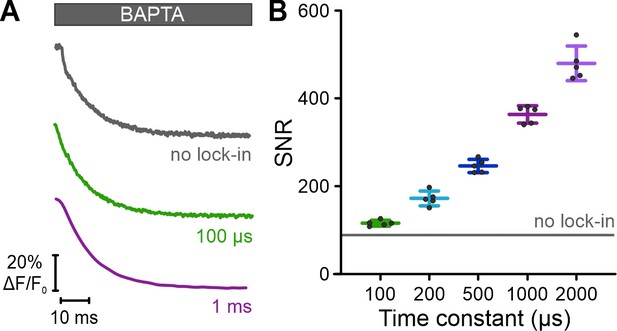
Signal-to-noise (S/N) ratio of fluorescence signals recorded upon mixing of Ca2+-bound Fura-2 with BAPTA.
(A) Kinetics of Ca2+ dissociation from Fura-2 recorded upon excitation at 340 nm. The kinetics were recorded with frequency- and spectrally-tuned multiplexing (FASTM) using either different lock-in amplifier time constants (100 µs and 1 ms) or without modulation of the excitation light and using a conventional amplifier (no lock-in). (B) S/N ratio determined by dividing the mean signal amplitude by the standard deviation of 200 data points. The gray line indicates the S/N ratio in recordings performed without modulation of the excitation light.
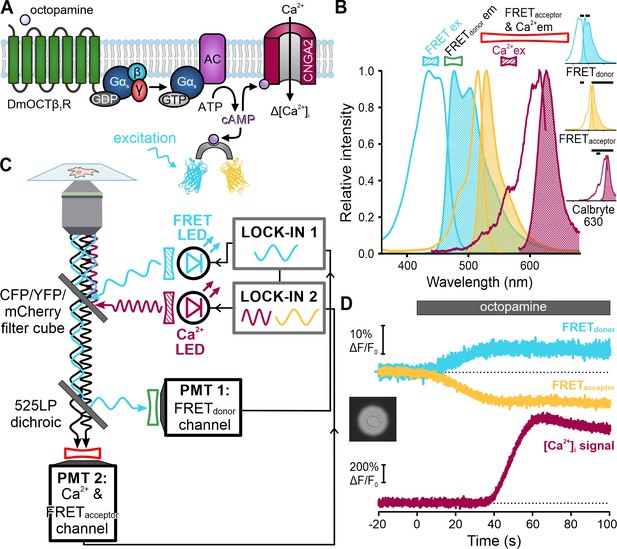
Single-cell frequency- and spectrally-tuned multiplexing (FASTM) fluorescence microscopy for simultaneous recording of cAMP and [Ca2+]i.
(A) Octopamine-signaling pathway in HEK cells coexpressing the DmOCTβ1 receptor, CNGA2-TM channel, and a FRET-based cAMP biosensor. (B) Superimposed excitation (outlined) and emission (filled) spectra of the FRET donor-acceptor pair cerulean-citrine, and the Ca2+-probe Calbryte 630. Bandpass filters used for excitation (filled) and emission (outlined) are depicted above the spectra. Inset: individual spectra and filters (black bars). (C) Schematic of the FASTM configuration for single-cell microscopy. (D) Changes in fluorescence (ΔF/F0 (%)) of the FRET donor and acceptor as well as Calbryte 630 evoked by octopamine (20 µM). Inset: image of the field of view with a single cell enclosed by an aperture.
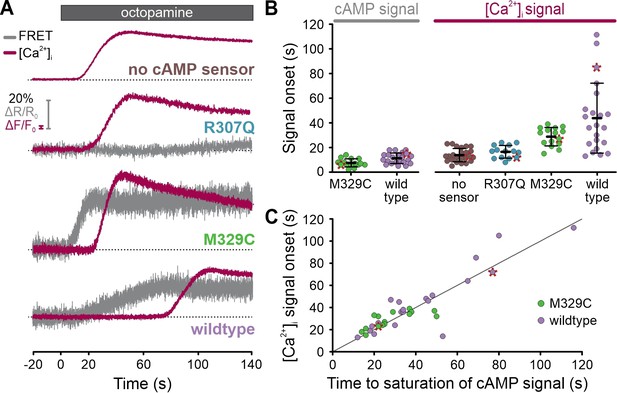
Simultaneous recording of cAMP and [Ca2+]i signals in single cells using frequency- and spectrally-tuned multiplexing (FASTM).
(A) Octopamine-induced (20 µM) [Ca2+]i signals and changes in the FRET ratio (donor/acceptor), that is, cAMP signals, in the absence or presence of a non-binding (R307Q), lower (M329C), or higher-affinity (wildtype) FRET-based cAMP biosensor. Data points corresponding to the representative traces are labeled with a red asterisk in (B) and (C). (B) Onset of the octopamine-induced cAMP and [Ca2+]i signals. (C) Comparison of the time to saturation of the cAMP signal and the onset of the [Ca2+]i signal. The gray line depicts the theoretical perfect correlation.
-
Figure 11—source data 1
Onset of the optopamine-induced cAMP and [Ca2+]i signals.
- https://cdn.elifesciences.org/articles/63129/elife-63129-fig11-data1-v2.xlsx
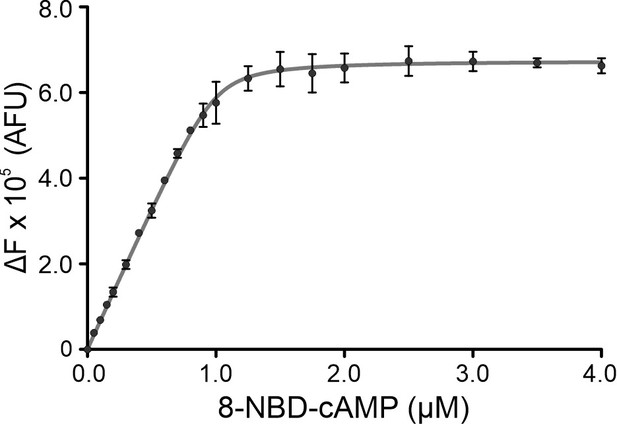
Affinity of mlCNBD-M329C for 8-NBD-cAMP.
Increase of 8‐NBD‐cAMP fluorescence (emission at 550 nm) upon binding to mlCNBD-M329C (1 μM). 8‐NBD‐cAMP fluorescence in the absence of mlCNBD-M329C was subtracted. The solid line represents a nonlinear least‐squares fit to ΔF = RL · x, with the receptor-ligand complex RL and the normalization factor x, that relates the concentration of bound 8-NBD-cNMP to the change in fluorescence (ΔF) (Cukkemane et al., 2007). The KD value was 0.12 µM.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (HEK293) | flp-In-293 | Invitrogen | #R750-07 | RRID:CVCL_U421 |
| Transfected construct (Drosophila melanogaster) | DmOCTβ1R | Balfanz et al., 2005 | ||
| Transfected construct (Bos taurus) | CNGA2-TM | Schröder-Lang et al., 2007 | ||
| Recombinant DNA reagent | pc3.1-ml CNBD-FRET | Mukherjee et al., 2016 | ||
| Recombinant DNA reagent | pc3.1-mlCNBD-FRET-R307Q | Mukherjee et al., 2016 | ||
| Recombinant DNA reagent | pc3.1-mlCNBD-FRET-M329C | This paper | Figure 11—figure supplement 1 and materials and methods part of this MS. | |
| Other | Pluronic F-127 | Sigma-Aldrich | P2443 | |
| Other | Fluo-4 AM | Thermo Fisher | F14202 | |
| Other | BCECF AM | Thermo Fisher | B1150 | |
| Other | Fura-2 AM | Thermo Fisher | F1201 | |
| Other | pHrodo Red AM | Thermo Fisher | P35372 | |
| Other | ANG-2 AM | MobiTec | 3502 | |
| Other | Calbryte 630 AM | AAT Bioquest | 20720 | |
| Other | Fura-FF, AM | AAT Bioquest | 21027 | |
| Other | VF2.1.Cl | Miller et al., 2012 | Sold by Thermo Fisher as FluoVolt | |
| Other | BeRST | Huang et al., 2015 | ||
| Other | RhoVR | Deal et al., 2016 | ||
| Other | Calbryte 630, potassium salt | AAT Bioquest | 20727 | |
| Other | Fluo-4, pentapotassium salt | Thermo Fisher | F14200 | |
| Other | Fura-2, pentapotassium salt | Thermo Fisher | F1200 |
Loading protocols for fluorescent probes in A. punctulata sperm and FASTM modulation frequencies.
| Fura-2, BCECF, RhoVR | ANG-2, pHrodo, BeRST | |||||
| Loading order | First | Second | Third | First | Second | Third |
| Name | Fura-2 AM | RhoVR | BCECF AM | ANG-2 AM | pHrodo Red AM | BeRST |
| Probe type | Ca2+ | Vm | pH | Na+ | pH | Vm |
| Concentration (µM) | 10 | 5 | 5 | 10 | 10 | 5 |
| Incubation (min) | 90 | 10 | 5 | 90 | 25 | 10 |
| FASTM modulation frequency (kHz) | 30.4 | 37.3 | 50 | 37 | 50 | 23 |
| Fura-2, ANG-2, RhoVR | Fura-2, pHrodo, VF2.1.Cl | |||||
| Loading order | First | Second | Third | First | Second | Third |
| Name | Fura-2 AM | ANG-2 AM | RhoVR | Fura-2 AM | pHrodo Red AM | VF2.1.Cl |
| Probe type | Ca2+ | Na+ | Vm | Ca2+ | pH | Vm |
| Concentration (µM) | 10 | 10 | 5 | 10 | 10 | 5 |
| Incubation (min) | 90 (added together) | 5 | 50 | 35 | 5 | |
| FASTM modulation frequency (kHz) | 50 | 23 | 37 | 50.3 | 25.3 | 17.1 |
| Fluo-4, phrodo, BeRST, BECMCM-cGMP | ||||||
| Loading order | First | Second | Third | Fourth | ||
| Name | BECMCM-cGMP | Fluo-4 AM | pHrodo Red AM | BeRST | ||
| Probe type | Caged cGMP | Ca2 | pH | Vm | ||
| Concentration (µM) | 10 | 10 | 10 | 5 | ||
| Incubation (min) | 15 | 10 | 35 | 10 | ||
| FASTM modulation frequency (kHz) | None | 37.3 | 30.1 | 50.3 | ||
Optical configurations for recording signals from A. punctulata sperm.
| Probe combination | Fluorescent probe | LED (Thorlabs) | Excitation filter (Semrock) | Dichroics | Emission filter(Semrock) |
|---|---|---|---|---|---|
| Fura-2, BCECF, RhoVR | Fura-2 | M375L4 | 379/34 | 470 LPXR (Chroma)HC BS 409 (Semrock) | 524/24 |
| BCECF | M490L4 | 485/20 | |||
| RhoVR | M455L4 | 438/24 | 607/36 | ||
| ANG-2, pHrodo, BeRST | ANG-2 | M490L4 | 485/20 | 525 LPXR (Chroma)470 LPXR (Chroma) | 542/20 |
| pHrodo | M565L3 | 575/19 | 593LP | ||
| BeRST | M455L4 | 438/24 | |||
| Fura-2, ANG-2, RhoVR | Fura-2 | M340L4 | 340/22 | 470 LPXR (Chroma)HC BS 409 (Semrock) | 542/20 |
| ANG-2 | M505L3 | 513/17 | |||
| RhoVR | M455L4 | 438/24 | 607/36 | ||
| Fura-2, pHrodo, VF2.1.Cl | Fura-2 | M340L4 | 340/22 | 470 LPXR (Chroma)HC BS 409 (Semrock) | 542/20 |
| VF2.1.Cl | M455L4 | 438/24 | |||
| pHrodo | M565L3 | 575/19 | 593LP | ||
| BECMCM- cGMP, Fluo-4, pHrodo, BeRST | BECMCM-cGMP | M340L4 | 340/22 | 525 LPXR (Chroma)470 LPXR (Chroma)HC BS 409 (Semrock) | |
| Fluo-4 | M490L4 | 494/20 | 542/20 | ||
| pHrodo | M565L3 | 575/19 | 593LP | ||
| BeRST | M455L4 | 438/24 |
Optical configuration for simultaneous ratiometric recording of [Ca2+]i and pHi signals in human sperm.
| Fluorescent probe | LED (Thorlabs) | FASTM modulation frequency (kHz) | Excitation filter (Semrock) | Dichroics | Emission filter(Semrock) |
|---|---|---|---|---|---|
| Fura-FF | M340L4 | 87.3 | 340/22 | HC BS 365 (Semrock)HC BS 409 (Semrock)470 LPXR (Chroma) | 524/24 |
| M375L4 | 73.51 | 370/10 | |||
| BCECF | M455L4 | 61.7 | 445/20 | ||
| M490L4 | 103.7 | 485/20 |
-
FASTM, frequency- and spectrally-tuned multiplexing.
Optical configuration for koff determination.
| Fluorescent probe | LED (Thorlabs) | FASTM modulation frequency (kHz) | Excitation filter (Semrock) | Dichroics | Emission filter(Semrock) |
|---|---|---|---|---|---|
| Fura-2 | M340L4 | 87.31 | 340/22 | HC BS 365 (Semrock)HC BS 409 (Semrock)525 LPXR (Chroma) | 524/24 |
| M375L4 | 103.7 | 370/10 | |||
| Fluo-4 | M490L4 | 59.51 | 485/20 | ||
| Calbryte 630 | M565L3 | 47.1 | 586/20 | 647/57 |
-
FASTM, frequency- and spectrally-tuned multiplexing.





