Sclerostin small-molecule inhibitors promote osteogenesis by activating canonical Wnt and BMP pathways
Figures
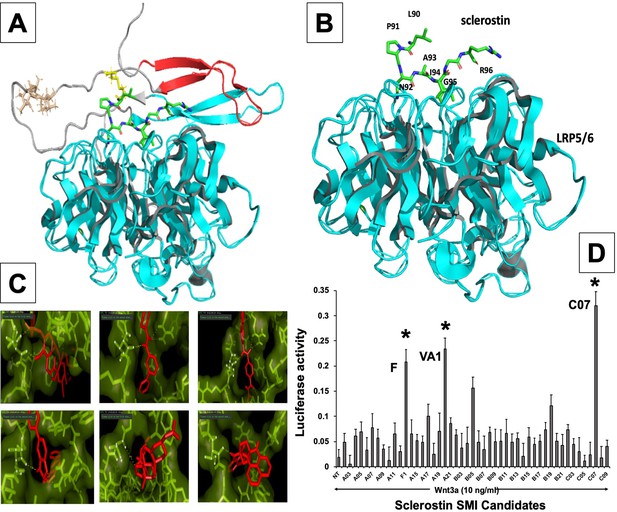
In silico design of sclerostin small-molecule inhibitors (SMIs).
Structural representation of sclerostin (PDB ID 2K8P) is shown (A). Three loops of sclerostin are color coded (red for loop 1, gray for loop 2, and cyan for loop 3). Also, the residues from loop 2 that are targeted for inhibitor selection are highlighted, including Leu 90, Pro 91, Asn 93, Ala 93, and Ile 94, which is shown in relation to the structure of the β-propeller domain of LRP6 (PDB ID 3SOV) (B). Candidate compounds were next selected in silico against the loop 2 region (91st to 95th amino acid position) and the cystine knot region (Cys 85 and Cys 143) of sclerostin; their poses are shown (C). Panel (D) shows the selection of the most effective compounds in vitro, with compounds F1, VA1, and C07 being the most potent sclerostin SMIs tested. All three were able to effectively enhance Wnt3a–induced reporter activity at suboptimal dose of Wnt3a. Treatment of transfected cells with compounds alone (without Wnt3a protein) did not show any significant induction of reporter activity.
-
Figure 1—source data 1
Relative luciferase activity of small-molecule screening.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig1-data1-v3.xlsx
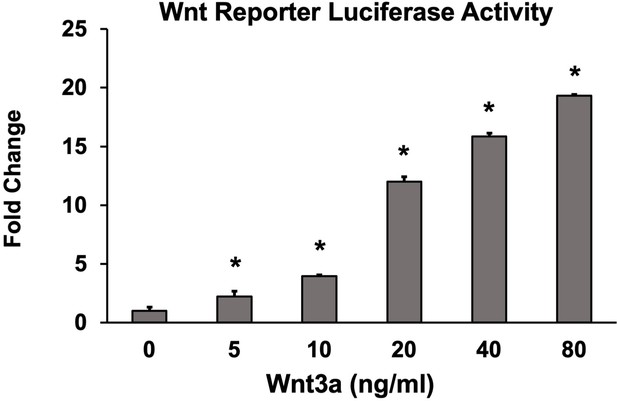
Optimization of suboptimal Wnt concentration in Wnt activity screening assay.
Wnt pathway potentiation by small-molecule inhibitors (SMIs) was determined in C2C12 cells. Based on these results, 10 ng/ml of Wnt3a was used to screen SMIs for Wnt activity in vitro. Luciferase activity was determined in triplicate (n = 3). Error bars represent standard error of mean (SEM) values. * denotes statistical significance (p<0.05) when compared to controls (BMP alone).
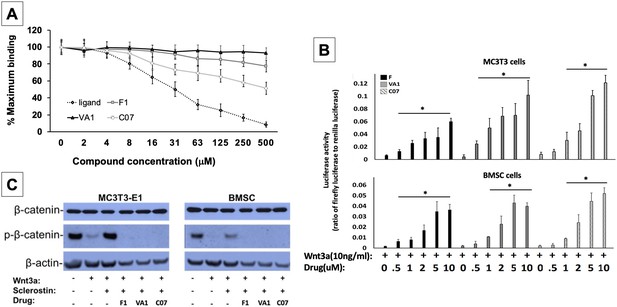
Sclerostin small-molecule inhibitors (SMIs) increase canonical Wnt signaling intensity in vitro.
An in vitro binding assay with purified recombinant LRP5 and sclerostin proteins was used to assess for SMI–LRP interaction (A). The sclerostin SMIs were able to complete with sclerostin to prevent up to 40% of the labeled sclerostin from binding to LRP5, confirming that our lead compounds significantly disrupt sclerostin binding to LRP5. Moreover, the sclerostin SMIs were able to potentiate the intensity of Wnt signaling in vitro in a luciferase reporter assay system (B). A TCF/LEF-responsive reporter was transfected into murine MC3T3-E1 and MSC cells. In the presence of a suboptimal dose of 10 ng/ml of Wnt3a, all three SMIs dose-dependently enhanced Wnt/β-catenin signaling. Finally, sclerostin SMIs inhibit the accumulation of the phosphorylated (inactive) form of β-catenin in both murine MC3T3-E1 and MSC cells (C). Cells were treated with or without 10 µM concentration of sclerostin inhibitors for 2 d. Western blotting with antibodies specific for the phosphorylated and unphosphorylated forms of β-catenin was performed. All sclerostin SMIs inhibited the accumulation of phosphorylated (inactive) β-catenin in murine MC3T3-E1s and MSCs.
-
Figure 2—source data 1
Raw β-catenin western blot data (Figure 2).
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig2-data1-v3.zip
-
Figure 2—source data 2
Raw sclerostin western blot data (Figure 2—figure supplement 1).
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig2-data2-v3.zip
-
Figure 2—source data 3
Relative luciferase activity of anti-sclerostin small molecules.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig2-data3-v3.xlsx
-
Figure 2—source data 4
Binding assay raw data.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig2-data4-v3.xlsx
-
Figure 2—source data 5
Raw data anti-sclerostin small-molecule candidate screening.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig2-data5-v3.xlsx
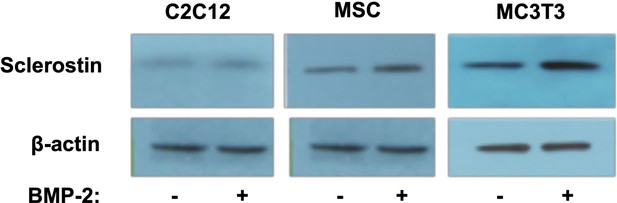
Verification of endogenous sclerostin protein in cell types used.
To ensure that sclerostin was in fact being expressed in these cells, we performed western blots using anti-sclerostin monoclonal antibodies (mAbs) both with and without BMP-2 treatment. All cells were treated with BMP-2 (200 ng/ml) for 2 d. Antibody dilution is 1:2000.
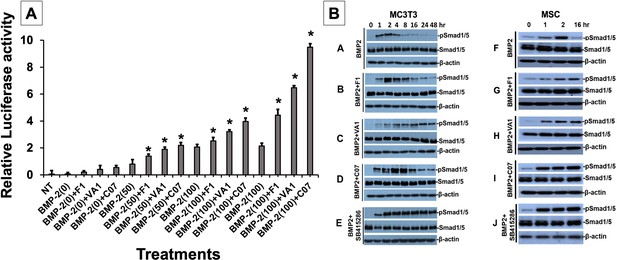
Sclerostin inhibitors potentiate the intensity and duration of BMP signaling in vitro.
Using an established BMP-responsive murine calvarial osteoblast reporter cell line, enhancement of BMP-2-induced luciferase reporter activity by sclerostin small-molecule inhibitors (SMIs) was observed (A). Three doses (50, 100, and 200 ng/ml) of BMP-2 were chosen to determine potentiating effect of the SMIs at 10 µM concentration. Cells were pretreated with compounds for 24 hr followed by another treatment by BMP ± SMIs for 3 hr. Measurements were determined in triplicate (n = 3). In addition to potentiating BMP signal intensity, sclerostin SMIs were also able to increase accumulation of phosphorylated (active) Smads1/5 (B). Cells were treated with or without 10 uM concentration of sclerostin SMIs for 2 d. The cell lysates were then subjected to western blotting with specific antibodies for phosphorylated and unphosphorylated forms of Smads1/5. The GSK3b inhibitor SB415286 was a positive control.
-
Figure 3—source data 1
Pulse chase western blot raw data, MC3T3.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig3-data1-v3.zip
-
Figure 3—source data 2
Pulse chase western blot raw data, MSC.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig3-data2-v3.zip
-
Figure 3—source data 3
Relative luciferase activity of small molecule + BMP.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig3-data3-v3.xlsx
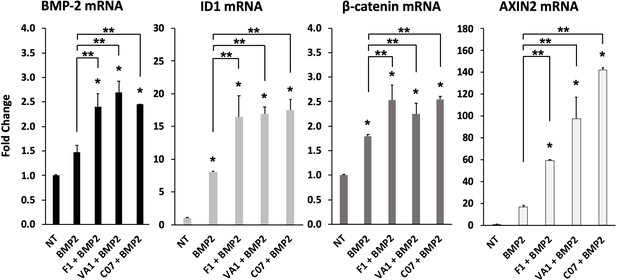
Sclerostin small-molecule inhibitors (SMIs) enhance the expression of osteogenic genes.
The expression level of several genes was assessed by RT-qPCR after MC3T3 cells were treated with 10 µM of F1, VA1, or C07 in the presence of a suboptimal dose of BMP-2 (35 ng/ml). All data were determined in triplicate (n = 3). NT, no treatment (control). * indicates statistical significance (p<0.05) compared to NT, while ** indicates significance (p<0.05) from BMP-2 treatment alone.
-
Figure 4—source data 1
Raw PCR data.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig4-data1-v3.xlsx
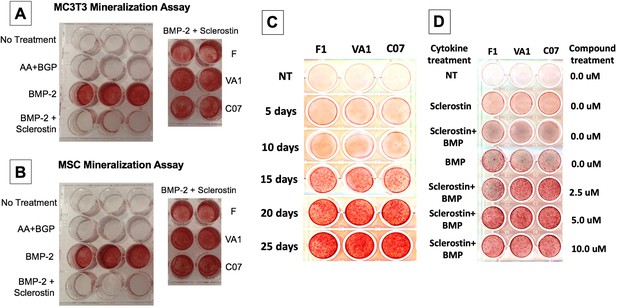
Sclerostin small-molecule inhibitors (SMIs) produce de novo mineralization in vitro.
Sclerostin SMIs reverse the inhibitory effects of sclerostin on the osteogenic potential of murine pre-osteoblasts (MC3T3-E1 cells, A) and bone marrow stromal cells (BMSCs) (B) in a mineralization assay in vitro. Recombinant murine sclerostin (80 ng/ml) completely reversed the osteoinductive effects of rhBMP-2 (20 ng/ml). This inhibitory effect was subsequently reversed by the addition of the three sclerostin SMI candidates at 10 μM. Next, a time-course experiment was run (C), which shows that the SMIs accelerate mineralization over time. The dose–response on mineralization was also assessed, showing a ceiling effect around 10 µM (D).
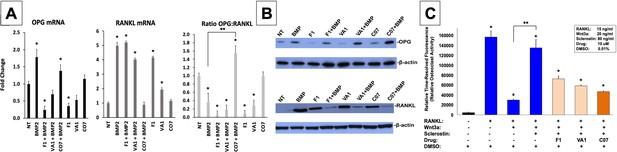
Sclerostin small-molecule inhibitors (SMIs) inhibit osteoclastic activity.
The expression level of OPG and RANKL was assessed by RT-qPCR after MC3T3 cells were treated with 10 µM of F1, VA1, or C07 in the presence of a suboptimal dose of BMP-2 (35 ng/ml) (A). All data were determined in triplicate (n = 3). NT, no treatment (control). * indicates statistical significance (p<0.05) compared to NT, while ** indicates significance (p<0.05) from BMP-2 treatment alone. These changes in OPG and RANKL levels were also confirmed at the protein level using western blot, particularly in the presence of a suboptimal dose of BMP-2 (100 ng/ml) (B). Finally, all sclerostin SMIs were tested for their ability to inhibit osteoclast activity in RAW cells in a bone resorption assay. All three SMIs were found to significantly (p<0.05) inhibit sclerostin-induced osteoclast activity, with C07 approaching the response to Wnt3a+RANKL (C).
-
Figure 6—source data 1
Raw RANKL western blot data.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig6-data1-v3.zip
-
Figure 6—source data 2
Raw OPG western blot data.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig6-data2-v3.zip
-
Figure 6—source data 3
Raw PCR data.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig6-data3-v3.xlsx
-
Figure 6—source data 4
Raw fluorescence data.
- https://cdn.elifesciences.org/articles/63402/elife-63402-fig6-data4-v3.xlsx
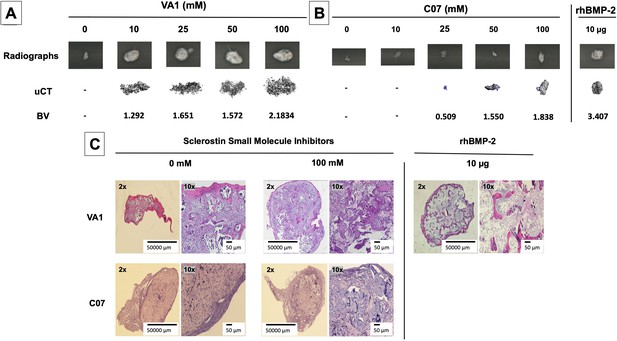
Standalone sclerostin small-molecule inhibitors (SMIs) result in de novo ectopic mineralization in vivo.
A dose-dependent increase in ectopic mineralization was observed when VA1 and C07 were placed subcutaneously in rats on a plain collagen sponge (A, B). Significant differences compared with control implants treated with the vehicle alone (DMSO) were found at all doses tested. Plain radiographs (XR) and μCT, along with the corresponding BV (bone volume) values, are shown for both VA1 (A) and C07 (B). Of not, both VA1 and C07 were able induce ectopic mineralization without the addition of exogenous BMP. A 10 μg rhBMP-2-positive control is also shown (B). Of note, the scale of each μCT scan is consistent across all images. Representative histological images (hematoxylin and eosin) of subcutaneous explants are also shown (C), with 100 mM of VA1 and C07 demonstrating ectopic bone formation without the adipogenesis also seen with BMP-2.
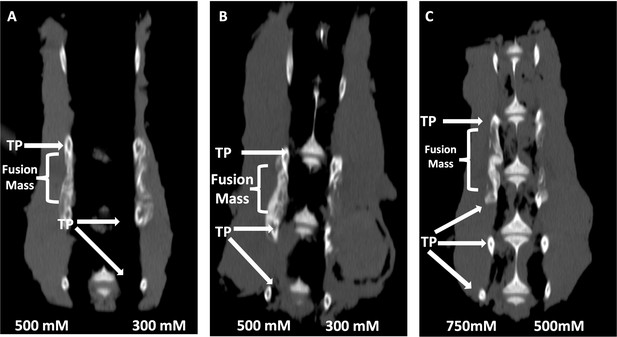
Locally delivered sclerostin small-molecule inhibitors (SMIs) produce successful spine fusions in vivo.
Coronal μCT reconstructions of rabbit spines 6 wk following posterolateral spine arthrodesis are shown. In rabbits that received C07 along with autologous Iliac crest bone graft (IBCG) (A), the posterolateral spine fusion rate was significantly increased compared to controls with iliac crest bone graft (ICBG) alone (83% vs. 66%, p<0.05). Similarly, the fusion rate in rabbits that received VA1 with autologous ICBG (B) was also significantly increased versus ICBG control (80% vs. 66%, p<0.05). Lastly, standalone C07 (no ICBG) at a high dose of 750 mM (C) produced a fusion rate of 80% (vs. 0% in controls, p<0.05). A continuous bridge of bone from TP to TP (noted as ‘Fusion Mass’) is shown on the side with the higher dose in all instances (A–C).
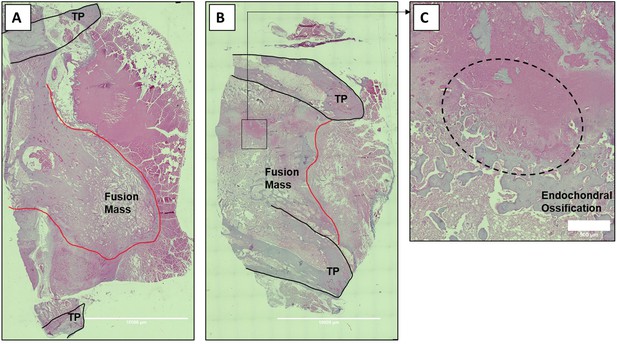
Histologic sections of C07-enhanced posterolateral spine fusions in vivo.
Mid-coronal histological sections (5× magnification) from rabbit spinal fusion beds 6 wk following posterolateral spine arthrodesis were H&E-stained and confirm successful spinal fusion, showing bridging bone from TP to TP. Sections from a rabbit that received C07 without (A) and with (B) autologous IBCG are shown. Panel (C) shows a ×10 magnification of the boxed area in panel (B) and shows the area of endochondral ossification in the middle of the developing fusion mass.
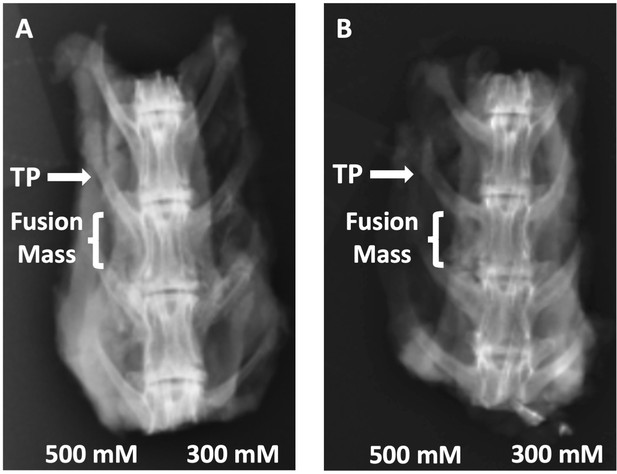
Radiographs of rabbit posterolateral spine fusions.
Representative posterior–anterior plain radiographs (XRs) are shown for C07 with (A) and without (B) autogenous iliac crest bone graft (ICBG). The posterolateral inter-TP fusion masses are shown on the left side of each spine.
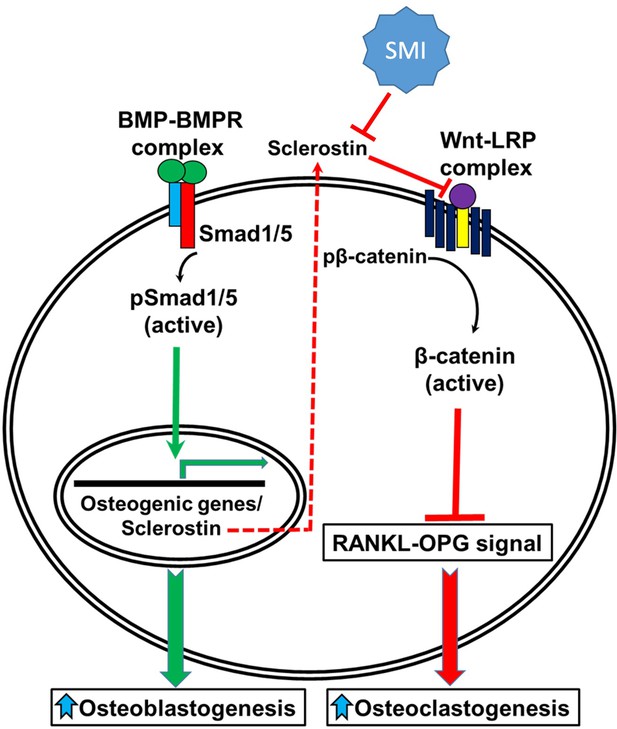
A schematic model of BMP-Wnt pathway crosstalk.
Canonical BMP signaling results in upregulation of sclerostin expression (the sclerostin promoter has BMP response elements) as part of a negative osteogenic feedback mechanism. Inhibition of sclerostin, such as with a sclerostin small-molecule inhibitor (SMI), allows for reactivation of normal canonical Wnt signaling with subsequent downregulation of osteoclastogenesis (via inhibition of the RANKL-OPG pathway and direct inhibitory effects on osteoclasts) and further potentiation of canonical BMP signaling via GSK3b. As such, sclerostin is a key bone mass-regulating factor. GSK3b, glycogen synthase kinase 3 beta.
Tables
Based off cell-based screening assays, these two FDA-approved compounds were determined to be good candidates to be repurposed as a sclerostin small-molecule inhibitor (SMI) for bone regeneration.
MOA, mechanism of action.
| Drug name | MOA | Current use | Dosing | Metabolism |
|---|---|---|---|---|
| Fluticasone (F) | Glucocorticoid receptor agonist | Topical anti-inflammatory | 100–2000 µg/day | Hepatic |
| Valproic acid (VA1) | Unknown | Anti-epileptic drug, migraines | 15–60 mg/kg/day | Hepatic |
Sclerostin small-molecule inhibitors (SMIs) increase posterolateral spinal fusion rates.
| Fusion rate (%) | |
|---|---|
| ICBG | 66 |
| VA1 + ICBG | 80 |
| C07 + ICBG | 83 |
| Control | 0 |
| VA1 | 17 |
| C07 | 33 |
| Control | 0 |
| VA1 | – |
| C07 | 80 |
-
Table 2—source data 1
Source data for Table 2.
- https://cdn.elifesciences.org/articles/63402/elife-63402-table2-data1-v3.xlsx
Additional files
-
Supplementary file 1
Primers used for PCR.
Details for the primers used in qPCR are listed. Primers for the genes in which a ‘Supplier’ is listed were purchased commercially.
- https://cdn.elifesciences.org/articles/63402/elife-63402-supp1-v3.xlsx
-
Supplementary file 2
Sclerostin small-molecule inhibitors (SMIs) show no signs of hepatic toxicity.
This table shows the results of liver function panels from peripheral blood drawn at the time of euthanasia for both rats after SQ implantation (4 wk) and rabbits after posterolateral spinal fusion (6 wk).
- https://cdn.elifesciences.org/articles/63402/elife-63402-supp2-v3.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/63402/elife-63402-transrepform1-v3.docx





