Glia actively sculpt sensory neurons by controlled phagocytosis to tune animal behavior
Figures
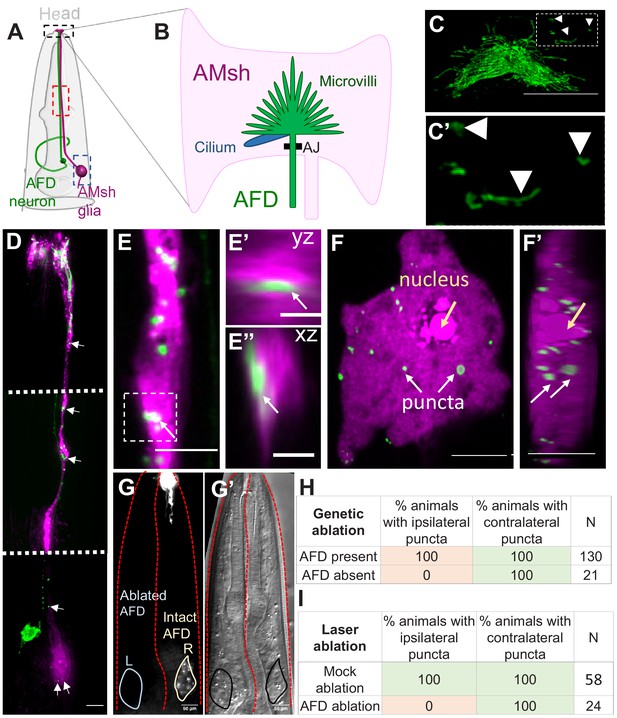
AMsh glia contain AFD–NRE-labeled puncta.
(A) Schematic of the C. elegans head region depicting AFD neuron and AMsh glial cell body and processes. Anterior is to the top. Black box: zoomed in (B, C); red box region zoomed in (E); blue box zoomed in (F). (B) The AMsh glia’s anterior ending ensheathes AFD–NRE dendrite, which comprises ~45 microvilli (green) and a single cilium (blue). AJ: adherens junction between AMsh glia and AFD neuron. (C, C’) PSRTX-1b:SRTX-1:GFP specifically labels AFD–NRE microvilli. Arrows indicate microvilli fragments disconnected from the main AFD–NRE structure, zoomed in (C'). Anterior is to the top. Scale bar: 5 μm. (D–F’) Fluorescence micrograph of AMsh glia (magenta) show AFD–NRE puncta throughout the cell (D) including the process (E) and soma (F). Image in (D) is a composite of three exposure settings of a single animal, stitched where indicated by dotted white line. Orthogonal slices of AMsh glial process (E’, E’’, scale bar: 2 μm) and cell body (F’) show AFD–NRE fragments completely within AMsh glia. Scale bar: 5 μm. (G, G’) Day 1 adult animal with left AFD neuron ablated by laser microsurgery during L1 larval stage. Left AMsh soma (blue outline) lacks AFD–NRE fragments, right AMsh soma (green outline) contains fragments. (G) Fluorescence micrograph, (G') differential intereference contrast (DIC) microscopy image. (H, I) Quantification of puncta in ipsilateral and contralateral AMsh glial cell soma with AFD neurons ablated by laser (H) or genetically (I). N: number of animals assayed; NRE: neuron-receptive ending.
-
Figure 1—source data 1
Raw data for Figure 1H, I.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig1-data1-v2.xlsx
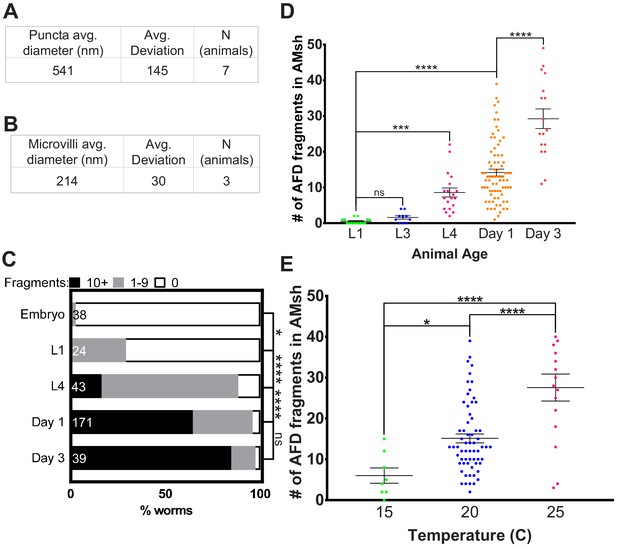
AMsh glia puncta engulf AFD–NRE.
(A) Quantification of average puncta diameter within AMsh glial cell soma. (B) Quantification of average AFD–NRE microvilli diameter from electron micrographs. (C) Population scores of wild-type animals with AFD–NRE-labeled fragments within AMsh soma at different developmental stages. X-axis: percent animals with fragments. Y-axis: developmental stage. Puncta numbers are quantified into three bins (≥10 fragments, black bar), (1–9 fragments, gray bar), (0 fragments, white bar). N: number of animals. Statistics: Fisher’s exact test. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005, ns = p>0.05. See Materials and methods for details. (D) Quantification of AFD–NRE-labeled fragments within AMsh soma at different developmental stages. X-axis: developmental stage. Y-axis: number of puncta per AMsh glial cell soma. Median puncta counts and N (number of animals): L1 larva (0.5 ± 0.2 puncta, n = 15 animals), L3 larva (1.6 ± 0.5 puncta, n = 10 animals), L4 larva (8.6 ± 1.2 puncta, n = 19 animals), day 1 adult (14.1 ± 1 puncta, n = 78 animals), and day 3 adult (29.2 ± 3 puncta, n = 17 animals). Statistics: one-way ANOVA w/ multiple comparison. *p<0.05, **p<0.005, ***p<0.0005, ****p<0.00005, ns = p>0.05. (E) Average number of fragments in animals cultivated at 15°C, 20°C, or 25°C. Refer (D) for data presentation details. Median puncta counts and N (number of animals): 15°C (6 ± 2 puncta, n = 8 animals), 20°C (14.1 ± 1 puncta, n = 78 animals), and 2 5°C (27.6 ± 3 puncta, n = 16 animals). NRE: neuron-receptive ending.
-
Figure 2—source data 1
AMsh glia puncta engulf AFD–NRE.
Raw data for Figure 2A–E. NRE: neuron-receptive ending.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig2-data1-v2.xlsx
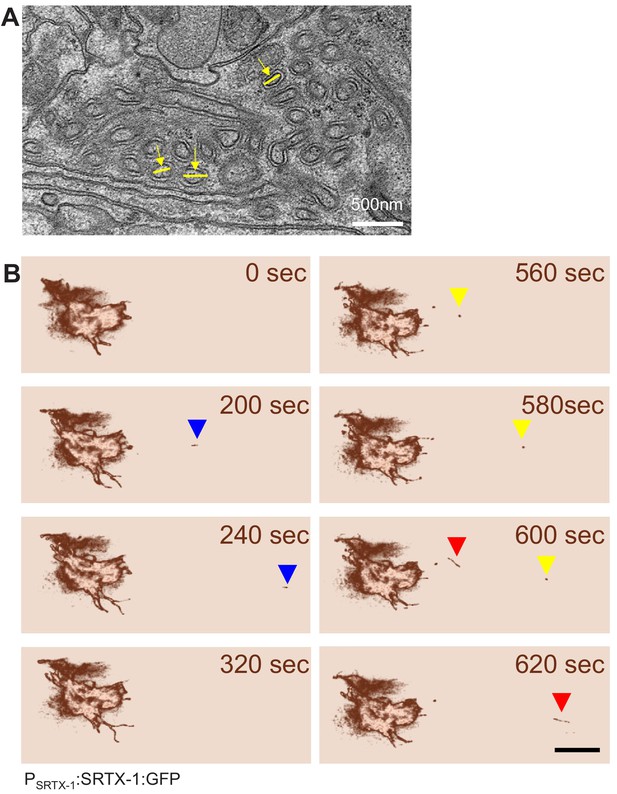
AMsh glia engulf AFD–NRE fragments.
(A) Electron micrograph through AFD–NRE microvilli of an animal. An individual microvillum taken for diameter measurement in Figure 2B is noted by yellow lines. Scale bar: 500 nm. (B) Time-stamped stills from Video 1 of AFD–NRE dissociation of fragments. Each colored arrowhead tracks an individual fragment moving away from AFD–NRE. Scale bar: 5 μm. NRE: neuron-receptive ending.
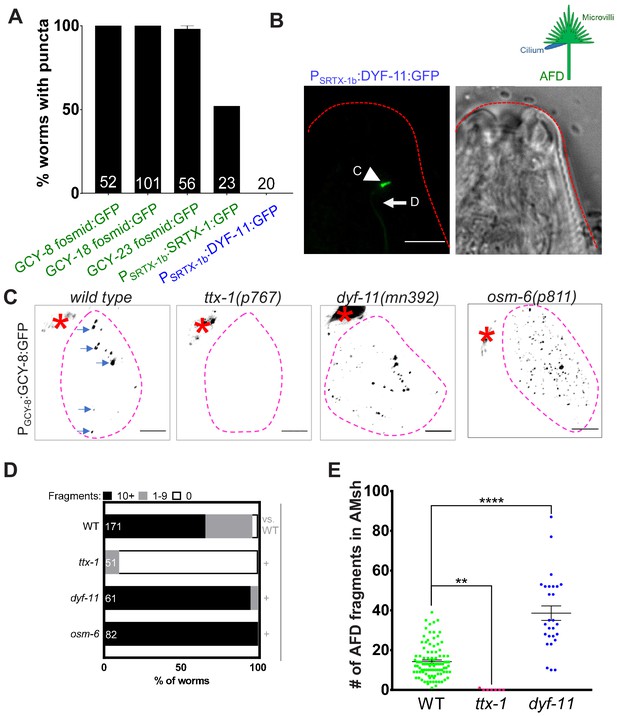
AMsh glia engulf AFD–NRE microvilli but not cilia.
(A) AFD–NRE-labeled fragments observed in different transgenic animal strains. Each strain has a different tagged fusion protein, driven by a different AFD-specific promoter, localizing to either microvilli (green) or cilium (blue). X-axis: genotype; Y-axis: percent animals with AFD–NRE-labeled puncta inside AMsh soma. N: number of animals analyzed. (B) Schematic depicting the two compartments of the AFD–NRE, which is an array of ~45 actin-based microvilli (green) and a single microtubule-based cilium (blue). Fluorescence and DIC micrographs showing expression of ciliary DYF-11:GFP, under an AFD neuron-specific promoter, in AFD cilia. C: cilia (arrowhead); D: AFD dendrite (arrow). (C) Fluorescence micrograph panel showing AFD–NRE tagged puncta (blue arrows) within AMsh glial cell soma (magenta outline) in different genetic backgrounds as noted. AFD cell body (red asterisk). Scale bar: 5 μm. (D) Population counts of animals with AMsh glial puncta. Refer Figure 2C for data presentation details. Alleles used: ttx-1(p767), dyf-11(mn392), and osm-6(p811). (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type. (E) Median puncta counts and N (number of animals): wild type (14 ± 1 puncta, n = 78 animals), ttx-1(p767) (0.1 ± 0.1 puncta, n = 7 animals), and dyf-11(mn392) (38.6 ± 3.6 puncta, n = 27 animals). Refer Figure 2D for data presentation details. NRE: neuron-receptive ending.
-
Figure 3—source data 1
AMsh glia engulf AFD–NRE microvilli but not cilia.
Raw data for Figure 3A, D, E. NRE: neuron-receptive ending.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig3-data1-v2.xlsx
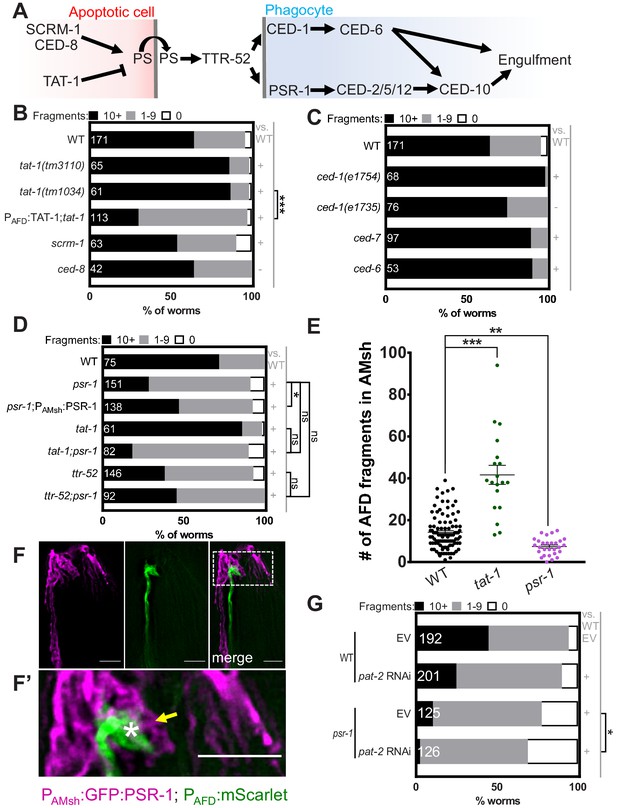
Engulfment of AFD–NRE by AMsh glia requires the phosphatidylserine receptor PSR-1 and integrin PAT-2.
(A) Schematic of the genetic pathway underlying apoptotic corpse engulfment in C. elegans. (B–D) Population counts of animals with AMsh glia puncta. Refer Figure 2C for data presentation details. (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type. (B) Alleles used in this graph: tat-1(tm3110), tat-1(tm1034), scrm-1(tm805), and ced-8(n1819). (C) Alleles used in this graph: ced-1(e1754), ced-1(e1735), ced-7(n2094), and ced-6(n1813). (D) Alleles used in this graph: psr-1(tm469), tat-1(tm1034), and ttr-52(tm2078). (E) Quantification of puncta within AMsh cell soma in listed mutants. Refer Figure 2D for data presentation details. Median puncta counts and N (number of animals): wild type (14 ± 1 puncta, n = 78 animals), psr-1(tm469) (7.4 ± 0.8 puncta, n = 28 animals), and tat-1 (41.6 ± 4.6 puncta, n = 19 animals). (F) Fluorescence micrograph of a transgenic animal with GFP tagged PSR-1 expressed specifically in AMsh glia (magenta) localizing on the apical membrane around AFD–NRE (green). GFP:PSR localizes to apical membrane in AMsh glia (yellow arrow) around AFD–NRE (asterisk). Scale bar: 5 μm. (F’) Zoom of box in two-color merged image. (G) RNAi (control pat-2) in wild-type or psr-1(tm469) mutant animals. Refer Figure 2C for data presentation details. EV: empty vector control. NRE: neuron-receptive ending.
-
Figure 4—source data 1
Engulfment of AFD–NRE by AMsh glia requires the phosphatidylserine receptor PSR-1 and integrin PAT-2.
Raw data for Figure 4B, C, D, E, G and Figure 4—figure supplement 1A–C. NRE: neuron-receptive ending.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig4-data1-v2.xlsx
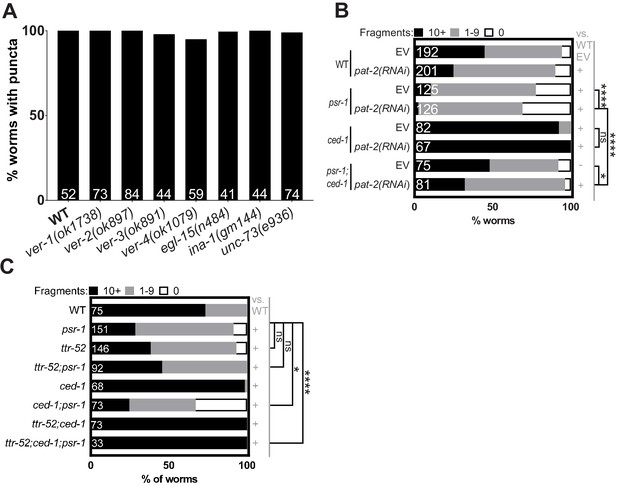
Engulfment of AFD–NRE by AMsh glia does not depend on some RTK or CED-1/MEGF10/Draper.
(A) Percent animals with AFD–NRE-labeled puncta in AMsh glia. X-axis: genotype; Y-axis: percent animals. N: number of animals examined. Alleles as noted. (B, C) Population counts of animals with AMsh glial puncta in animals as noted in the genotype. Refer Figure 2C for data presentation details. Alleles used in either graph: psr-1(tm469), ced-1(e1754), and ttr-52(tm2078). EV: empty vector control. (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type. NRE: neuron-receptive ending.
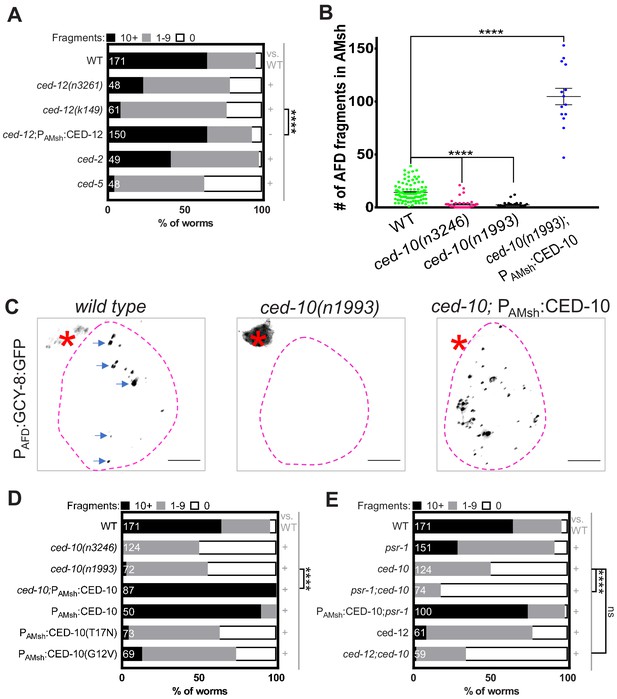
Phagocytosis pathway components, glial CED-10 levels, and actin remodeling actively control rate of engulfment.
(A) Population counts of animals with AMsh glial puncta in the indicated genetic backgrounds. Refer Figure 2C for data presentation details. (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type. Alleles used in this graph: ced-12(n3261), ced-12(k149), ced-2(e1752), and ced-5(n1812). (B) Quantification of puncta within AMsh cell soma in phagocytosis pathway mutants. Refer Figure 2D for data presentation details. Median puncta counts and N (number of animals): wild type (14 ± 1 puncta, n = 78 animals), ced-10(n1993) (2.4 ± 0.6 puncta, n = 24 animals), ced-10(n3246) (3.08 ± 0.79, n = 39), andPAMsh:CED-10 (104.7 ± 7.8 puncta, n = 14 animals). (C) Panel showing AFD–NRE tagged puncta (blue arrows) within AMsh glial cell soma (magenta outline) in different genetic backgrounds as noted. AFD cell body (red asterisk). Scale bar: 5 μm. (D, E) Population counts of animals with AMsh glial puncta in genetic backgrounds indicated. Refer Figure 2C for data presentation details. (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type. (D) Alleles used in this graph: ced-10(n3246) and ced-10(n1993). CED-10G12V and CED-10T17N is a constitutively active or dominant negative form of CED-10, respectively. (E) Alleles used in this graph: psr-1(tm469), ced-10(n3246), and ced-12 (k149). NRE: neuron-receptive ending.
-
Figure 5—source data 1
Phagocytosis pathway components, glial CED-10 levels, and actin remodeling actively control rate of engulfment.
Raw data for Figure 5A, B, D, E and Figure 5—figure supplement 1A–C.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig5-data1-v2.xlsx
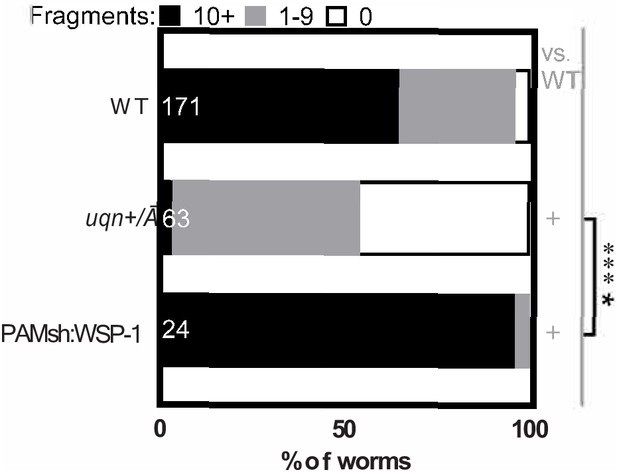
The actin regulator WSP-1 can regulate engulfment cell-autonomously in AMsh glia.
(A) Population counts of animals with AMsh glial puncta in animals as noted in the genotype. Allele used: wsp-1(gm324). Refer Figure 2C for data presentation details. (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type.
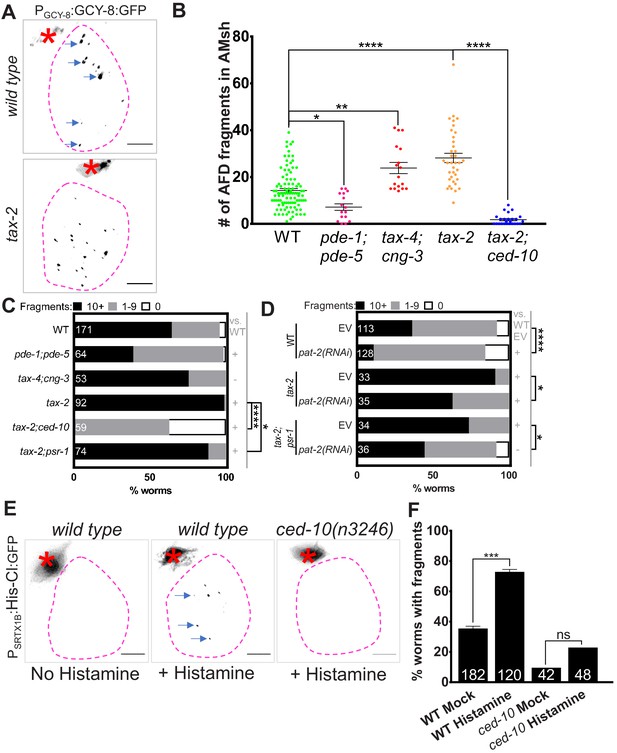
Glial phagocytic pathway tracks neuron activity to regulate AFD–NRE engulfment rate.
(A) Panel showing AFD–NRE tagged puncta (blue arrows) within AMsh glial cell soma (magenta outline) in different genetic backgrounds, as noted. AFD cell body (red asterisk). Scale bar: 5 μm. (B) Quantification of puncta within AMsh cell soma in phagocytosis pathway mutants. Refer Figure 2D for data presentation details. Median puncta counts and N (number of animals): wild type (14 ± 1 puncta, n = 78 animals), pde-1(nj57) pde-5(nj49) double mutant animals (7.1 ± 1.4, n = 11 animals), tax-4(p678);cng-3(jh113) double mutants (23.8 ± 2.4 puncta, n = 17 animals), tax-2(p691) (28.1 ± 2 puncta, n = 37 animals), and ced-10(n3246); tax-2(p691) double mutants (1.8 ± 0.5 puncta, n = 25 animals). (C, D) Population counts of animals with AMsh glial puncta in genetic backgrounds indicated. Refer Figure 2C for data presentation details. (+) p<0.05 compared to wild type, (–) p≥0.05 compared to wild type. (C) Alleles used in this graph: pde-1(nj57), pde-5(nj49), tax-4(p678), cng-3(jh113), tax-2(p691), ced-10(n3246), and psr-1(tm469). (D) Alleles used in this graph: tax-2(p691), and psr-1(tm469). EV: empty vector control. (E) Percent wild type or ced-10(n3246) mutant animals with observable GFP+ puncta with or without histamine. N: number of animals. (F) Quantification of percent animals with puncta in AMsh glia (Y-axis) in transgenic strains carrying a histamine-gated chloride channel, with/out histamine activation as noted (X-axis). NRE: neuron-receptive ending.
-
Figure 6—source data 1
Glial phagocytic pathway tracks neuron activity to regulate AFD–NRE engulfment rate.
Raw data for Figure 6B–D, F. NRE: neuron-receptive ending.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig6-data1-v2.xlsx
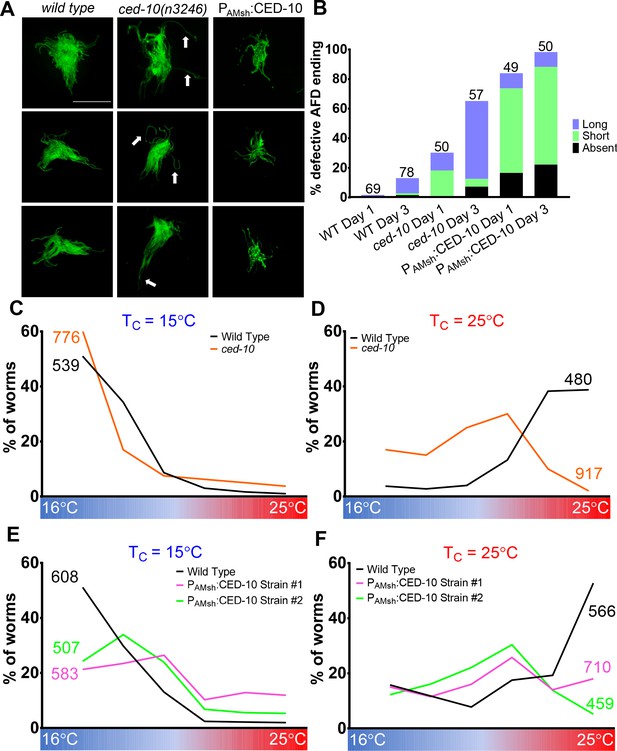
AMsh glial engulfment of AFD–NRE modulates AFD–NRE shape and animal thermosensory behavior.
(A) AFD–NRE microvilli labeled with GFP in day 3 adult animals of genotypes as indicated. Three representative images are shown for each genotype. Scale bar: 5 μm (B) Quantification of percent animals with defective AFD–NRE microvilli shape. N: number of animals scored. (C–F) Thermotaxis behavior assays for animals of indicated genotype raised at 15°C (C, E) or 25°C (D, F). Animals assayed 24 hr post-mid-L4 larval stage. N: number of animals. NRE: neuron-receptive ending.
-
Figure 7—source data 1
AMsh glial engulfment of AFD–NRE modulates AFD–NRE shape and animal thermosensory behavior.
Raw data for Figure 7B–F and Figure 7—figure supplement 1B– D. NRE: neuron-receptive ending.
- https://cdn.elifesciences.org/articles/63532/elife-63532-fig7-data1-v2.xlsx
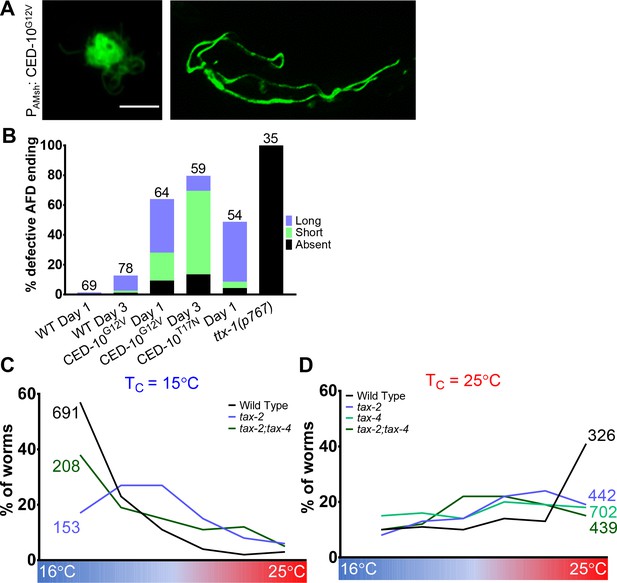
AMsh glial CED-10 tracks neuron activity to regulate AFD–NRE engulfment.
(A) Day 1 AFD–NRE defects in animals expressing constitutive active CED-10G12V in AMsh glia. (B) Proportion of worms with defective AFD–NRE shape on days 1 and 3 of adulthood in animals expressing constitutive active CED-10G12V or dominant negative CED-10T17N. ttx-1 (p767) mutant analysis included for reference. (C, D) Thermotaxis behavior assays for animals of indicated genotype raised at 15°C (C) or 25°C (D). Animals assayed 24 hr post-mid-L4 larval stage. N: number of animals. Genotype as noted. Alleles used for assays: tax-2(p691) and tax-4(p678). NRE: neuron-receptive ending.
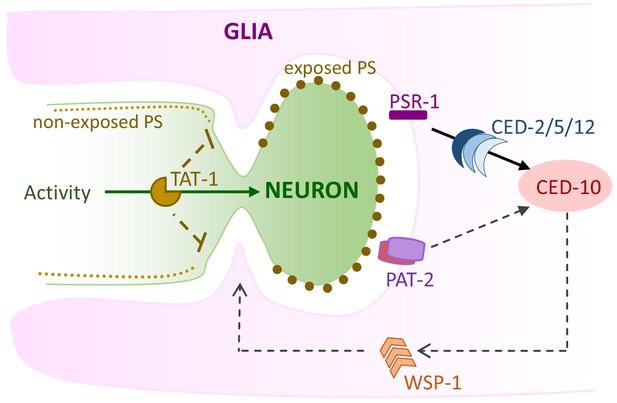
Model of AMsh glial engulfment of AFD–NRE.
Model depicting molecular machinery driving engulfment of AFD neuron microvilli by AMsh glia. TAT-1 maintains phosphatidylserine on the inner plasma leaflet. Neuron activity negatively regulates engulfment. The phosphatidylserine receptor PSR-1 signals via ternary GEF complex CED-2/5/12 to activate Rac1 GTPase CED-10, along with PAT-2/integrin. CED-10 and its downstream effector, WSP-1, drive engulfment of AFD neuron microvilli fragments. NRE: neuron-receptive ending.
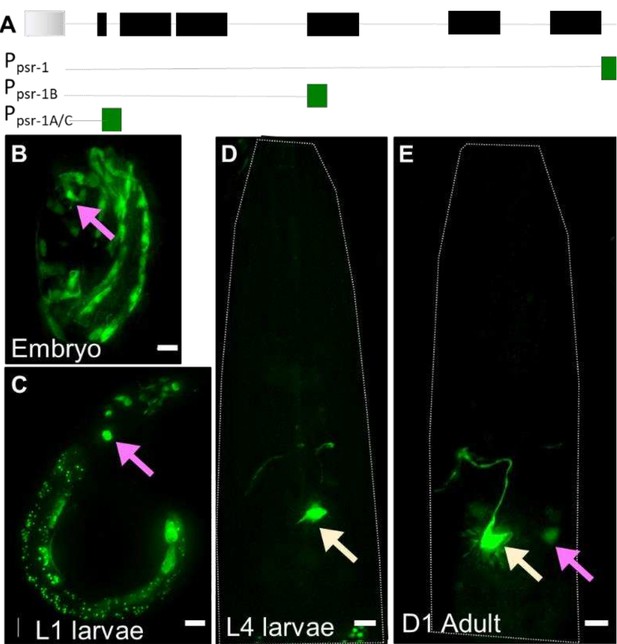
(A) psr-1 genomic organization and transcriptional reporters tested.
Gray: gene upstream; black = psr-1 exons; green = GFP. (B-E). Ppsr-1A:GFP transcriptional reporter across different life stages, as noted on each panel. Presumptive neural cells in the head region of the animal (magenta arrow), and interneuron (yellow arrow) are seen. Scale bar = 5µm.
Videos
Dissociation of AFD–NRE fragments.
Movie of an animal’s AFD–NRE, labeled with GFP and imaged in vivo at 7 frames/s, shows fragments blebbing at regular intervals. NRE: neuron-receptive ending.
AFD–NRE fragments are engulfed by AMsh glia.
Movie of an animal’s AFD–NRE (green) and AMsh glia (magenta) imaged in vivo at 7 frames/s shows fragments blebbing at regular intervals. NRE: neuron-receptive ending.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Caenorhabditis elegans) | nsIs481 | This paper | Singhvi Lab Database ID: OS8556 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Integration of nsEx3945. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsIs482 | This paper | Singhvi Lab Database ID: OS8557 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Integration of nsEx3945. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsIs483 X | This paper | Singhvi Lab Database ID: OS8558 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Integration of nsEx3945. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsIs484 | This paper | Singhvi Lab Database ID: OS8502 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Integration of nsEx3945. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsIs645 IV | This paper | Singhvi Lab Database ID: OS10884 | [50 ng/µl pAS322 (Psrtx-1B:STRX-1:GFP) + Punc-122:RFP]. Integration of nsEx4078. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsIs647 | This paper | Singhvi Lab Database ID: OS10805 | [50 ng/µl pAS322 (Psrtx-1B:STRX-1:GFP) + Punc-122:RFP]. Integration of nsEx4078. Request from corresponding author. |
| Strain, strain background(C. elegans) | dnaIs1 | This paper | Singhvi Lab Database ID: ASJ160 | [50 ng/µl pAS540 (Psrtx-1B:HisCl1:SL2:GFP) + elt-2:mCherry]. Integration of nsEx5340. Request from corresponding author. |
| Strain, strain background(C. elegans) | dnaIs2 | This paper | Singhvi Lab Database ID: ASJ161 | [50 ng/µl pAS540 (Psrtx-1B:HisCl1:SL2:GFP) + elt-2:mCherry]. Integration of nsEx5340. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaIs3 | This paper | Singhvi Lab Database ID: ASJ271 | [50 ng/µl pAS540 (Psrtx-1B:HisCl1:SL2:GFP) + elt-2:mCherry]. Integration of nsEx5340. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaIs4 | This paper | Singhvi Lab Database ID: ASJ280 | [50 ng/µl pAS540 (Psrtx-1B:HisCl1:SL2:GFP) + elt-2:mCherry]. Integration of nsEx5340. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaIs7 | This paper | Singhvi Lab Database ID: ASJ360 | [5 ng/µl pAS275 (PF53F4.13:CED-10:SL2:mCherry) + Pmig-24:Venus]. Integration of nsEx5365. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaIs8 | This paper | Singhvi Lab Database ID: ASJ359 | [5 ng/µl pAS275 (PF53F4.13:CED-10:SL2:mCherry) + Pmig-24:Venus]. Integration of nsEx5365. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsIs143X | Procko et al., 2011 | OS9176 | PF16F9.3:DsRed. |
| Strain, strain background (C. elegans) | nsIs109 | Bacaj et al., 2008b | OS1932 | PF16F9.3:DTA(G53E). |
| Strain, strain background (C. elegans) | nsEx3944 | Singhvi et al., 2016 | Singhvi Lab Database ID: OS7171 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Request from either corresponding author or Dr. Shai Shaham (The Rockefeller University, USA). |
| Strain, strain background (C. elegans) | nsEx3945 | Singhvi et al., 2016 | Singhvi Lab Database ID: OS7172 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Request from either corresponding author or Dr. Shai Shaham (The Rockefeller University, USA). |
| Strain, strain background (C. elegans) | nsEx3946 | Singhvi et al., 2016 | Singhvi Lab Database ID: OS7173 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Request from either corresponding author or Dr. Shai Shaham (The Rockefeller University, USA). |
| Strain, strain background (C. elegans) | nsEx3947 | Singhvi et al., 2016 | Singhvi Lab Database ID: OS7174 | [20 ng/µl 02097061181003035 C08 (Pgcy-8:gcy-8:GFP) + Pelt-2:mCherry]. Request from either corresponding author or Dr. Shai Shaham (The Rockefeller University, USA). |
| Strain, strain background (C. elegans) | nsEx4733 | This paper | Singhvi Lab Database ID: OS9078 | [20 ng/µl 9735267524753001 E03 (Pgcy-18:gcy-18:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4734 | This paper | Singhvi Lab Database ID: OS9079 | [20 ng/µl 9735267524753001 E03 (Pgcy-18:gcy-18:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4857 | This paper | Singhvi Lab Database ID: OS9406 | [20 ng/µl 9735267524753001 E03 (Pgcy-18:gcy-18:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4763 | This paper | Singhvi Lab Database ID: OS9164 | [20 ng/µl 9735267524753001 E03 (Pgcy-18:gcy-18:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4803 | This paper | Singhvi Lab Database ID: OS9276 | [20 ng/µl 6523378417130642 E08 (Pgcy-23:gcy-23:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4765 | This paper | Singhvi Lab Database ID: OS9166 | [20 ng/µl 6523378417130642 E08 (Pgcy-23:gcy-23:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4392 | This paper | Singhvi Lab Database ID: OS8257 | [20 ng/µl pAS428 (Psrtx-1B:DYF-11:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4393 | This paper | Singhvi Lab Database ID: OS8258 | [20 ng/µl pAS428 (Psrtx-1B:DYF-11:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4394 | This paper | Singhvi Lab Database ID: OS8259 | [20 ng/µl pAS428 (Psrtx-1B:DYF-11:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4446 | This paper | Singhvi Lab Database ID: OS8330 | [20 ng/µl pAS428 (Psrtx-1B:DYF-11:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4051 | This paper | Singhvi Lab Database ID: OS7443 | [50 ng/µl pAS322 (Psrtx-1B:SRTX-1:GFP) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4077 | This paper | Singhvi Lab Database ID: OS7541 | [50 ng/µl pAS322 (Psrtx-1B:SRTX-1:GFP) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4078 | This paper | Singhvi Lab Database ID: OS7542 | [50 ng/µl pAS322 (Psrtx-1B:SRTX-1:GFP) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4570 | This paper | Singhvi Lab Database ID: OS8598 | [25 ng/µl pAS447 (Psrtx-1:EGL-1) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4616 | This paper | Singhvi Lab Database ID: OS8767 | [25 ng/µl pAS447 (Psrtx-1:EGL-1) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx4688 | This paper | Singhvi Lab Database ID: OS8970 | [25 ng/µl pAS447 (Psrtx-1:EGL-1) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5266 | This paper | Singhvi Lab Database ID: OS10640 | [50 ng/µl pAS540 (Psrtx-1:HisCl1:SL2:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5340 | This paper | Singhvi Lab Database ID: OS10735 | [50 ng/µl pAS540 (Psrtx-1:HisCl1:SL2:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5356 | This paper | Singhvi Lab Database ID: OS10761 | [50 ng/µl pAS540 (Psrtx-1:HisCl1:SL2:GFP) + Pelt-2:mCherry]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5365 | This paper | Singhvi Lab Database ID: OS10781 | [5 ng/µl pAS275 (PF53F4.13:CED-10B:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5381 | This paper | Singhvi Lab Database ID: OS10826 | [5 ng/µl pAS275 (PF53F4.13:CED-10B:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5382 | This paper | Singhvi Lab Database ID: OS10877 | [5 ng/µl pAS275 (PF53F4.13:CED-10B:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx1 | This paper | Singhvi Lab Database ID: ASJ06 | [5 ng/µl pASJ11-pSAR1 (PF53F4.13:CED-12B:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx2 | This paper | Singhvi Lab Database ID: ASJ07 | [5 ng/µl pASJ11-pSAR1 (PF53F4.13:CED-12B:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx3 | This paper | Singhvi Lab Database ID: ASJ08 | [5 ng/µl pASJ11-pSAR1 (PF53F4.13:CED-12B:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx19 | This paper | Singhvi Lab Database ID: ASJ104 | [5 ng/µl pASJ23-pSAR7 (PF53F4.13:PSR-1C:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx30 | This paper | Singhvi Lab Database ID: ASJ143 | [5 ng/µl pASJ23-pSAR7 (PF53F4.13:PSR-1C:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx33 | This paper | Singhvi Lab Database ID: ASJ147 | [5 ng/µl pASJ23-pSAR7 (PF53F4.13:PSR-1C:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx29 | This paper | Singhvi Lab Database ID: ASJ142 | [5 ng/µl pASJ29-pSAR8 (PF53F4.13:CED-10BG12V:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx51 | This paper | Singhvi Lab Database ID: ASJ218 | [5 ng/µl pASJ37 (pSAR11) (PF53F4.13:CED-10BT17N:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx57 | This paper | Singhvi Lab Database ID: ASJ225 | [5 ng/µl pASJ37 (pSAR11) (PF53F4.13:CED-10BT17N:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx59 | This paper | Singhvi Lab Database ID: ASJ230 | [5 ng/µl pASJ37 (pSAR11) (PF53F4.13:CED-10BT17N:SL2:mCherry) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5268 | This paper | Singhvi Lab Database ID: OS10642 | [5 ng/µl pAS247 (PF53F4.13: WSP-1:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5363 | This paper | Singhvi Lab Database ID: OS10779 | [5 ng/µl pAS247 (PF53F4.13:WSP-1:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | nsEx5380 | This paper | Singhvi Lab Database ID: OS10825 | [5 ng/µl pAS247 (PF53F4.13:WSP-1:SL2:mCherry) + Pmig-24:Venus]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx160 | This paper | Singhvi Lab Database ID: ASJ488 | [45 ng/µl pASJ114-pSAR35 (Psrtx-1B:TAT-1A) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx162 | This paper | Singhvi Lab Database ID: ASJ498 | [45 ng/µl pASJ114-pSAR35 (Psrtx-1B:TAT-1A) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx70 | This paper | Singhvi Lab Database ID: ASJ266 | [2.5 ng/µl pASJ56-pSAR18 (PF53F4.13:GFP:PSR-1C) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx71 | This paper | Singhvi Lab Database ID: ASJ267 | [2.5 ng/µl pASJ56-pSAR18 (PF53F4.13:GFP:PSR-1C) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | dnaEx74 | This paper | Singhvi Lab Database ID: ASJ273 | [2.5 ng/µl pASJ56-pSAR18 (PF53F4.13:GFP:PSR-1C) + Punc-122:RFP]. Request from corresponding author. |
| Strain, strain background (C. elegans) | Wild type | CGC | Singhvi Lab Database ID: N2 | Reference strain. |
| Strain, strain background (C. elegans) | tax-2(p691) I | CGC | Singhvi Lab Database ID: PR691 | |
| Strain, strain background (C. elegans) | ced-12(n3261) I | CGC | Singhvi Lab Database ID: MT11068 | |
| Strain, strain background (C. elegans) | ced-12(k149) I | CGC | Singhvi Lab Database ID: NF87 | |
| Strain, strain background (C. elegans) | psr-1(tm469) I | CGC | Singhvi Lab Database ID: CU1715 | |
| Strain, strain background (C. elegans) | ced-1(e1754) I | CGC | Singhvi Lab Database ID: CB3261 | |
| Strain, strain background (C. elegans) | ced-1(e1735) I | CGC | Singhvi Lab Database ID: CB3203 | |
| Strain, strain background (C. elegans) | unc-73(e936) I | CGC | Singhvi Lab Database ID: CB936 | |
| Strain, strain background (C. elegans) | scrm-1(tm805) I | CGC | Singhvi Lab Database ID: CU2945 | |
| Strain, strain background (C. elegans) | ttr-52(tm2078) III | NBRP | Singhvi Lab Database ID: FX002078 | Kang et al., 2012. |
| Strain, strain background (C. elegans) | ced-6(n1813) III | CGC | Singhvi Lab Database ID: MT4433 | |
| Strain, strain background (C. elegans) | tat-1(tm1034) III | NBRP | Singhvi Lab Database ID: FX001034 | Darland-Ransom et al., 2008. |
| Strain, strain background (C. elegans) | tax-4(p678) III | CGC | Singhvi Lab Database ID: PR678 | |
| Strain, strain background (C. elegans) | ced-7(n2094) III | CGC | Singhvi Lab Database ID: MT8886 | |
| Strain, strain background (C. elegans) | ver-1(ok1738) III | CGC | Singhvi Lab Database ID: VC1263 | Consortium, C.e.D.M, 2012. |
| Strain, strain background (C. elegans) | ver-2(ok897) III | CGC | Singhvi Lab Database ID: RB983 | Consortium, C.e.D.M, 2012. |
| Strain, strain background (C. elegans) | ina-1(gm144) III | CGC | Singhvi Lab Database ID: NG144 | |
| Strain, strain background (C. elegans) | ced-10(n3246)IV | CGC | Singhvi Lab Database ID: MT9958 | |
| Strain, strain background (C. elegans) | ced-10(n1993) IV | CGC | Singhvi Lab Database ID: MT5013 | |
| Strain, strain background (C. elegans) | ced-2(e1752) IV | CGC | Singhvi Lab Database ID: CB3257 | |
| Strain, strain background (C. elegans) | ced-5(n1812) IV | CGC | Singhvi Lab Database ID: MT4434 | |
| Strain, strain background (C. elegans) | cng-3(jh113) IV | CGC | Singhvi Lab Database ID: KJ462 | |
| Strain, strain background (C. elegans) | ttx-1(p767) V | CGC | Singhvi Lab Database ID: PR767 | |
| Strain, strain background (C. elegans) | osm-6(p811) V | CGC | Singhvi Lab Database ID: PR811 | |
| Strain, strain background (C. elegans) | dyf-11(mn392) X | CGC | Singhvi Lab Database ID: SP1713 | |
| Strain, strain background (C. elegans) | ced-8(n1891) X | CGC | Singhvi Lab Database ID: MT5006 | |
| Strain, strain background (C. elegans) | ver-3(ok891) X | CGC | Singhvi Lab Database ID: VC610 | Consortium, C.e.D.M, 2012. |
| Strain, strain background (C. elegans) | ver-4(ok1079) X | CGC | Singhvi Lab Database ID: RB1100 | Consortium, C.e.D.M, 2012. |
| Strain, strain background (C. elegans) | egl-15(n484) X | CGC | Singhvi Lab Database ID: OS10586 | |
| Genetic reagent (Escherichia coli) | pat-2 RNAi | Kamath and Ahringer, 2003 | Singhvi Lab Database ID: pASJ_RNAi_1D1 | Ahringer RNAi library: WBGene00018832. |





